Abstract
Aversive visceral stimuli, such as those associated with sickness, suppress appetite. Yet an understanding of the neural mechanisms underlying illness-related anorexia has remained elusive. Carter et al. (2013) now identify a specific hindbrain → amygdala circuit that contributes to illness-induced loss of appetite.
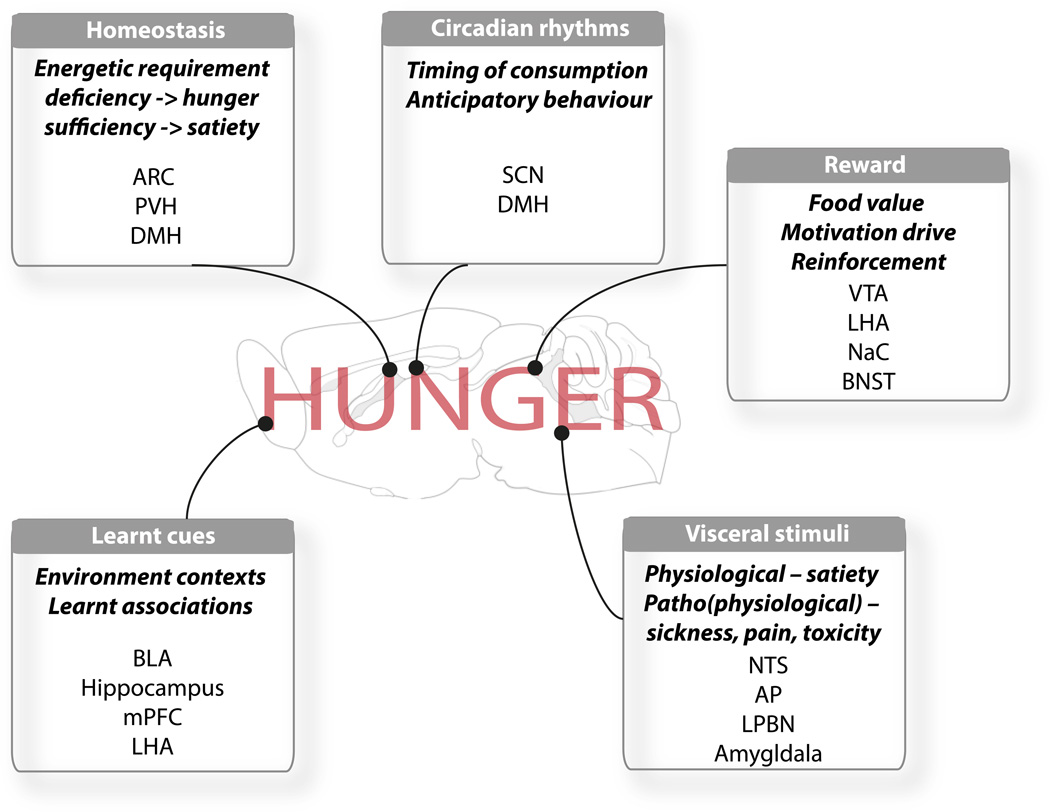
Hunger is a motivational state operationally defined as the tendency to seek out, work for, and eat food. Many stimuli converge to affect hunger including homeostatic deficiency-related cues (fasting increases hunger), the rewarding aspects of food (dessert is readily eaten after an otherwise energetically sufficient meal), circadian rhythms (eating occurs at certain times of day), learned environmental cues associated with foods or aversive experiences (we eat more during the holidays and less when fearful), and finally, internal stimuli associated with illness or visceral distress (Figure 1). Indeed, while the question of why we do eat is of immediate salience to the modern day obesity crisis, the latter point speaks to the potentially more vital question of why, under certain conditions, do we not eat? Carter et al. (2013) now provide a neurological basis for the pathophysiological ‘loss of appetite’ through their identification of “illness-activated” calcitonin gene related peptide (CGRP)-expressing neurons in the lateral parabrachial nucleus (LPBN).
Figure 1. Neuroanatomical integration of behavioural and physiological cues influencing hunger.
The drive to eat is underscored by the concerted action of numerous neuroanatomical microcircuits reactive to both internal (physiologic and pathophysiologic) signals and external environmental cues. Abbreviations: AP, area posterma; ARC, arcuate nucleus of the hypothalamus; BLA, basolateral amygdala; BNST bed nucleus of the stria terminalis; DMH, dorsomedial nucleus of the hypothalamus; LHA, lateral hypothalamic area; LPBN, lateral parabrachial nucleus; mPFC, medial prefrontal cortex; PVH, NaC, nucleus accumbens; NTS, nucleus of the solitary tract; paraventricular nucleus of the hypothalamus; SCN, suprachiasmatic nucleus; VTA, ventral tegmental area.
Hunger-promoting GABAergic AgRP neurons are indispensible for feeding. Located in the arcuate nucleus, they are activated by fasting and are linked to various aspects of energy homeostasis (Cansell et al 2012). Their targeted ablation in mice (AgRPDTR) leads to complete aphagia (Luquet et al 2005) and induces chronic neuronal activation and excitotoxicity in downstream neuroanatomical targets, including the LPBN (Wu et al 2008). Importantly, starvation in these animals was rescued by LPBN administration of a GABA-receptor agonist (Wu et al 2009). Thus, inducible ablation of AgRP neurons serves as a unique model of anorexia that has consistently pointed Palmiter’s group towards the LPBN as a site of functional outflow.
As a somatosensory relay for peripherally derived information the LPBN integrates sensory input from spinal afferents (directly) and vagal afferents (via the nucleus of the solitary tract) and is reactive to a number of (patho)physiologic visceroceptive modalities. Discreet subsets of LPBN neurons are activated by specific visceral stressors, including lipopolysaccharide (infection), lithium chloride (toxicity) and nociceptive stimuli (pain), all known to suppress food intake. Building upon their previous work, Carter et al now show that LPBN CGRP-expressing neurons contribute to aphagia in AgRPDTR mice, and suppress appetite in response to illness. Indeed, these cells were stimulated by AgRP neuron ablation and visceral stressors (LPS and LiCl). Functionally, LPBN CGRP cells are sufficient to suppress food consumption when artificially activated at times when animals would normally be motivated to feed (nocturnal and post-fast-refeeding). Furthermore, optogenetic terminal field stimulation of CGRP-projections within the central nucleus of the amygdala, the laterocapsular division (CeLC), recapitulated feeding suppression, identifying these projections as those underlying CGRP neuron-induced anorexia. To provide physiological relevance, Carter et al tested the necessity of LPBN CGRP neurons to suppress feeding within basal and aversive contexts. CGRP neuron silencing failed to promote a reciprocal increase in food consumption under basal conditions. However, within the context of visceral distress, inhibition of these cells partially ameliorated LPS and LiCl-induced anorexia, suggesting that LPBN CGRP neurons are part of the neurocircuitry through which illness suppresses feeding. Finally, and consistent with their earlier work, the authors reveal that silencing of CGRP neurons completely rescues the lethal aphagia phenotype of AgRPDTR mice.
Illness-associated anorexia is caused by an averse stimulus that reduces the drive to eat. In contrast, anorexia arising from satiety can be viewed as relief from deficiency-related hunger. Traditionally, AgRP neurons are associated with the homeostatic control of hunger, activated by states of energetic-depletion and suppressed by energetic-repletion. Consistently, their artificial activation in the nutritionally replete state or their inhibition in the deficient state, stimulates and inhibits feeding, respectively (Krashes et al 2011; Aponte et al 2011). Furthermore, this functional reciprocity holds true for the downstream paraventricular hypothalamic neurons through which AgRP neurons regulate homeostasis-directed feeding (Atasoy et al 2012; our unpublished observations). This tenet of deficiency-related hunger is not upheld by LPBN CGRP neurons; while their activation induces anorexia, their inhibition does not promote intake in a replete state, suggesting that CGRP neurons influence context-specific (illness-associated) anorexia, but not homeostatic control of appetite.
This functional dichotomy raises an interesting question: How is a population of deficiency-related hunger neurons (AgRP) functionally integrated into a somatosensory network associated with illness-induced anorexia (CGRP)? This issue is best addressed by considering the two components of the system in isolation. In the first instance, the capacity of CGRP neurons to suppress feeding under genuine pathophysiological conditions is a fascinating finding and the first to delineate a neurological framework for illness-associated appetite loss. Secondly, and divorced from this observation, is the involvement of AgRP neurons and their physiological relevance to CGRP neuron-induced anorexia. It is possible, if not likely, that AgRP neuron function transcends (patho)physiological context, such that via specific and distinct projections they promote feeding under various circumstances. Thus, during illness, when food consumption seems aversive, LPBN-projecting AgRP neurons temper CGRP neuron-induced anorexia to ensure long-term viability of the organism. However, this presumes a functional connection between hunger-promoting AgRP neurons and anorexia-promoting CGRP neurons. Through the identification of cFOS induction, as a marker of neuronal activation, in LPBN CGRP neurons of AgRPDTR mice, Carter et al. provide indirect evidence of this connection. Yet, given that these mice exhibit LPBN excitotoxicity, gliosis and inflammation, is it possible that AgRP neuron ablation somehow promotes chronic activation of resident CGRP neurons (and subsequent anorexia), secondary to withdrawal of AgRP neuron-derived inhibition? Undoubtedly, this model was invaluable in leading Carter et al to discover CGRP neuron function, but within the context of a neurologically intact animal the physiological role of AgRP neurons in their regulation remains unclear. To this end, some questions remain. Are AgRP neurons in synaptic contact with LPBN CGRP neurons? Would synaptic silencing of LPBN AgRP terminals (without ablation) promote anorexia via dis-inhibition of CGRP neurons, or their activation under visceral distress inhibit CGRP neurons and ameliorate illness-induced anorexia? Finally, is CGRP neuron activation aversive in its own right? Although the authors noted no overt signs of distress, given the context under which they are activated, it would be interesting to know if CGRP neuron stimulation elicits conditioned place aversion and/or taste aversion
The data provided by Carter et al allude to the neuroanatomical integration of hypothalamic AgRP neurons into the somatosensory parabrachio-amygdalaoid axis. If substantiated by further studies it would afford new insight into how interoceptive awareness of visceral state and the homeostatic need for food are reconciled. Independently, the involvement of CGRP neurons in illness-induced anorexia is of immediate clinical relevance. Infectious and inflammatory conditions, even those not impacting upon digestive function, are associated with suppressed appetite. The cellular components of this LPBN→CeLC microcircuit may yield novel therapeutic targets for the re-establishment of appetite, potentially aiding recuperation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansell C, Denis RG, Joly-Amado A, Castel J, Luquet S. Arcuate AgRP neurons and the regulation of energy balance. Front Endocrinol (Lausanne) 2012;3:169. doi: 10.3389/fendo.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Wu Q, Howell MP, Palmiter RD. Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions. J Neurosci. 2008;28:9218–9226. doi: 10.1523/JNEUROSCI.2449-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]