Abstract
Susceptibility to motion sickness is a predictor of postoperative nausea and vomiting, and studies in humans suggest that genetic factors determine sensitivity to motion sickness. The aim of the current study was to determine if a preclinical model could be selectively bred for motion-induced emesis and to assess a potential relationship to anesthesia-induced emesis. Musk shrews were tested for motion-induced emesis using a shaker plate (10 min, 1 Hz, and 4 cm of lateral displacement). Animals were rank ordered for motion-induced emesis and selectively bred to produce high and low response strains. Shrews were also tested with nicotine (5 mg/kg, sc), copper sulfate (CuSO4; 120 mg/kg, ig), and isoflurane anesthesia (10 min; 3%) to determine responses to a panel of emetic stimuli. High response strain shrews demonstrated significantly more emetic responses to motion exposure compared to low response strain animals in the F1 and F2 generations. In F2 animals, there were no significant differences in total emetic responses or emetic latency between strains after nicotine or CuSO4 injection. However, isoflurane exposure stimulated more emesis in F1 and F2 high versus low strain animals, which suggests a relationship between vestibular- and inhalational anesthesia-induced emesis. Overall, these results indicate genetic determinants of motion sickness in a preclinical model and a potential common mechanism for motion sickness and inhalational anesthesia-induced emesis. Future work may include genetic mapping of potential “emetic sensitivity genes” to develop novel therapies or diagnostics for patients with high risk of nausea and vomiting.
Keywords: Emesis, Vomiting, Nausea, Suncus, Genetics
1. Introduction
As many as 35% of US adults have experienced vestibular dysfunction, and prevalence increases with age [1]. Motion sickness has significant adverse effects on cognitive and physical performance [2-5]. Although motion sickness involves several divergent brain pathways and functional components (e.g., pallor, cold sweating, and disorientation) and a link to pronounced activation of stress response systems [6-8], a cardinal feature is the activation of nausea and vomiting (NV) [9]. The incidence of NV in medical settings can reach 80%, particularly in individuals at highest risk, such as patients with a history of sensitivity to motion sickness [10, 11], and a twin study estimates that motion sickness has a heritability of 57-70% [12]. Currently used anti-motion sickness drugs (e.g., histamine and muscarinic antagonists) do not always control NV and can result in sedation, blurred vision, and dizziness [13-15]. Research to date has focused on these older drugs, often with non-specific receptor targets, in heterogeneous human cohorts and preclinical models [14, 15]. A high-throughput approach in an easily manipulated preclinical model could provide greater mechanistic insight and identify more effective therapeutic strategies for the control motion sickness.
The focus of the current study was to selectively breed an animal model of motion-induced emesis that could be applied to future molecular-genetic studies. Musk shrews were used for these experiments because, unlike mice and rats [16, 17], they are capable of vomiting and are a well-characterized species for motion-induced emesis [18-24] using standardized behavioral test conditions [20]. Furthermore, musk shrews can be tested with this standardized approach in high-throughput screening (> 40 animals per day); breed rapidly; have a short time to maturity (~35 days to adulthood); and, at 40 to 80 g, are only slightly larger than mice, which allows high density housing. Animals were tested for vestibular-induced emesis by placing test cages on a shaker plate (10 min, 1 Hz, and 4 cm of lateral displacement). Shrews were ranked from high to low emetic responses to motion and selectively bred to produce high and low response strains. Animals were also tested with nicotine (5 mg/kg, sc), copper sulfate (120 mg/kg, ig), and isoflurane anesthesia (10 min; 3%) to determine responses to emetic stimuli acting on additional neural pathways. Circulating nicotine and intragastric copper sulfate (CuSO4) are believed to activate the area postrema and gut vagal afferent pathways, respectively [25-29]. In contrast, little is known about the mechanism for anesthesia-induced emesis.
2. Materials and methods
2.1. Animals
Musk shrews were descendants from breeding stock obtained from the Chinese University of Hong Kong; a Taiwanese strain of Suncus murinus [30]. Studies used 30 females and 30 males in the parental generation, 16 females and 20 males in the F1 generation, and 15 females and 16 males in the F2 generation (a total of 127 animals). Animals were housed in clear plastic cages (28 × 17 × 12 cm), with a filtered air supply, under a 12 h standard light cycle (lights on: 0700 h), in a temperature (~23°C) and humidity (~40%) controlled environment. Food and drinking water were freely available except during the brief test periods (~45 min). Food consisted of a mixture of 75% Purina Cat Chow Complete Formula and 25% Complete Gro-Fur mink food pellets [31]. All experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care international-accredited animal care facility.
2.2. Chemicals
Nicotine ((-)-Nicotine, catalog # 36733) and CuSO4 (copper (II) sulfate pentahydrate, catalog # 209198) were obtained from Sigma-Aldrich. Nicotine was made as a 2.5 mg/ml solution in sterile saline (0.15 M NaCl; subcutaneous injection = 5 mg/kg/2 ml) and copper sulfate was dissolved in filtered water (Milli-Q) at a concentration of 24 mg/ml (gavage injection = 120 mg/kg/5 ml). Isoflurane was provided via an enclosed chamber (allowed to fill for 2 min before use). Flow rates of gas were 6 L/min (from 100% O2 compressed air canister flowing through an isoflurane vaporizer; Matrx, TEC-3). Percentage of isoflurane was set by the dial on the vaporizer. The enclosed induction chamber was 10 × 8.5 cm, height and diameter.
2.3. Emetic testing procedures
Emetic tests were conducted with a 3 to 4 week time interval between tests to allow for recover (test order: motion, nicotine, CuSO4, and then isoflurane) [32, 33]. Animals were tested between 0800 and 1200 h (light phase). Testing for males and females was balanced to control for time of day effects. For motion, nicotine, and CuSO4 tests, animals had 15 min of adaption in the test chambers before the emetic stimulus. For motion exposure, the test chambers (28 × 17 × 12 cm) had a clear acrylic lid placed directly on the top. These chambers were placed on a reciprocating shaker (Taitec, Double Shaker R-30, Taiyo Scientific Industrial). Horizontal motion (4 cm displacement; 2 cm left and 2 cm right; 1 Hz) was applied for 10 min based on other studies that have determined optimal parameters [20]. In nicotine or CuSO4 tests, animals were subcutaneously injected with nicotine (5 mg/kg) or using a gavage needle for CuSO4 (120 mg/kg), based on previous studies [21, 34, 35]. Cohorts of F1 and F2 generations were also tested for isoflurane-induced emesis. Only a subset of F1 animals were used for the isoflurane test because other members of this cohort were euthanized to collect blood for future genetic analysis. Animals were placed in a transparent induction chamber for 10 min of isoflurane exposure, and then transferred to a transparent observation chamber using our published procedure [32].
All animal behavior was recorded with a digital video camera (Sony DCR-SR300 or HDRXR550V, wide field lenses) placed above each test chamber and connected to a computer for storage (Media Recorder; Noldus Information Technology). A trained observer was positioned outside the transparent test chambers to record the occurrence of an emetic episode (with or without a vomit), abdominal contraction, or a swaying movement using a notebook computer installed with coding software (JWatcher; http://www.jwatcher.ucla.edu/). Emetic episodes (with or without expulsion) were defined as a sequence of contractions of the abdominal region associated with forward movements of the head and separated by other episodes by a minimum of 2 s. In past studies, we have noted the occurrence of abdominal contractions (a single contraction of the abdominal region) and swaying movements (swaying the abdominal portion of the body from side to side) in association with emesis, and they were included in this report to determine potentially subtle differences between conditions.
2.4. Breeding procedures
Parental animals were ranked for emetic responses to motion exposure and divided into upper and lower 1/3 responders (n=10/group for males and females). The cutoffs were ≥ 15 (High group) and ≤ 10 (Low group) episodes for males; and ≥ 13 (High group) and ≤ 7 (Low group) for females. These High and Low group motion response parental males and females (i.e., the P-split) were selectively bred (i.e., high with high, and low with low). Because of fewer animals in F1 breeding we used a High cutoff ≥ 13 emetic episodes for both males and females. To reduce the fixation of genes that are associated with inbreeding (and likely not with the phenotype of emesis), we only bred animals that were not siblings and did not share a parent. For breeding, one adult musk shrew female is placed in the home cage of an adult male overnight. The gestation period is a total of approximately 30 days. The average litter size was 2 pups. The pups were housed with their mother until 21 days of age. From that time, animals were housed singly and tested for emetic responses during adulthood (i.e., > 35 days of age).
2.5. Data analysis
Dependent measures included emetic episodes, episodes with vomiting, episodes without vomiting, duration (time from first to last emetic event), emetic rate (episodes/min), standard deviation of the emetic interval (SD-I), abdominal contractions, and swaying were analyzed. SD-I was calculated as the standard deviation of the intervals between emetic episodes and can be used as a measure of the variability in the rhythm of emesis during the emetic duration. In each generation, data were analyzed with ANOVA for each emetic stimulus and variable (strain by sex factorial design). Hom-Sidak tests were used to compare means after ANOVA. It is frequently difficult to apply parametric statistics to behavioral latency data, which are often skewed. In the F1 isoflurane test, females and males were combined to form High and Low groups because of the lower power in this experiment (e.g., only n = 4 High females); T-tests were used to compare groups. To address this issue we used survival plots and Cox regression analysis for comparison of latency data (time to the first emetic event). This approach permits the use of all data, including censored values (i.e., animals that did not show emesis during the test period) [36]. P < 0.05 was used to determine statistical significance for all tests.
3. Results
3.1. Parental and P-split generation responses to motion, nicotine, and CuSO4
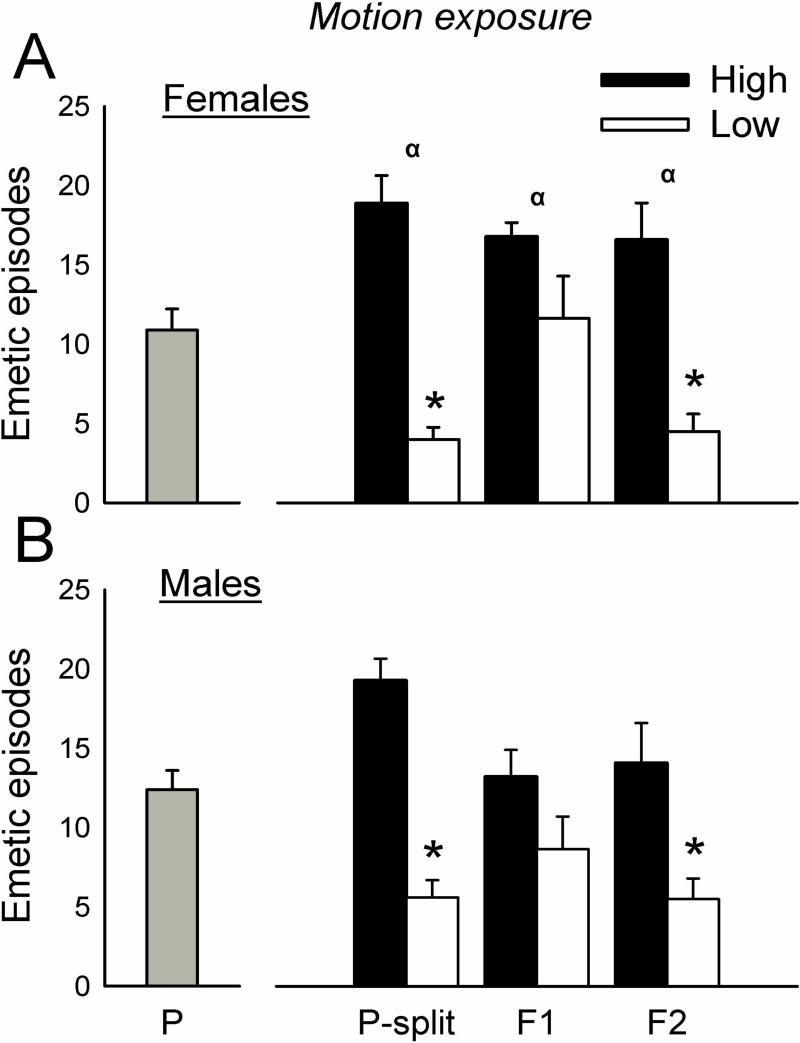
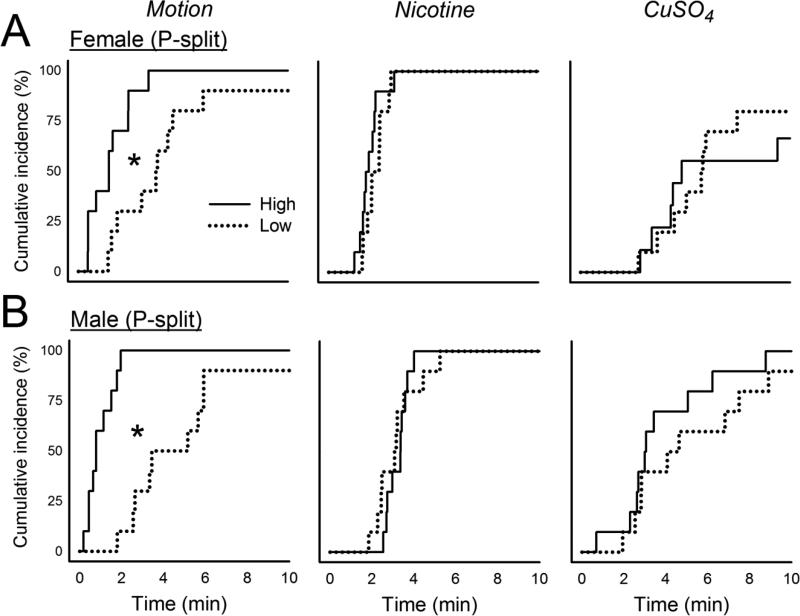
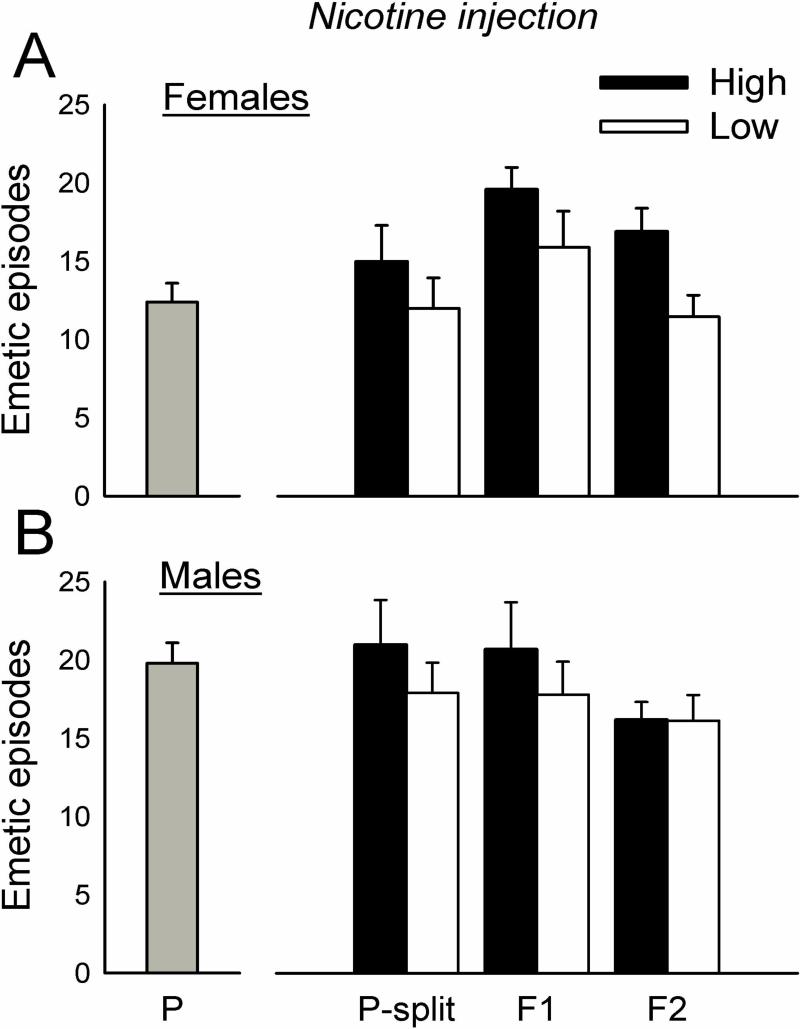
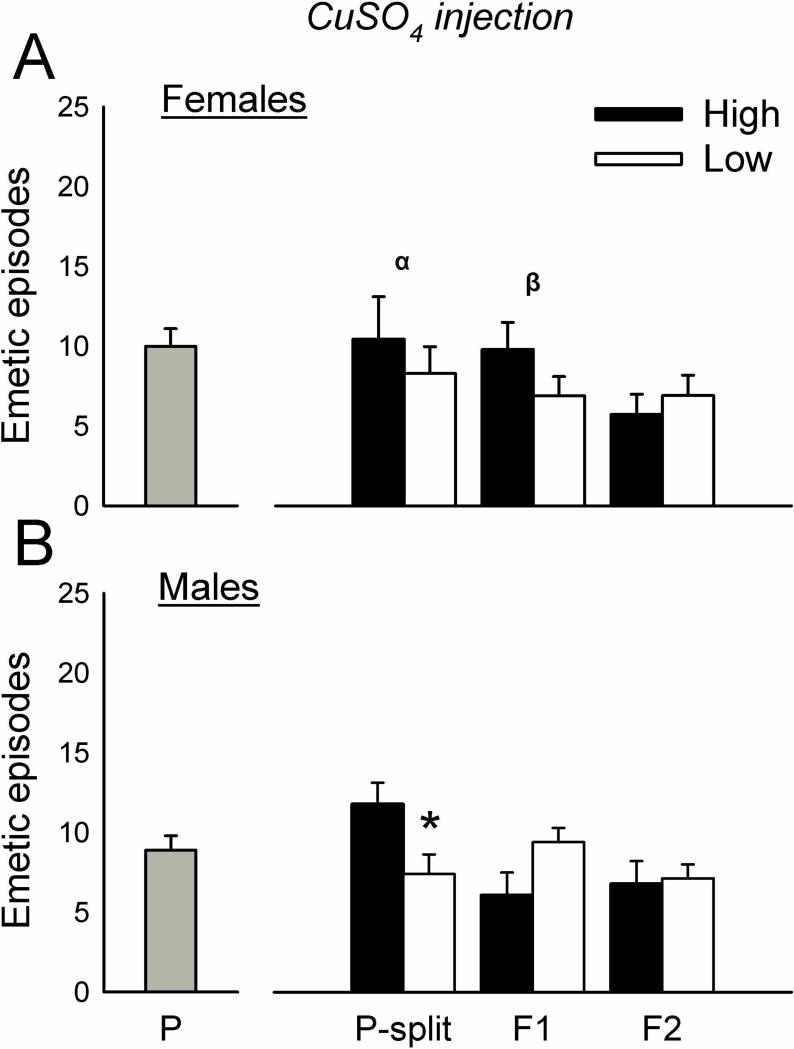
The initial cohorts of parental animals displayed 0 to 29 total emetic responses (10.9 ± 7.1, mean ± standard deviation) for females and 0 to 27 responses (12.4 ± 6.5, mean ± standard deviation) for males (Fig.1) to motion exposure. Animals were ranked for emetic responses to motion and divided into higher and lower 1/3 responders (n=10 per group for males and females). This selection (i.e., the P-split animals) produced significantly more total emetic episodes, episodes with a vomits, episodes without a vomit, duration, rate, and SD-I in High versus Low groups after motion exposure [F's(1,32) ≥ 7.5, p's ≤ 0.01, ANOVA, main effects of group; p's < 0.05, Hom-Sidak tests; Fig.1 and Table 1]. Motion exposure also produced a shorter emetic latency in the High compared to Low group (p < 0.05, Cox regression, Fig. 4). There were no significant differences in emetic responses after nicotine injection in selected groups (Fig. 2, Fig. 4, and Table 1). P-split animals displayed significantly more CuSO4-induced emetic episodes in High versus Low group males [F(1,35) = 6.5, p < 0.02, ANOVA, main effect of group; p < 0.05, Hom-Sidak test; Fig. 3], an effect that was related to the increased number of emetic episodes without a vomit [F(1,35) = 4.3, p < 0.05, ANOVA, interaction effect; p < 0.05, Hom-Sidak test; Table 1]. There was no statistically significant difference in emetic latency after CuSO4 injection (Fig. 4).
Fig. 1.
Total emetic episodes (with and without a vomit) to motion exposure (10 min, 1 Hz, 4 cm lateral displacement) in the parental (P), upper and lower responders from P (P-split), and two generations of offspring (F1 and F2). The P generation was divided into upper (High) and lower (Low) 1/3 responders for motion-induced emesis (P-split) and then bred. A) Females. B) Males. * = p < 0.05, Hom-Sidak test, High versus Low. α = p < 0.05, ANOVA, main effect of strain. Values are mean ± SEM.
Table 1.
Emetic responses to Motion, Nicotine, and CuSO4
| Motion | Nicotine | CuSO4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | ||||||||||
| High | Low | High | Low | High | Low | High | Low | High | Low | High | Low | ||||
| Episodes with vomit | |||||||||||||||
| P-Split | α | 5.2 (±1.3)* | 1.0 (±0.5) | 3.8 (±0.7)* | 1.4 (±0.5) | 1.9 (±0.6) ( | 0.8 (±0.5) | 1.2 (±0.4) | 1.7 (±0.6) | 4.4 (±1.3) | 2.1 (±0.7) | 3.5 (±0.8) | 3.5 (±0.3) | ||
| F1 | 4.4 (±0.7) | 3.5 (±0.8) | 4.7 (±0.9) | 2.7 (±0.8) | α | 1.8 (±0.5) | 1.2 (±0.4) | 2.8 (±0.7)* | 0.8 (±0.3) | 3.0 (±1.3) | 3.2 (±0.8) | 2.2 (±0.5) | 4.2 (±0.5) | ||
| F2 | α | 3.9 (±0.7)* | 1.0 (±0.3) | 4.6 (±0.7)* | 1.1 (±0.3) | 1.0 (±0.4) | 1.1 (±0.3) | 1.4 (±0.4) | 1.0 (±0.3) | 2.3 (±0.6) | 2.1 (±0.4) | 2.8 (±0.9) | 2.7 (±0.4) | ||
| Episodes w/out vomit | |||||||||||||||
| P-Split | α | 13.8 (±1.5)* | 3.0 (±0.6) | 14.0 (±1.7)* | 4.2 (±1.0) | 12.5 (±1.9) | 9.8 (±1.4) | 19.2 (±2.5) | 14.9 (±1.6) | α,β | 6.0 (±1.6) | 5.5 (±1.3) | 10.9 (±2.2)* | 3.7 (±1.1) | |
| F1 | 12.4 (±1.1) | 8.1 (±1.9) | 8.6 (±1.5) | 5.9 (±1.5) | 17.8 (±1.7) | 14.7 (±2.1) | 17.9 (±3.1) | 17.0 (±2.2) | β | 6.8 (±0.9) | 3.7 (±0.9) | 3.9 (±1.2) | 5.2 (±0.9) | ||
| F2 | α | 12.7 (±2.2)* | 3.5 (±1.0) | 9.5 (±2.5)* | 4.5 (±1.1) | β | 15.9 (±1.5)* | 10.4 (±1.2) | 14.8 (±1.0) | 15.1 (±1.5) | 3.5 (±0.9) | 4.8 (±1.3) | 4.0 (±0.9) | 4.5 (±1.0) | |
| Duration (time from 1st to last episode; min) | |||||||||||||||
| P-Split | α | 7.7 (±0.3)* | 4.3 (±0.6) | 7.6 (±0.4)* | 4.6 (±0.4) | 3.7 (±0.8) | 3.7 (±1.0) | 7.6 (±1.8) | 7.8 (±1.5) | 12.0 (±3.8) | 11.1 (±2.9) | 8.9 (±2.2) | 6.4 (±2.7) | ||
| F1 | α | 7.8 (±0.5) | 6.9 (±0.6) | 7.5 (±0.5)* | 5.5 (±0.6) | 6.9 (±1.0) | 5.1 (±0.7) | 8.4 (±1.4) | 8.3 (±1.6) | 11.2 (±1.4) | 11.7 (±1.9) | 8.3 (±3.2) | 11.5 (±3.0) | ||
| F2 | α | 7.3 (±0.5)* | 4.0 (±0.7) | 7.2 (±0.6) | 5.8 (±1.2) | 4.6 (±0.6) | 4.2 (±0.5) | 6.1 (±1.2) | 9.1 (±1.5) | 6.4 (±1.3) | 10.8 (±2.2) | 7.9 (±1.5) | 6.7 (±1.3) | ||
| Rate (episodes/min) | |||||||||||||||
| P-Split | α | 2.4 (±0.2)* | 1.0 (±0.2) | 2.2 (±0.2)* | 1.3 (±0.2) | 4.3 (±0.5) | 3.2 (±0.4) | 3.3 (±0.5) | 2.5 (±0.4) | 1.6 (±1.5) | 1.5 (±0.6) | 2.8 (±0.9) | 2.6 (±0.8) | ||
| F1 | 2.1 (±0.2) | 1.6 (±0.3) | 1.6 (±0.2) | 1.7 (±0.3) | 3.1 (±0.5) | 3.0 (±0.3) | 2.7 (±0.4) | 2.7 (±0.4) | 0.8 (±0.2) | 0.7 (±0.1) | 1.9 (±0.6) | 1.4 (±0.4) | |||
| F2 | α | 2.1 (±0.2)* | 1.4 (±0.3) | 1.9 (±0.2) | 1.3 (±0.2) | α | 3.6 (±0.2) | 2.6 (±0.3) | 3.5 (±0.6) | 2.5 (±0.4) | 1.9 (±1.1) | 0.6 (±0.1) | 1.0 (±0.4) | 1.6 (±0.3) | |
| SD-I (min) | |||||||||||||||
| P-Split | α | 0.5 (±0.1)* | 1.1 (±0.2) | 0.6 (±0.1) | 0.9 (±0.2) | 0.3 (±0.1) | 0.4 (±0.2) | 0.7 (±0.2) | 0.8 (±0.2) | 1.8 (±0.4) | 2.6 (±0.7) | 1.6 (±0.4) | 1.8 (±0.9) | ||
| F1 | 0.6 (±0.1) | 0.9 (±0.2) | 0.8 (±0.1) | 0.8 (±0.2) | 0.5 (±0.1) | 0.3 (±0.1) | 0.8 (±0.2) | 1.0 (±0.3) | 2.5 (±0.5) | 3.7 (±0.7) | 3.3 (±2.1) | 2.7 (±0.7) | |||
| F2 | 0.6 (±0.1) | 0.8 (±0.3) | 0.6 (±0.1) | 0.9 (±0.2) | 0.2 (±0.0) | 0.5 (±0.1) | 0.7 (±0.3) | 1.0 (±0.2) | 2.2 (±0.5) | 2.9 (±0.4) | 2.2 (±0.4) | 2.2 (±0.6) | |||
= p < 0.05, Hom-Sidak test, High vs. Low;
= p < 0.05, ANOVA, main effect of strain
= p < 0.05, ANOVA, interaction effect
Fig. 4.
Cumulative latency to the first emetic episode after motion exposure (10 min, 1 Hz), nicotine injection (5 mg/kg, sc), or CuSO4 injection (120 mg/kg, ig) in animals divided into upper (High) and lower (Low) 1/3 responders for motion-induced emesis (P-split). A) Females. B) Males. * p < 0.05, Cox regression, High versus Low.
Fig. 2.
Total emetic episodes (with and without a vomit) to nicotine (5 mg/kg, sc) in the parental (P), upper and lower responders from P (P-split), and two generations of offspring (F1 and F2). The P generation was divided into upper (High) and lower (Low) 1/3 responders for motion-induced emesis (P-split) and then bred. A) Females. B) Males. Values are mean ± SEM.
Fig. 3.
Total emetic episodes (with and without a vomit) to CuSO4 (120 mg/kg, ig) in the parental (P), upper and lower responders from P (P-split), and two generations of offspring (F1 and F2). The P generation was divided into upper (High) and lower (Low) 1/3 responders for motion-induced emesis (P-split) and then bred. A) Females. B) Males. * = p < 0.05, Hom-Sidak test, High versus Low. α = p < 0.05, ANOVA, main effect of strain. β = p < 0.05, ANOVA, interaction effect. Values are mean ± SEM.
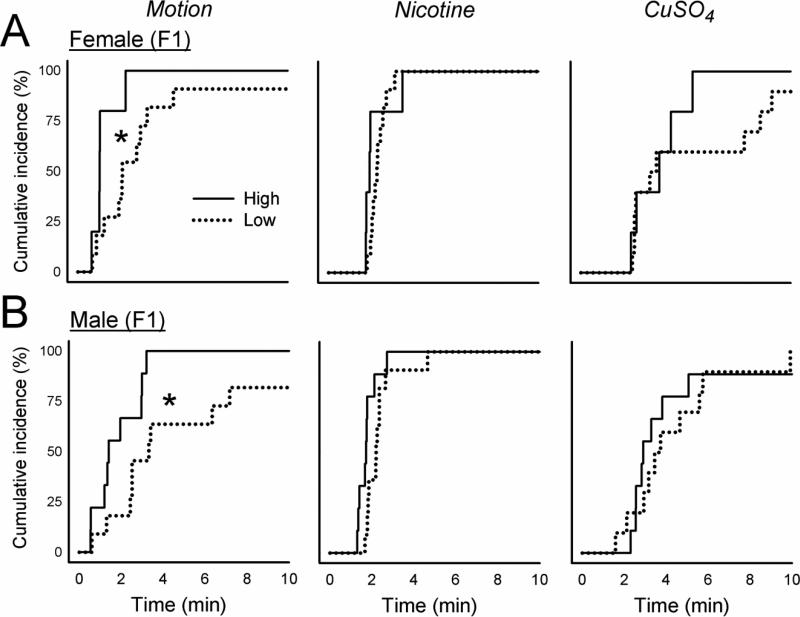
3.2. F1 generation responses to motion, nicotine, and CuSO4
Breeding the P-split generation High females with High males, and Low females with Low males, produced n = 6 High female, n = 9 High male, n = 11 Low female, and n = 11 Low male F1 animals. The High strain offspring (i.e., the F1) showed significantly greater total emetic responses and duration to motion exposure compared to Low strain offspring [F's(1,32 or 29) ≥ 4.2, p's ≤ 0.05, ANOVA, main effect of group; p < 0.05, Hom-Sidak test; Fig. 1 and Table 1]. This effect was also demonstrated as a reduced emetic latency after motion exposure in High versus Low strain males and females (p < 0.05, Cox regression, Fig. 5). There were no significant differences between High and Low F1 strains for total emetic episodes or emetic latency after nicotine injection (Fig. 2 and Fig. 5), but nicotine produced more emetic episodes with vomiting in High versus Low males [F(1,32) = 7.0, p < 0.02, ANOVA, main effect of group; p < 0.05, Hom-Sidak test; Table 1]. Significant interaction effects (sex by strain) were detected for CuSO4 injection in the number of emetic episodes (total and without a vomit) [F's(1,30) ≥ 4.3, p's < 0.05, ANOVA, interaction effects; Fig. 3 and Table 1], but with no significant effects on emetic latency (Fig. 5).
Fig. 5.
Cumulative latency to the first emetic episode after motion exposure (10 min, 1 Hz), nicotine injection (5 mg/kg, sc), or CuSO4 injection (120 mg/kg, ig) in the F1 generation of strains selectively bred for motion-induced emesis. A) Females. B) Males. * p < 0.05, Cox regression, High versus Low.
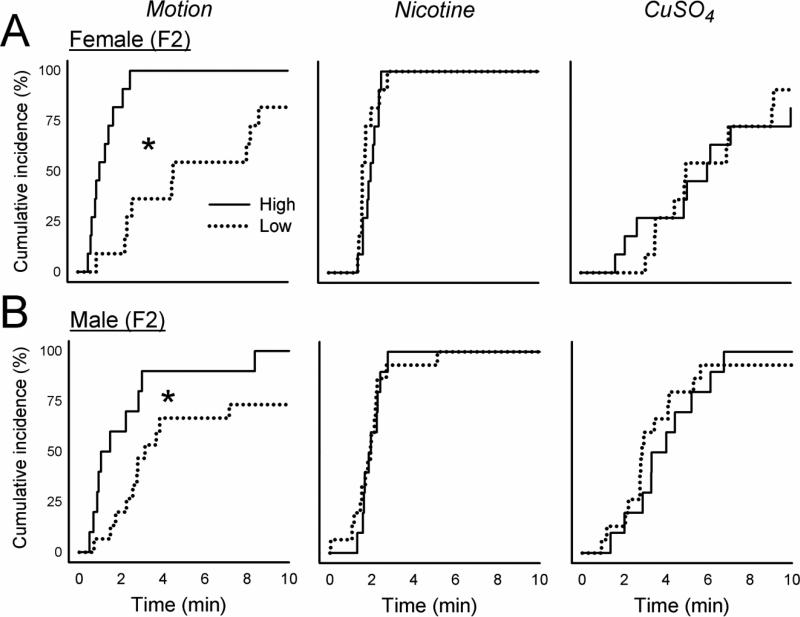
3.3. F2 generation responses to motion, nicotine, and CuSO4
Breeding the F1 High females with the High males (n = 4 and n = 5, respectively, i.e., those meeting the cutoff), and Low females with the Low males (n = 6 and n = 6, respectively), produced n = 11 High female, n = 10 High male, n = 11 Low female, and n = 15 Low male F2 animals. The High strain offspring (i.e., the F2) of F1 breeding showed significantly greater total emetic episodes, episodes with a vomit, episodes without a vomit, duration, and rate after motion exposure compared to Low strain offspring [F's (1,36) ≥ 7.8, p < 0.01, ANOVA, main effects of group; p < 0.05, Hom-Sidak tests; Fig. 1 and Table 1]. Similarly, High strain males and females showed a shorter emetic latency compared to Low strain animals exposed to motion (p < 0.05, Cox regression; Fig. 6). Nicotine injection produced a significant increase in episodes without a vomit in High versus Low females [F(1,43) = 4.3, p < 0.05, ANOVA interaction effect; p < 0.05, Hom-Sidak test; Table 1], and a faster emetic rate in High compared to Low strain animals [F(1,42) = 6.8, p < 0.05, ANOVA, main effect; Table 1]. There were no significant effects of CuSO4 injection on emesis in the F2 strain (Fig. 2, 3, and 6).
Fig. 6.
Cumulative latency to the first emetic episode after motion exposure (10 min, 1 Hz), nicotine injection (5 mg/kg, sc), or CuSO4 injection (120 mg/kg, ig) in the F2 generation of strains selectively bred for motion-induced emesis. A) Females. B) Males. * p < 0.05, Cox regression, High versus Low.
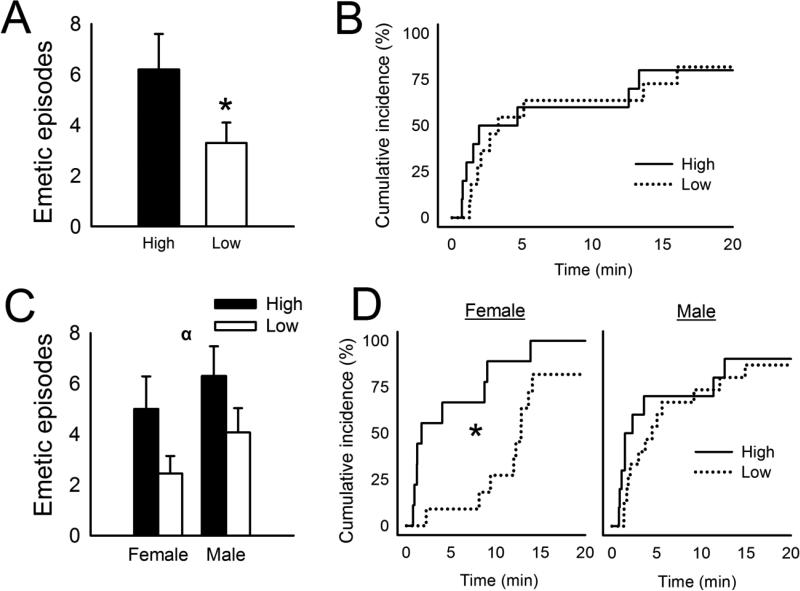
3.4. F1 and F2 generation responses to isoflurane
High strain F1 animals (n = 4 females plus n = 6 males) tested with isoflurane displayed more total emetic episodes and abdominal contractions than Low strain F1 animals (n = 4 females plus n = 7 males) [t(19) = 4.3, p < 0.04, t-test, one-tailed, Fig. 7A and Table 2], but no difference in emetic latency (Fig. 7B). High strain F2 shrews showed more total emetic episodes, episodes with a vomit, and a longer emetic duration than Low strain animals [F's(1,34) ≥ 6.9, p's < 0.05, ANOVA, main effects; p < 0.05, Hom-Sidak test; Fig. 7C and Table 2]. High strain F2 females showed a shorter emetic latency compared to Low strain females exposed to isoflurane (p < 0.05, Cox regression; Fig. 7D).
Fig. 7.
Isoflurane-induced emesis in the F1 and F2 generation of musk shrews selectively bred for motion-induced emesis (High and Low response strains). A) Total number of emetic episodes to isoflurane exposure (10 min; 3%) in the F1 generation. * = p < 0.05, t-test. Values are mean ± SEM. B) Cumulative latency to the first emetic episode to isoflurane exposure in the F1 generation. C) Total number of emetic episodes to isoflurane exposure (10 min; 3%) in the F2 generation females and males. α = p < 0.05, ANOVA, main effect of strain. Values are mean ± SEM. D) Cumulative latency to the first emetic episode to isoflurane exposure in the F2 generation females and males. * p < 0.05, Cox regression, High versus Low.
Table 2.
Emetic responses to isoflurane
| F1 | F2 | ||||||
|---|---|---|---|---|---|---|---|
| Female | Male | ||||||
| High | Low | High | Low | High | Low | ||
| Episodes with vomit | 1.9 (±0.8) | 0.5 (±0.3) | α | 1.8 (±0.7) | 0.8 (±0.3) | 2.4 (±0.5)* | 0.6 (±0.2) |
| Episodes w/out vomit | 4.3 (±0.9) | 2.7 (±0.7) | 3.2 (±0.9) | 1.6 (±0.5) | 3.9 (±1.0) | 3.5 (±0.9) | |
| Duration (min) | 10.4 (±1.8) | 9.7 (±3.4) | α | 11.1 (±1.2) | 3.7 (±1.2) | 12.3 (±1.5) | 10.3 (±1.0) |
| Rate (episodes/min) | 0.9 (±0.3) | 0.7 (±0.3) | β | 0.4 (±0.1)* | 1.1 (±0.3) | 0.6 (±0.1) | 0.5 (±0.1) |
| SD-I (min) | 2.8 (±0.6) | 2.6 (±0.9) | 3.9 (±1.0) | 1.3 (±0.5) | 3.8 (±1.3) | 3.7 (±0.4) | |
| AbCon | 10.3 (±2.6)* | 3.1 (±0.7) | 6.8 (±1.7) | 6.8 (±0.8) | 8.8 (±0.8) | 8.5 (±1.9) | |
F1: = p < 0.05, t-test, one-tailed, High vs. Low
F2: = p < 0.05, Hom-Sidak test, High vs. Low
= p < 0.05, ANOVA, main effect of strain
= p < 0.05, ANOVA, interaction effect
4. Discussion
These data show that musk shrews can be successfully bred for emetic sensitivity to motion exposure. Strain differences in motion-induced emesis did not translate into consistent differences in response to nicotine and CuSO4 injections. Ultimately F2 strains showed a largely specific response to motion exposure, with a greater amount of motion-induced emesis in High versus Low group animals. Moreover, as predicted, isoflurane exposure produced more emesis in F1 and F2 High strain offspring compared to Low strain animals.
The significant difference observed in total number of emetic responses to motion exposure in the parental P-split animals was reduced in F1 generation males and females. Larger differential responses to motion exposure returned in the F2 generation. In reference to other measures of emesis (i.e., emetic latency, episodes with and without vomits, duration, and rate in Fig. 6 and Table 1), P-split and F2 animals also displayed more statistically significant categories of emetic responses compared to F1 animals. Notably there was a shorter latency to emesis (1 vs. 4 min), a longer duration (7 vs. 4 min), and a faster rate (2 vs. 1 episodes/min) in High versus Low F2 animals. Relatively few, small, and inconsistent effects were observed between High and Low strain animals injected with nicotine or CuSO4.
Selective breeding for motion-induced emesis also resulted in a greater amount of emetic responses in High versus Low animals exposed to isoflurane in the F1 and F2 generations. It is unknown how inhalational anesthesia produces emesis. Inhalational anesthesia using fluranes (e.g., sevoflurane, isoflurane) produces postoperative nausea and vomiting (PONV), and a longer duration of exposure to these agents is associated with more PONV [37]. Patients are reported to show more dizziness after sevoflurane, which suggests that inhalational anesthesia could affect the vestibular system [38]. The current data support the hypothesis that isoflurane exposure acts on the vestibular system because animals selectively bred for motion-induced emesis also displayed higher levels of isoflurane-induced emesis. The effect of isoflurane on emesis appears to be more difficult to statistically assess in this model because it produces a low level of emesis (i.e., a mean of approximately 6 emetic episodes) compared to motion, nicotine, and CuSO4 conditions, with average responses of greater than 10 emetic episodes.
Based on our current knowledge, this study represents the first time that musk shrews have been specifically bred for motion-induced emesis. Our prior study showed that geographically distinct strains of musk shrews have differential emetic responses to motion exposure, with Taiwan-derived animals displaying more motion-induced emesis than Guam-derived shrews [33]. Other investigators have reported selective breeding of musk shrews for veratrine-induced emesis [39], a plant alkaloid toxin believed to act on the vagus nerve [40, 41]. These veratrine-sensitive animals were also subsequently reported to display differential emetic responses to motion exposure [42, 43]; this would indicate that these high and low response animals have a general sensitivity to emetic activation – independent of the type of emetic input. In contrast, the current study suggests that our High and Low strain musk shrews have specific sensitivity to activation of the vestibular system. The current data demonstrate this difference by testing nicotine and CuSO4 responses, which are indicated to act on the area postrema and vagal afferents, respectively [25-29]. An array of emetic measures were also used, including number of episodes (with and without vomiting), duration, rate, SD-I, and latency (a Cox regression approach) to further define the specific behavioral differences between High and Low strain animals.
The current results suggest that genetic factors play a significant role in emetic sensitivity to motion, and potentially isoflurane exposure. Similarly, a human twin study estimates that motion sickness has a heritability of 57-70% [12], and polymorphisms of the alpha-2-adrenergic receptor are associated with motion sickness in humans [6]. Moreover, human studies show that genetic polymorphisms in neurotransmitter receptors (serotonin type 3, mu-opioid, muscarinic type 3, and dopamine 2) are related to the severity of PONV [44-49]. Musk shrew strains with differential sensitivity to emetic stimuli could potentially serve as a laboratory model to determine the biological mechanisms for differences in the experience of emesis. Future studies could potentially focus on delineating the genetic differences of selectively sensitive musk shrews using DNA and RNA sequencing and bioinformatics.
Highlights.
> Musk shrews were selectively bred (High & Low) for sensitivity to motion-induced emesis
> Isoflurane exposure produced more emesis in High compared to Low strain animals
> Results suggest a common mechanism for motion and inhalational anesthesia-induced emesis
Acknowledgements
The authors wish to thank the University of Pittsburgh, Division of Laboratory Animal Re- search (DLAR) for the care of the musk shrew colony. This work was supported by an NIH grant to the University of Pittsburgh Cancer Institute, P30 CA047904 (Cancer Center Support Grant). This project used the UPCI Animal Facility, which was also supported in part by the P30CA047904 award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Arch. Intern. Med. 2009;169:938–44. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- 2.Griffin M. Handbook of human vibration. Academic Press; London: 1990. [Google Scholar]
- 3.Stevens SC, Parsons MG. Effects of motion at sea on crew performance: A survey. Marine Technology. 2002;39:29–47. [Google Scholar]
- 4.Arnold RD, Phillips JB. Causes of student attrition in US naval aviation training: A five year review from FY 2003 to FY 2007. NAMRL Technical Memorandum. 2008 [Google Scholar]
- 5.Rickert D. C41 Mobile Operational Prototype (CMOP), User Jury 8 Summary Report General Dynamics Amphibious Systems. Woodbridge, VA: 2000. [Google Scholar]
- 6.Finley JC, Jr., O'Leary M, Wester D, MacKenzie S, Shepard N, Farrow S, et al. A genetic polymorphism of the alpha2-adrenergic receptor increases autonomic responses to stress. J. Appl. Physiol. 2004;96:2231–9. doi: 10.1152/japplphysiol.00527.2003. [DOI] [PubMed] [Google Scholar]
- 7.Strewe C, Feuerecker M, Nichiporuk I, Kaufmann I, Hauer D, Morukov B, et al. Effects of parabolic flight and spaceflight on the endocannabinoid system in humans. Rev. Neurosci. 2012;0:1–8. doi: 10.1515/revneuro-2012-0057. [DOI] [PubMed] [Google Scholar]
- 8.Chouker A, Kaufmann I, Kreth S, Hauer D, Feuerecker M, Thieme D, et al. Motion sickness, stress and the endocannabinoid system. PloS one. 2010;5:e10752. doi: 10.1371/journal.pone.0010752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull. 1998;47:395–406. doi: 10.1016/s0361-9230(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 10.Feyer P, Jordan K. Update and new trends in antiemetic therapy: the continuing need for novel therapies. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:30–8. doi: 10.1093/annonc/mdq600. [DOI] [PubMed] [Google Scholar]
- 11.Fero KE, Jalota L, Hornuss C, Apfel CC. Pharmacologic management of postoperative nausea and vomiting. Expert Opinion Pharmacother. 2011;12:2283–96. doi: 10.1517/14656566.2011.598856. [DOI] [PubMed] [Google Scholar]
- 12.Reavley CM, Golding JF, Cherkas LF, Spector TD, MacGregor AJ. Genetic influences on motion sickness susceptibility in adult women: a classical twin study. Aviation, space, and environmental medicine. 2006;77:1148–52. [PubMed] [Google Scholar]
- 13.Horn CC. Is there a need to identify new anti-emetic drugs? Drug Discovery Today: Therapeutic Strategies. 2007;4:183–7. doi: 10.1016/j.ddstr.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanger GJ, Andrews PL. Treatment of nausea and vomiting: gaps in our knowledge. Auton. Neurosci. 2006;129:3–16. doi: 10.1016/j.autneu.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Spinks A, Wasiak J. Scopolamine (hyoscine) for preventing and treating motion sickness. Cochrane Database Syst Rev. 2011:CD002851. doi: 10.1002/14651858.CD002851.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125:100–15. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, et al. Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS ONE. 2013;8:e60537. doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueno S, Matsuki N, Saito H. Suncus murinus as a new experimental model for motion sickness. Life Sci. 1988;43:413–20. doi: 10.1016/0024-3205(88)90520-6. [DOI] [PubMed] [Google Scholar]
- 19.Kaji T, Saito H, Ueno S, Matsuki N. Comparison of various motion stimuli on motion sickness and acquisition of adaptation in Suncus Murinus. Jikken Dobutsu. 1990;39:75–9. doi: 10.1538/expanim1978.39.1_75. [DOI] [PubMed] [Google Scholar]
- 20.Javid FA, Naylor RJ. Variables of movement amplitude and frequency in the development of motion sickness in Suncus murinus. Pharmacol.Biochem.Behav. 1999;64:115–22. doi: 10.1016/s0091-3057(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 21.Rudd JA, Ngan MP, Wai MK. Inhibition of emesis by tachykinin NK1 receptor antagonists in Suncus murinus (house musk shrew). Eur J Pharmacol. 1999;366:243–52. doi: 10.1016/s0014-2999(98)00920-0. [DOI] [PubMed] [Google Scholar]
- 22.Cluny NL, Naylor RJ, Whittle BA, Javid FA. The effects of cannabidiol and tetrahydrocannabinol on motion-induced emesis in Suncus murinus. Basic Clin Pharmacol Toxicol. 2008;103:150–6. doi: 10.1111/j.1742-7843.2008.00253.x. [DOI] [PubMed] [Google Scholar]
- 23.Percie du Sert N, Chu KM, Wai MK, Rudd JA, Andrews PL. Telemetry in a motion-sickness model implicates the abdominal vagus in motion-induced gastric dysrhythmia. Exp Physiol. 2010;95:768–73. doi: 10.1113/expphysiol.2009.052001. [DOI] [PubMed] [Google Scholar]
- 24.Andrews P, Dovey E, Hockaday J, Hoyle CH, Woods AJ, Matsuki N. The development of the emetic reflex in the house musk shrew, Suncus murinus. Brain Res.Dev.Brain Res. 2000;121:29–34. doi: 10.1016/s0165-3806(00)00022-5. [DOI] [PubMed] [Google Scholar]
- 25.Beleslin DB, Krstic SK. Further studies on nicotine-induced emesis: nicotinic mediation in area postrema. Physiol Behav. 1987;39:681–6. doi: 10.1016/0031-9384(87)90250-2. [DOI] [PubMed] [Google Scholar]
- 26.Beleslin DB, Krstic SK, Dozic S. Central nicotinic receptors: vomiting, ear twitching and panting. Brain Res.Bull. 1983;11:299–302. doi: 10.1016/0361-9230(83)90164-8. [DOI] [PubMed] [Google Scholar]
- 27.Jovanovic-Micic D, Strbac M, Krstic SK, Japundzic N, Samardzic R, Beleslin DB. Ablation of the area postrema and emesis. Metab Brain Dis. 1989;4:55–60. doi: 10.1007/BF00999494. [DOI] [PubMed] [Google Scholar]
- 28.Fukui H, Yamamoto M, Sasaki S, Sato S. Involvement of 5-HT3 receptors and vagal afferents in copper sulfate- and cisplatin-induced emesis in monkeys. Eur J Pharmacol. 1993;249:13–8. doi: 10.1016/0014-2999(93)90656-3. [DOI] [PubMed] [Google Scholar]
- 29.Makale MT, King GL. Surgical and pharmacological dissociation of cardiovascular and emetic responses to intragastric CuSO4. Am J Physiol. 1992;263:R284–91. doi: 10.1152/ajpregu.1992.263.2.R284. [DOI] [PubMed] [Google Scholar]
- 30.Wang CH. Introduction: A new experimental animal, Suncus murinus. In: Saito H, Wang CH, Chen CY, editors. Proceeding of ROC-Japan Symposium on Suncus murinus: New experimental animal, its speciality and usefulness. Chia Nan Junior College of Pharmacy Press (Chia Nan, Taiwan ROC); Tainan, Taiwan, R.O.C.: 1994. [Google Scholar]
- 31.Temple JL. The musk shrew (Suncus murinus): a model species for studies of nutritional regulation of reproduction. ILAR J. 2004;45:25–34. doi: 10.1093/ilar.45.1.25. [DOI] [PubMed] [Google Scholar]
- 32.Horn CC, Meyers K, Pak D, Nagy A, Apfel CC, Williams BA. Post-anesthesia vomiting: Impact of isoflurane and morphine on ferrets and musk shrews. Physiol Behav. 2012;106:562–8. doi: 10.1016/j.physbeh.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horn CC, Still L, Fitzgerald C, Friedman MI. Food restriction, refeeding, and gastric fill fail to affect emesis in musk shrews. Am J Physiol Gastrointest Liver Physiol. 2010;298:G25–G30. doi: 10.1152/ajpgi.00366.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan SW, Rudd JA, Lin G, Li P. Action of anti-tussive drugs on the emetic reflex of Suncus murinus (house musk shrew). Eur.J.Pharmacol. 2007;559:196–201. doi: 10.1016/j.ejphar.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Ngan MP, Takeda N, Yamatodani A, Rudd JA. Differential activity of drugs to induce emesis and pica behavior in Suncus murinus (house musk shrew) and rats. Physiol Behav. 2004;83:151–6. doi: 10.1016/j.physbeh.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Jahn-Eimermacher A, Lasarzik I, Raber J. Statistical analysis of latency outcomes in behavioral experiments. Behav. Brain Res. 2011;221:271–5. doi: 10.1016/j.bbr.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, Rauch S, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth. 2002;88:659–68. doi: 10.1093/bja/88.5.659. [DOI] [PubMed] [Google Scholar]
- 38.Raeder J, Gupta A, Pedersen FM. Recovery characteristics of sevoflurane- or propofol-based anaesthesia for day-care surgery. Acta Anaesthesiol. Scand. 1997;41:988–94. doi: 10.1111/j.1399-6576.1997.tb04825.x. [DOI] [PubMed] [Google Scholar]
- 39.Ebukuro S, Wakana S, Hioki K, Nomura T. Selective breeding of house musk shrew (Suncus murinus) lines in relation to emesis induced by veratrine sulfate. Comp Med. 2000;50:281–3. [PubMed] [Google Scholar]
- 40.Borison HL, Fairbanks VF. Mechanism of veratrum-induced emesis in the cat. The Journal of pharmacology and experimental therapeutics. 1952;105:317–25. [PubMed] [Google Scholar]
- 41.Bobkov YG. Role of Nodose Ganglion in Vomiting Reflex to Aconitine and Veratrine. Fed. Proc. Transl. Suppl. 1965;24:325–8. [PubMed] [Google Scholar]
- 42.Uchino M, Ishii K, Kuwahara M, Ebukuro S, Tsubone H. Role of autonomic nervous system for development and suppression of motion sickness in Suncus murinus. Auton Neurosci. 2001;94:46–51. doi: 10.1016/S1566-0702(01)00344-7. [DOI] [PubMed] [Google Scholar]
- 43.Ito H, Nishibayashi M, Maeda S, Seki M, Ebukuro S. Emetic responses and neural activity in young musk shrews during the breast-feeding/weaning period: comparison between the high and low emetic response strains using a shaking stimulus. Exp.Anim. 2005;54:301–7. doi: 10.1538/expanim.54.301. [DOI] [PubMed] [Google Scholar]
- 44.Rueffert H, Thieme V, Wallenborn J, Lemnitz N, Bergmann A, Rudlof K, et al. Do variations in the 5-HT3A and 5-HT3B serotonin receptor genes (HTR3A and HTR3B) influence the occurrence of postoperative vomiting? Anesth. Analg. 2009;109:1442–7. doi: 10.1213/ane.0b013e3181b2359b. [DOI] [PubMed] [Google Scholar]
- 45.Goecke TW, Ekici AB, Niesler B, Loehberg CR, Hammer C, Rappold G, et al. Two naturally occurring variants of the serotonin receptor gene HTR3C are associated with nausea in pregnancy. Acta Obstet. Gynecol. Scand. 2010;89:7–14. doi: 10.3109/00016340903322727. [DOI] [PubMed] [Google Scholar]
- 46.Sia AT, Lim Y, Lim EC, Goh RW, Law HY, Landau R, et al. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109:520–6. doi: 10.1097/ALN.0b013e318182af21. [DOI] [PubMed] [Google Scholar]
- 47.Kolesnikov Y, Gabovits B, Levin A, Voiko E, Veske A. Combined catechol-O- methyltransferase and mu-opioid receptor gene polymorphisms affect morphine postoperative analgesia and central side effects. Anesth. Analg. 2011;112:448–53. doi: 10.1213/ANE.0b013e318202cc8d. [DOI] [PubMed] [Google Scholar]
- 48.Janicki PK, Vealey R, Liu J, Escajeda J, Postula M, Welker K. Genome-wide Association study using pooled DNA to identify candidate markers mediating susceptibility to postoperative nausea and vomiting. Anesthesiology. 2011;115:54–64. doi: 10.1097/ALN.0b013e31821810c7. [DOI] [PubMed] [Google Scholar]
- 49.Nakagawa M, Kuri M, Kambara N, Tanigami H, Tanaka H, Kishi Y, et al. Dopamine D2 receptor Taq IA polymorphism is associated with postoperative nausea and vomiting. Journal of anesthesia. 2008;22:397–403. doi: 10.1007/s00540-008-0661-z. [DOI] [PubMed] [Google Scholar]