Abstract
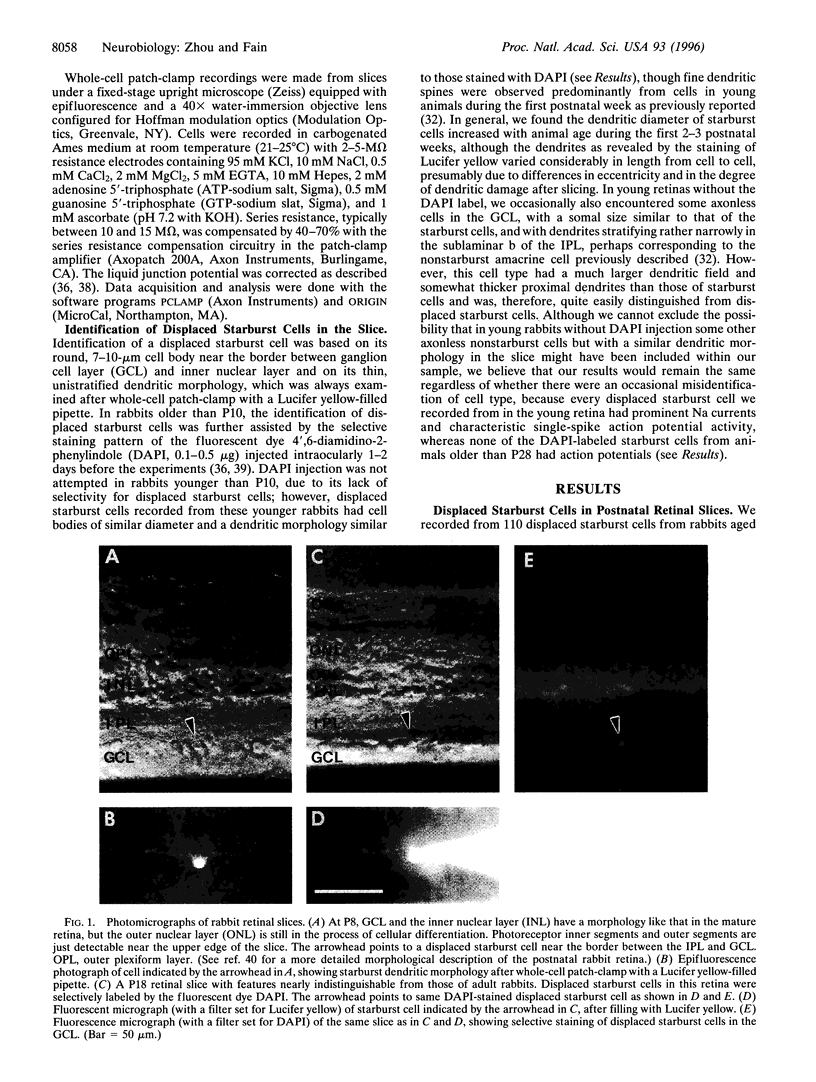
The membrane excitability of cholinergic (starburst) amacrine cells was studied in the rabbit retina during postnatal development. Whole-cell patch-clamp recordings were made from 110 displaced starburst cells in a thin retina] slice preparation of rabbits between postnatal days P1 and P56 old. We report that displaced starburst cells undergo a dramatic transition from spiking to nonspiking, caused by a loss of voltage-gated Na currents. This change in membrane excitability occurred just after eye opening (P10), such that all of the starburst cells tested before eye opening had conspicuous tetrodotoxin-sensitive Na currents and action potentials, but none tested after the first 3 postnatal weeks had detectable Na currents or spikes. Our results suggest that starburst cells use action potentials transiently during development and probably play a functional role in visual development. These cells then cease to spike as the retina matures, presumably consistent with their role in visual processing in the mature retina.
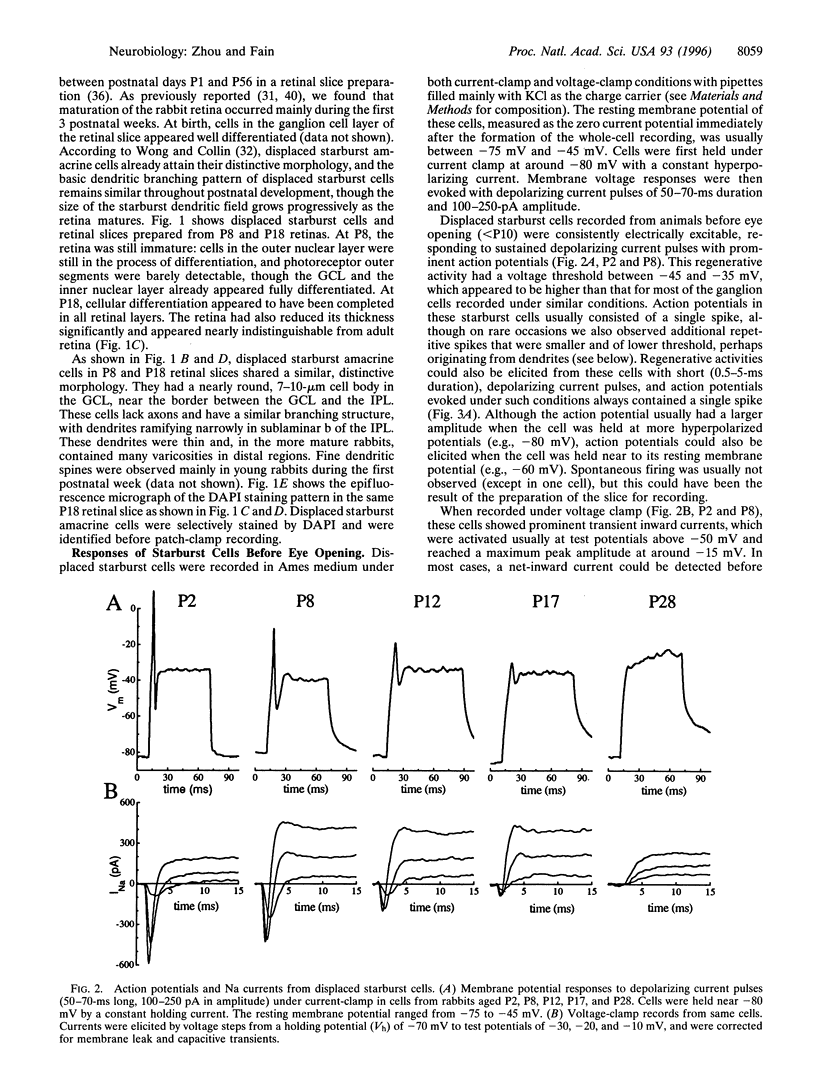
Full text
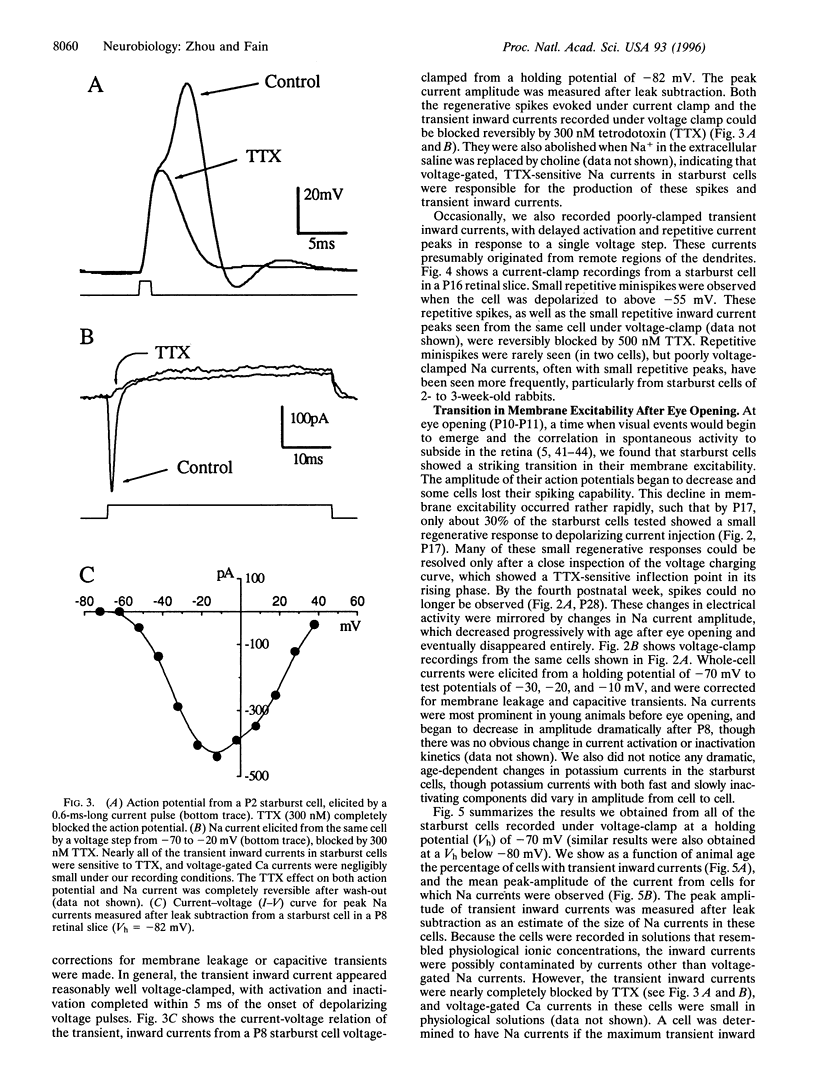
PDF
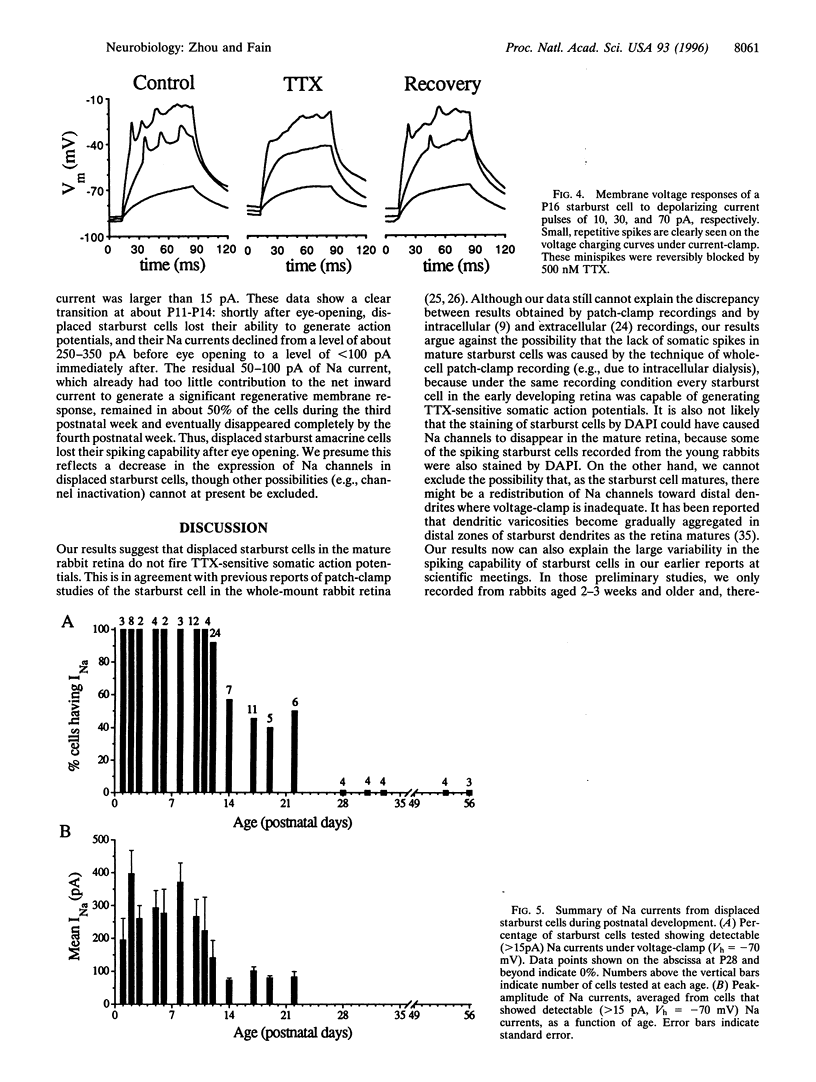

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Nesbett F. B. In vitro retina as an experimental model of the central nervous system. J Neurochem. 1981 Oct;37(4):867–877. doi: 10.1111/j.1471-4159.1981.tb04473.x. [DOI] [PubMed] [Google Scholar]
- Ariel M., Daw N. W. Pharmacological analysis of directionally sensitive rabbit retinal ganglion cells. J Physiol. 1982 Mar;324:161–185. doi: 10.1113/jphysiol.1982.sp014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S., Werblin F. Gated currents generate single spike activity in amacrine cells of the tiger salamander retina. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1509–1512. doi: 10.1073/pnas.83.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield S. A. Relationship between receptive and dendritic field size of amacrine cells in the rabbit retina. J Neurophysiol. 1992 Sep;68(3):711–725. doi: 10.1152/jn.1992.68.3.711. [DOI] [PubMed] [Google Scholar]
- Brandon C. Cholinergic neurons in the rabbit retina: dendritic branching and ultrastructural connectivity. Brain Res. 1987 Nov 17;426(1):119–130. doi: 10.1016/0006-8993(87)90431-8. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M., Cline H. T., Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Cook P. B., Werblin F. S. Spike initiation and propagation in wide field transient amacrine cells of the salamander retina. J Neurosci. 1994 Jun;14(6):3852–3861. doi: 10.1523/JNEUROSCI.14-06-03852.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux R. F., Miller R. F. An intracellular electrophysiological study of the ontogeny of functional synapses in the rabbit retina. I. Receptors, horizontal, and bipolar cells. J Comp Neurol. 1981 May 10;198(2):307–326. doi: 10.1002/cne.901980209. [DOI] [PubMed] [Google Scholar]
- Dacheux R. F., Miller R. F. An intracellular electrophysiological study of the ontogeny of functional synapses in the rabbit retina. II. Amacrine cells. J Comp Neurol. 1981 May 10;198(2):327–334. doi: 10.1002/cne.901980210. [DOI] [PubMed] [Google Scholar]
- Eliasof S., Barnes S., Werblin F. The interaction of ionic currents mediating single spike activity in retinal amacrine cells of the tiger salamander. J Neurosci. 1987 Nov;7(11):3512–3524. doi: 10.1523/JNEUROSCI.07-11-03512.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr 'Starburst' amacrine cells and cholinergic neurons: mirror-symmetric on and off amacrine cells of rabbit retina. Brain Res. 1983 Feb 14;261(1):138–144. doi: 10.1016/0006-8993(83)91293-3. [DOI] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr On and off pathways through amacrine cells in mammalian retina: the synaptic connections of "starburst" amacrine cells. Vision Res. 1983;23(11):1265–1279. doi: 10.1016/0042-6989(83)90102-5. [DOI] [PubMed] [Google Scholar]
- Famiglietti E. V. Starburst amacrine cells: morphological constancy and systematic variation in the anisotropic field of rabbit retinal neurons. J Neurosci. 1985 Feb;5(2):562–577. doi: 10.1523/JNEUROSCI.05-02-00562.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti E. V. Synaptic organization of starburst amacrine cells in rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. J Comp Neurol. 1991 Jul 1;309(1):40–70. doi: 10.1002/cne.903090105. [DOI] [PubMed] [Google Scholar]
- Feller M. B., Wellis D. P., Stellwagen D., Werblin F. S., Shatz C. J. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996 May 24;272(5265):1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C. S., Shatz C. J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993 Jan;72 (Suppl):77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Greiner J. V., Weidman T. A. Embryogenesis of the rabbit retina. Exp Eye Res. 1982 May;34(5):749–765. doi: 10.1016/s0014-4835(82)80035-3. [DOI] [PubMed] [Google Scholar]
- Huba R., Hofmann H. D. Identification of GABAergic amacrine cell-like neurons developing in chick retinal monolayer cultures. Neurosci Lett. 1990 Sep 4;117(1-2):37–42. doi: 10.1016/0304-3940(90)90116-q. [DOI] [PubMed] [Google Scholar]
- Jensen R. J. Receptive-field properties of displaced starburst amacrine cells change following axotomy-induced degeneration of ganglion cells. Vis Neurosci. 1995 Jan-Feb;12(1):177–184. doi: 10.1017/s0952523800007409. [DOI] [PubMed] [Google Scholar]
- Kalil R. E. The influence of action potentials on the development of the central visual pathway in mammals. J Exp Biol. 1990 Oct;153:261–276. doi: 10.1242/jeb.153.1.261. [DOI] [PubMed] [Google Scholar]
- Kallen R. G., Cohen S. A., Barchi R. L. Structure, function and expression of voltage-dependent sodium channels. Mol Neurobiol. 1993 Fall-Winter;7(3-4):383–428. doi: 10.1007/BF02769184. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMasson G., Marder E., Abbott L. F. Activity-dependent regulation of conductances in model neurons. Science. 1993 Mar 26;259(5103):1915–1917. doi: 10.1126/science.8456317. [DOI] [PubMed] [Google Scholar]
- Maffei L., Galli-Resta L. Correlation in the discharges of neighboring rat retinal ganglion cells during prenatal life. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2861–2864. doi: 10.1073/pnas.87.7.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiafava P. L. Centrifugal actions on amacrine and ganglion cells in the retina of the turtle. J Physiol. 1976 Feb;255(1):137–155. doi: 10.1113/jphysiol.1976.sp011273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland R. H., Mills J. W., Cassidy C. The functions of acetylcholine in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984 Nov 22;223(1230):121–139. doi: 10.1098/rspb.1984.0086. [DOI] [PubMed] [Google Scholar]
- Masland R. H., Mills J. W., Hayden S. A. Acetylcholine-synthesizing amacrine cells: identification and selective staining by using radioautography and fluorescent markers. Proc R Soc Lond B Biol Sci. 1984 Nov 22;223(1230):79–100. doi: 10.1098/rspb.1984.0084. [DOI] [PubMed] [Google Scholar]
- McArdle C. B., Dowling J. E., Masland R. H. Development of outer segments and synapses in the rabbit retina. J Comp Neurol. 1977 Oct 1;175(3):253–274. doi: 10.1002/cne.901750302. [DOI] [PubMed] [Google Scholar]
- Meister M., Wong R. O., Baylor D. A., Shatz C. J. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991 May 17;252(5008):939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Millar T. J., Morgan I. G. Cholinergic amacrine cells in the rabbit retina synapse onto other cholinergic amacrine cells. Neurosci Lett. 1987 Mar 9;74(3):281–285. doi: 10.1016/0304-3940(87)90310-7. [DOI] [PubMed] [Google Scholar]
- Nelson R., von Litzow A., Kolb H., Gouras P. Horizontal cells in cat retina with independent dendritic systems. Science. 1975 Jul 11;189(4197):137–139. doi: 10.1126/science.1138370. [DOI] [PubMed] [Google Scholar]
- Peters B. N., Masland R. H. Responses to light of starburst amacrine cells. J Neurophysiol. 1996 Jan;75(1):469–480. doi: 10.1152/jn.1996.75.1.469. [DOI] [PubMed] [Google Scholar]
- Skaliora I., Scobey R. P., Chalupa L. M. Prenatal development of excitability in cat retinal ganglion cells: action potentials and sodium currents. J Neurosci. 1993 Jan;13(1):313–323. doi: 10.1523/JNEUROSCI.13-01-00313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi M., Masland R. H. Local order among the dendrites of an amacrine cell population. J Neurosci. 1985 Sep;5(9):2494–2501. doi: 10.1523/JNEUROSCI.05-09-02494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi M., Masland R. H. The shape and arrangement of the cholinergic neurons in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984 Nov 22;223(1230):101–119. doi: 10.1098/rspb.1984.0085. [DOI] [PubMed] [Google Scholar]
- Taylor W. R., Wässle H. Receptive field properties of starburst cholinergic amacrine cells in the rabbit retina. Eur J Neurosci. 1995 Nov 1;7(11):2308–2321. doi: 10.1111/j.1460-9568.1995.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Thackeray J. R., Ganetzky B. Developmentally regulated alternative splicing generates a complex array of Drosophila para sodium channel isoforms. J Neurosci. 1994 May;14(5 Pt 1):2569–2578. doi: 10.1523/JNEUROSCI.14-05-02569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G., Abbott L. F., Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994 May 13;264(5161):974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- Vaney D. I. 'Coronate' amacrine cells in the rabbit retina have the 'starburst' dendritic morphology. Proc R Soc Lond B Biol Sci. 1984 Feb 22;220(1221):501–508. doi: 10.1098/rspb.1984.0016. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wong R. O., Chernjavsky A., Smith S. J., Shatz C. J. Early functional neural networks in the developing retina. Nature. 1995 Apr 20;374(6524):716–718. doi: 10.1038/374716a0. [DOI] [PubMed] [Google Scholar]
- Wong R. O., Collin S. P. Dendritic maturation of displaced putative cholinergic amacrine cells in the rabbit retina. J Comp Neurol. 1989 Sep 8;287(2):164–178. doi: 10.1002/cne.902870203. [DOI] [PubMed] [Google Scholar]
- Wong R. O., Meister M., Shatz C. J. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993 Nov;11(5):923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Wässle H., Boycott B. B. Functional architecture of the mammalian retina. Physiol Rev. 1991 Apr;71(2):447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Zhou Z. J., Fain G. L. Neurotransmitter receptors of starburst amacrine cells in rabbit retinal slices. J Neurosci. 1995 Jul;15(7 Pt 2):5334–5345. doi: 10.1523/JNEUROSCI.15-07-05334.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]




