Abstract
The measurement of antiretroviral concentrations in hair is emerging as an important technology to objectively quantify adherence to combination antiretroviral therapy. Hair levels of antiretrovirals are the strongest independent predictor of virologic success in large prospective cohorts of HIV-infected patients and surpass self-report in predicting outcomes. Hair is easy to collect and store, but validated methods to analyze antiretroviral levels in hair using liquid chromatography tandem mass spectrometry (LC-MS/MS) are expensive. We report here on the development of a thin-layer chromatography (TLC) assay for the semiquantitative analysis of nevirapine in hair. TLC assay results from 11 samples were consistent with results using LC-MS/MS [Spearman correlation coefficient 0.99 (95% CI 0.95–0.996)]. This simple, low-cost method of analyzing nevirapine concentrations in hair may provide a novel monitoring tool for antiretroviral adherence in resource-limited settings and merits further study in clinical settings.
Adherence to antiretroviral (ARV) therapy is essential for the realization of virologic, immunologic, and clinical benefits, but the limitations of self-report and other commonly used adherence measures are well-described.1 The measurement of ARV concentrations in hair as a biological measure of adherence is emerging as an important technology to objectively quantify adherence to combination antiretroviral therapy (cART).2 Hair levels of ARVs are the strongest independent predictor of virologic success in large prospective cohorts of HIV-infected patients,3–6 and surpass self-report in predicting outcomes.3 Moreover, hair levels can serve as a measure of exposure to maternal ARVs in infants during pregnancy and breastfeeding.7
Since drugs accumulate in hair over prolonged periods,8, 8a a single measurement of an ARV in a hair sample provides a longer-term measure of adherence than a single plasma level, which captures only short-term use.9–13 Nevirapine (NVP) is widely prescribed in cART regimens for the treatment of HIV-infected adults and children in global settings,14 and plays a role in worldwide perinatal transmission prevention. A low-cost testing method for analyzing NVP levels in small hair samples that could be performed in local laboratories would therefore be useful for monitoring adherence to NVP-based regimens in resource-limited settings.
Hair samples are simple and inexpensive to collect and can be stored at room temperature prior to analysis. Unlike phlebotomy, hair collection is noninvasive and does not require specific skills or sterile equipment. These features provide obvious cost and feasibility advantages for hair collection and storage, over plasma but validated methods of analyzing ARV levels in hair samples use liquid chromatography/tandem mass spectrometry (LC-MS/MS).15,16 The LC-MS/MS method for analyzing NVP in hair samples is highly sensitive,16 but the equipment required is expensive, limiting its suitability in resource-limited settings. In contrast, thin-layer chromatography (TLC) is a simple and inexpensive analytical tool that has been used for the detection of NVP concentrations in fixed-dose combination tablets, human plasma, saliva, and umbilical cord blood.17–20 We report on the development of a low-cost method to analyze NVP in human hair samples using TLC.
Nevirapine hemihydrate, nevirapine-D5, atazanavir, efavirenz, emtricitabine, lamivudine, raltegravir, ritonavir, stavudine, tenofovir, and zidovudine, along with organic solvents, were obtained from relevant commercial laboratories. Drug-free blank human hair samples were obtained from six HIV-noninfected volunteers. The positive control hair samples were obtained from five patients with HIV infection who were on NVP-based treatment (Table 1). Collection of hair from these five treated individuals was performed as part of a larger protocol where heads were shaved from treated individuals to develop assays for analyzing ARV levels in hair from 2006 to 2011; the protocol was approved by the Institutional Review Board of the University of California, San Francisco (UCSF). High-performance TLC plates were Silica gel 60 F254 (Merck KGaA, Germany) and an ultraviolet (UV) lamp for viewing the TLC plates was obtained from CAMAG (Chemie-Erzeugnisse & Adsorptionstechnik AG & Co., Germany).
Table 1.
Comparison of Hair Concentrations of Nevirapine Analyzed by Thin-Layer Chromatography and by Liquid Chromatography/Tandem Mass Spectrometry Methods in Five HIV-Infected Patients on Nevirapine-Based Therapy
| Sample ID | Antiretrovirals coadministered with NVP | Semiquantitative concentration of NVP with the TLC method (ng/mg hair) | Concentration of NVP with the LC-MS/MS method (ng/mg hair) |
|---|---|---|---|
| 1 |
Tenofovir, emtricitabine |
10–25 |
21.7 |
| 2 |
Tenofovir, emtricitabine |
25–50 |
33.4 |
| 3 |
Atazanavir/ritonavir; lamivudine, tenofovir |
10–25 |
16.3 |
| 4 |
Atazanavir/ritonavir; tenofovir |
25–50 |
43.9 |
| 5 | Stavudine, lamivudine | 25–50 | 44.2 |
NVP, nevirapine; TLC, thin-layer chromatography; LC-MS/MS, liquid chromatogrpahy/tandem mass spectrometry.
Human hair samples were cut to about 1- to 3-mm segments with scissors. Approximately 20 mg of each cut hair sample was weighed and placed into a glass test tube (16×125 mm). Two milliliters of methanol/trifluoroacetic acid (9/1) solution was added and the sample was incubated at 37°C overnight (>12 h) in a shaking water bath. The organic solvent was then evaporated to dryness by nitrogen gas. Extracted NVP was further cleaned up by liquid–liquid (liq-liq) extraction as described previously.16 Briefly, 0.50 ml of 0.20 M sodium phosphate buffer (pH 9.4) was added and the sample was vortexed for 30 s. Three milliliters of methyl t-butyl ether/ethyl acetate (1:1) was then added and the mixture was vortexed three times, each for 1 min. The liquid sample was then centrifuged at 3,000 rpm for 10 min and the sample was frozen in a dry ice/methanol bath prior to transfer of the supernatant to a fresh test tube (13×100 mm). Fifty microliters of 1% trifluoroacetic acid in methanol was then added and the sample was evaporated to dryness by nitrogen gas.
The liq-liq cleaned-up sample was then mixed with 200 μl of methanol, transferred to a small test tube (10×75 mm), evaporated by nitrogen gas again, and reconstituted with 10 μl of methanol. A total of 4 μl of the solution was blotted onto TLC plates for analysis. A series of standard samples was generated for use as negative and positive controls in the TLC assays. Blank hair samples were “spiked” with different concentrations of NVP solution (ranging from 0 μg/ml to 12 μg/ml to 400 μg/ml) to achieve standard hair samples ranging from 0 ng/mg to 200 ng/mg.
TLC plates (10×10 mm high performance TLC plate) were marked using a soft lead pencil with an origin line 2 cm from the base of the plate. All samples were spotted with 1 μl of the extracted sample at a time using a micropipette, allowing each microliter to dry before spotting the next microliter (for a total of 4 μl applied to each plate). The plate was then air dried and developed in toluene/ethyl acetate (1:1) containing 0.4% trifluoroacetic acid for 10 min in a covered TLC tank. The solvent front was marked with a soft lead pencil and the plate was subsequently air dried. The drug spot was visualized under UV light at a wavelength of 254 nm. NVP concentrations in hair samples as estimated under TLC conditions were compared to concentrations derived from LC-MS/MS measurements for the 11 hair samples (six blank and five from patients on NVP-containing cART) by calculating Spearman correlation coefficients with 95% confidence intervals (CIs).
Six drug-free blank human hair samples from HIV-noninfected volunteers were used for specificity testing. Three concentrations of NVP in hair samples (6, 10, and 25 ng/mg hair) were used for testing the detection limit of NVP concentrations in hair via TLC. For each extracted sample tested via TLC, a retention factor (Rf) was calculated for each spot. The Rf values were calculated by dividing the distance from the origin to the sample spot for each extracted sample by the distance from the origin to the solvent front. No endogenous interference from the TLC testing of the blank hair samples was detected at the same Rf as the NVP spot, indicating selective detection of NVP in hair. The estimated semiquantitative limit of detection for NVP in human hair samples via TLC was 10 ng/mg hair.
The commonly used antiretroviral medications of emtricitabine, lamivudine, ritonavir, tenofovir, zidovudine, efavirenz, atazanavir, stavudine, and raltegravir were tested for potential interference with the NVP TLC assay. The above drugs at 200 ng/μl in pure solution were tested for Rf values in the TLC system. The Rf of each tested drug was as follows: emtricitabine, 0.04; lamivudine, 0.02; ritonavir, 0.02; tenofovir, <0.01; zidovudine, 0.12; efavirenz, 0.70; atazanavir, 0.11; stavudine, 0.04; and raltegravir, 0.02. The Rf of NVP in the system was 0.30. The above nine antiretroviral medications were also spiked into blank hair at 100 ng/mg, then extracted in the same manner as NVP, and analyzed via the above-described TLC method. None of these medications was found to show interference with the detection of NVP in the system.
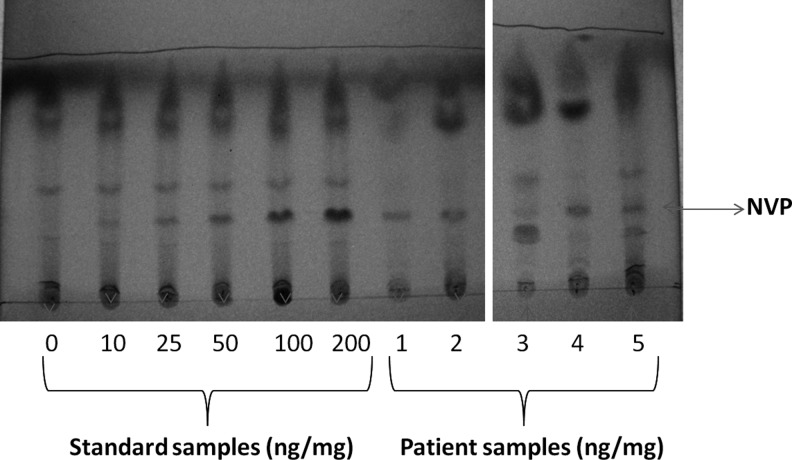
To test the range of the TLC assay to detect NVP in hair, the standard samples described above were tested in the system. The density of each NVP spot on the TLC plates as estimated semiquantitatively was proportional to its concentration in hair samples from 10 ng/mg to 200 ng/mg (Fig. 1), indicating detection of NVP in hair samples using TLC across a wide range. As depicted in Fig. 1, NVP could be detected in all five human hair samples from patients on NVP-containing cART (ID numbers: 1, 2, 3, 4, and 5). The semiquantitative concentrations are summarized in Table 1. Of note, imaging processing applications such as image J® provided free of charge by the National Institutes of Health (http://rsbweb.nih.gov/ij/) can provide semiquantitative concentrations from gel bands using optical density calibration. The NVP concentrations in these samples as analyzed by the validated LC-MS/MS method are also shown in Table 1. The Spearman correlation coefficient (r) for the TLC hair assay versus the LC-MS/MS assay in the 11 hair samples (five patients samples and six blank) was 0.99 (95% CI 0.95–0.996).
FIG. 1.
Thin-layer chromatography (TLC) plate assays for the detection of nevirapine (NVP) in hair from five HIV-infected patients on NVP-containing regimens and standard samples across a wide range.
In conclusion, this article describes briefly a simple and specific method for the detection of NVP in small hair samples using thin-layer chromatography. When compared to the validated method of analyzing NVP concentrations in hair samples using LC-MS/MS, the semiquantitative results using TLC showed strong correlations with results using more expensive equipment. The method was specific and no endogenous substances or commonly used antiretrovirals interfered with the NVP TLC detection method. Moreover, the distinct Rf values for other commonly used antiretrovirals in global settings, such as efavirenz (Rf 0.70), suggests that TLC assays for other agents could be developed as inexpensive monitoring tools for adherence using similar methods. Given the limited availability of second and third line regimens to treat HIV in the global setting, assessing adherence to initial nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimens using a pharmacologic biomarker could allow for adherence counseling and closer monitoring to hopefully optimize the duration of first-line cART. This simple, inexpensive assay for the semiquantitative determination of NVP in human hair samples mandates further study as a monitoring tool for adherence in resource-limited settings.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID)/NIH (RO1 AI098472 to M.G.). The authors gratefully acknowledge the intellectual contributions of Ruth Greenblatt, MD, professor of Clinical Pharmacy and Medicine at UCSF, to this project.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Berg KM. and Arnsten JH: Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr 2006;43(Suppl 1):S79–S87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi M. and Greenblatt RM: Hair it is: The long and short of monitoring antiretroviral treatment. Ann Intern Med 2002;137(8):696–697 [DOI] [PubMed] [Google Scholar]
- 3.Gandhi M, Ameli N, Bacchetti P, et al.: Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis 2011;52(10):1267–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Zyl GU, van Mens TE, McIlleron H, et al.: Low lopinavir plasma or hair concentrations explain second line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr 2011;56(4):333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi M, Ameli N, Bacchetti P, et al.: Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS 2009;23(4):471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi M, Ameli N, Bacchetti P, et al.:. Concentrations of Efavirenz in Hair Are Strongly Correlated with Virologic Response. 16th Conference on Retroviruses and Opportunistic Infections (CROI), Montreal, Canada, 2009; paper 692 [Google Scholar]
- 7.Gandhi M, Mwesigwa J, Aweeka F, et al.: Hair and plasma data show that lopinavir, ritonavir and efavirenz all transfer from mother to infant in utero, but only efavirenz transfers via breastfeeding. J Acquir Immune Defic Syndr 2013;63(5):578–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beumer J, Bosman I, and Maes R: Hair as a biological specimen for therapeutic drug monitoring. Int J Clin Pract 2001;55:353–357 [PubMed] [Google Scholar]
- 8a.Gandhi M, Greenblatt RM, Bacchetti P, et al.: A single nucleotide polymorphism in CYP2B6 leads to >3-fold increases in efavirenz concentrations in intensive PK curves and hair samples in HIV-infected women. Journal of Infectious Diseases 2012;(9):1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nettles RE, Kieffer TL, Parsons T, et al.: Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis 2006;42(8):1189–1196 [DOI] [PubMed] [Google Scholar]
- 10.Clevenbergh P, Garaffo R, Durant J, and Dellamonica P: PharmAdapt: A randomized prospective study to evaluate the benefit of therapeutic monitoring of protease inhibitors: 12 week results. AIDS 2002;16:2311–2315 [DOI] [PubMed] [Google Scholar]
- 11.Wertheimer BZ, Freedberg KA, Walensky RP, Yazdanapah Y, and Losina E: Therapeutic drug monitoring in HIV treatment: A literature review. HIV Clin Trials 2006;7(2):59–69 [DOI] [PubMed] [Google Scholar]
- 12.Cramer JA, Scheyer RD, and Mattson RH: Compliance declines between clinic visits. Arch Intern Med 1990;150(7):1509–1510 [PubMed] [Google Scholar]
- 13.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, and Hanna GJ: “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials 2008;9(4):238–246 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization: Consolidated guidelines on the use of ARV drugs for treating and preventing HIV infection. June30, 2013. www.who.int/hiv/pub/guidelines/arv2013/short_summary/en/index.html [PubMed]
- 15.Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, and Messenkoff N: Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir, and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom 2008;22(21):3401–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Yang Q, Yoon K, et al.: Microanalysis of the antiretroviral nevirapine in human hair from HIV-infected patients by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 2011;401(6):1923–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shewiyo DH, Kaale E, Ugullum C, et al.: Development and validation of a normal-phase HPTLC method for the simultaneous analysis of lamivudine, stavudine, and nevirapine in fixed-dose combination tablets. J Pharm Biomed Anal 2011;54(3):445–450 [DOI] [PubMed] [Google Scholar]
- 18.L'Homme RF, Muro EP, Droste JA, et al.: Therapeutic drug monitoring of nevirapine in resource-limited settings. Clin Infect Dis 2008;47(10):1339–1344 [DOI] [PubMed] [Google Scholar]
- 19.Dubuisson JG, King JR, Stringer JS, Turner ML, Bennetto C, and Acosta EP: Detection of nevirapine in plasma using thin-layer chromatography. J Acquir Immune Defic Syndr 2004;35(2):155–157 [DOI] [PubMed] [Google Scholar]
- 20.Chi BH, Lee A, Acosta EP, Westerman LE, Sinkala M, and Stringer JS: Field performance of a thin-layer chromatography assay for detection of nevirapine in umbilical cord blood. HIV Clin Trials 2006;7(5):263–269 [DOI] [PubMed] [Google Scholar]