Abstract
Background: This study estimated temporal trends of metabolic control over 12 years in a national cohort of childhood-onset type 1 diabetes.
Subjects and Methods: Data from the prospective childhood-onset diabetes register, which included 886 case subjects from 0 to 17.99 years of age at diagnosis and at least 1 year of follow-up until the age of 22.99 years, were analyzed using multivariable linear and logistic regression models in the observational period between 2000 and 2011.
Results: Hemoglobin A1c (HbA1c) significantly decreased over 12 years, from 78 mmol/mol (interquartile range [IQR], 68–88 mmol/mol) (9.26% [IQR, 8.41–10.24%]) in the year 2000 to 61 mmol/mol (IQR, 55–67 mmol/mol) (7.75% [IQR, 7.20–8.30%]) in the year 2011 (P<0.001). HbA1c was significantly associated with age, treatment modality, and duration of diabetes (P<0.001), with females having on average 1.02% higher HbA1c (P=0.01; 95% confidence interval [CI] 1.005–1.035). The overall use of insulin pumps was 74%. The incidence rate of severe acute complications was low: 1.07 per 100 patient-years for severe diabetic ketoacidosis (95% CI 0.81–1.40) and 1.21 per 100 patient-years for severe (requiring intravenous or intramuscular therapy) hypoglycemia (95% CI 0.81–1.40).
Conclusions: The metabolic control of the entire nationwide pediatric type 1 diabetes population significantly improved during the 12-year observational period with a low rate of severe acute complications events. The improvement was associated with the treatment modality. Additional efforts and solutions are necessary to further improve metabolic control and the quality of life of young people with type 1 diabetes.
Introduction
The primary goal in the treatment of type 1 diabetes (T1D) is to maintain blood glucose levels as close to normal as possible with the aim of reducing the risk for developing chronic complications.1 The strategies for the treatment of infants and young children may differ from those intended for teenagers or adults, but finally aim at the same primary goal.2
Current guidelines for adult3 and pediatric2 T1D populations suggest that the hemoglobin A1c (HbA1c) level should be below 53 mmol/mol (7%) or 58 mmol/mol (7.5%), respectively. Only a minority of patients can safely achieve these goals.4 The increase of severe hypoglycemia (SH) rate, commonly associated with a reduction in HbA1c,1 precludes many patients from reaching the target metabolic control.
Intensified insulin delivery usually consists of either multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII) and is based on self-monitoring of blood glucose and recently also on real-time continuous glucose monitoring.5 CSII is considered as safe and effective as MDI in all pediatric age groups6 and is in some7,8—but not all9—pediatric T1D population studies associated with better metabolic control compared with MDI. Its use is steadily increasing.10,11 Recent data indicate that in many countries outside the United States only a few pediatric T1D patients are treated with CSII.10,12,13 However, treatment modality is not always associated with an improvement in HbA1c level.14
The aim of this study was to analyze temporal trends of metabolic control and possible factors influencing metabolic control, including treatment modality, in the Slovene pediatric T1D population over the last 12 years.
Study Design and Methods
Data on the entire pediatric T1D population, which included 886 case subjects from 0 to 17.99 years of age at diagnosis and at least 1 year of follow-up until the age of 22.99 years, were collected from the prospective Slovene childhood-onset T1D register.15,16 The observational period was from January 1, 2000 to December 31, 2011. All outpatient visits and measurements took place approximately every 3 months at the University Children's Hospital Ljubljana, Ljubljana, Slovenia. Six full-time endocrinologists and six diabetes educators (148 patients per full-time equivalent) were working in the multidisciplinary team together with two psychologists, one dietitian, and one social worker.
In total, 21 patients who had other forms of diabetes mellitus, concomitant Addison's disease, cancer, or autoimmune neurological disease or were transferred to a center outside Slovenia after less than 1 year of follow-up were excluded from this analysis. Attrition from the register before the age of 18 years was followed.
Data for individual patients were available for at least three visits annually. The HbA1c level was determined centrally with the same immunochemical method throughout the observation period using the DCA 2000+ analyzer (Bayer Diagnostics, Tarrytown, NY). The accuracy of the DCA 2000+ analyzer was verified routinely every 3 months by the hospital laboratory quality-assurance program and annually by the provider, who issued a certificate. Data on severe acute complications were collected from medical records. Diabetic ketoacidosis (DKA) was defined as an event requiring hospitalization and intravenous therapy, and SH was defined as an event with a loss of consciousness and/or seizures requiring hospitalization and intramuscular and/or intravenous therapy.17 The study protocol was approved (number 22/12/09) by the Slovene Medical Ethics Committee. Patients and/or parents gave their informed consent for the anonymous use of data.
Statistical methods
Numerical variables were presented as median and interquartile range (IQR) or as mean and SD values. The primary outcome measure was HbA1c. The secondary outcomes were body mass index (BMI) SD score (SDS), daily insulin dose (units/kg), and severe DKA and SH events. Repeated outcome measurements obtained from the same patient were averaged over 6-month periods.
Multivariable linear regression models were used to examine the association between HbA1c and patient characteristics; HbA1c was log2-transformed to reduce the skewness of its distribution. The covariates were gender, age, year of measurement, treatment modality, BMI SDS, and duration of diabetes. The association of the same covariates with the probability of suboptimal (HbA1c >7.5%) and poor (HbA1c >9%) metabolic control2 was assessed using logistic regression models. Model selection was not performed, and all the covariates were included in the regression models as fixed effects. To avoid the implicit assumption that the effect of the continuous covariates on outcome was linear (on the log-transformed scale or on the logit scale), restricted cubic splines18 were used to flexibly model the relationship between covariates and outcome (five knots were used, placed at the 5th, 25th, 50th, 75th, and 95th percentiles). To take into account the multiple measurements repeated in each patient, the analyses were adjusted for a subject variable as a random effect. The association of the covariates with outcomes and the nonlinear relationship between continuous covariates and outcome were assessed by likelihood ratio tests. The estimated shape of the relationship between each continuous covariate and outcome was represented graphically, using specified values for other covariates, reported in the figure legends. It is important that these choices did not influence the estimated shape of the relationship between the covariates and outcome, but only the estimated value of the outcome and the size of the confidence intervals. For this reason all the figures were rescaled, and the starting point on the y-axes was set to 0. Therefore, the values on the y-axes can be interpreted as estimated differences from the starting point.
Results of multiple regression analyses were presented as multiple adjusted HbA1c mean ratios or odds ratios (OR), with 95% confidence intervals (CIs).
BMI was transformed into SDS for gender and age according to United Kingdom Cole reference tables. For the regression models, when BMI SDS was missing, we imputed the average BMI SDS value of the previous and next available measurement.
Incidence rates of severe DKA and SH were calculated as events per 100 patient-years, and their 95% CI was based on the Poisson distribution.
The P values of all statistical tests were two-sided, and 95% CI were reported. For all analyses differences were considered significant at values of P≤0.05. Statistical analyses were performed using R statistical language.19 The multiple mixed-effect regression models were fitted using the nlme and lme4 R package.19
Results
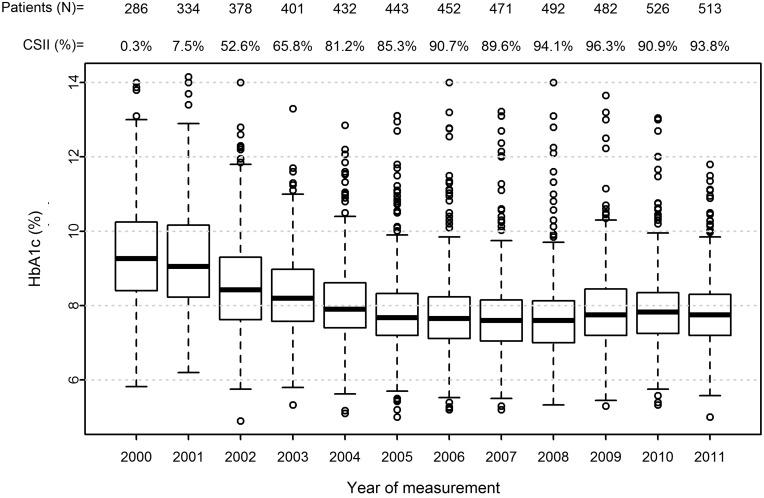
The median HbA1c decreased from 78 mmol/mol (IQR, 68–88 mmol/mol) to 61 mmol/mol (IQR, 55–67 mmol/mol) (from 9.26% [IQR, 8.41–10.24%] to 7.75% [IQR, 7.20–8.30%]) (P<0.001, Mann–Whitney test) (Fig. 1). The mean values were 79 mmol/mol (9.42%) (SD, 1.5) and 62 mmol/mol (7.80%) (SD, 0.96), respectively (P<0.001, two-sample t test).
FIG. 1.
Median hemoglobin A1c (HbA1c) (with interquartile range) by year of measurement (year of measurement/median HbA1c) with total number of patients under observation (N) and continuous subcutaneous insulin infusion (CSII) percentage by each year from 2000 to 2011.
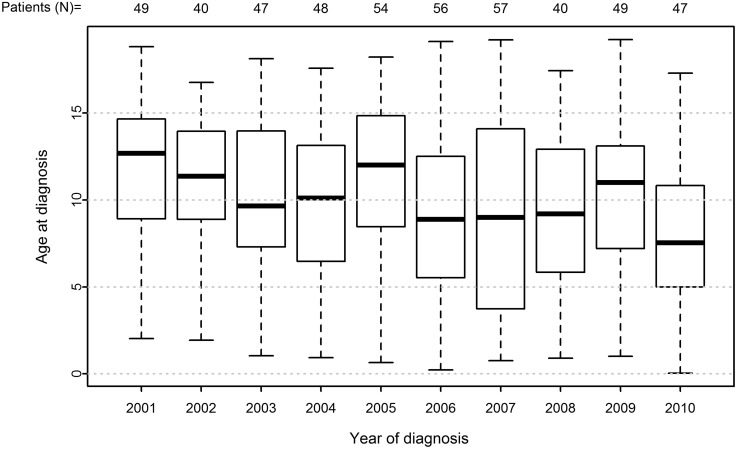
Median age of the cohort at diabetes onset was 9.61 years (IQR, 5.90–13.08) and decreased significantly with time: it was 12.68 years (IQR, 8.91–14.66) in the year 2001 and 7.53 years (IQR, 4.99–10.83) in the year 2010 (P<0.001) (Fig. 2). Median follow-up time was 5.00 years (IQR, 2.50–8.00). The numbers of available 6-month averages of measurements per patient were 10 for HbA1c (IQR, 6–16), nine for BMI SDS (IQR, 5–14), and eight for daily insulin dose (IQR, 4–12). Of the total, 4.2% (n=37) patients were lost to our follow-up.
FIG. 2.
Median age (with interquartile range) at diabetes onset (year of measurement/age at diagnosis) with total number of patients with newly diagnosed type 1 diabetes (N) in each year from 2000 to 2011.
The median BMI SDS increased from 0.40 (IQR, −0.17 to 0.99) to 0.5 (IQR, −0.15 to 1.18) (P=0.47), whereas daily insulin dose decreased from 0.76 units/kg (IQR, 0.60–0.88) to 0.70 units/kg (IQR, 0.60–0.80) (P<0.001).
All patients were treated with either MDI or CSII, of whom 8.9% (79 patients) were treated exclusively with MDI, including 28 patients (4.3% of CSII) who decided not to use CSII after a short trial. The overall use of CSII was 74%. When patients were treated with CSII, median time from disease onset to the start of CSII was 8.81 (IQR, 6.32–10.93) years in 2001 and 0.59 (IQR, 0.14–2.00) years in 2010 (P<0.001).
In the entire group of patients, the incidence rate of severe acute complications was 1.07 per 100 patient-years for severe DKA (95% CI 0.81–1.40) and 1.21 per 100 patient-years for SH (95% CI 0.65–1.21). Forty-four (5.0%) patients had at least one episode of DKA, and 47 (5.3%) patients had at least one episode of SH. Data on severe acute complications rate per each year are presented in Table 1.
Table 1.
Severe Acute Complications in Each Observational Year
| |
Year |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | |
| DKA | ||||||||||||
| N |
6 |
3 |
3 |
3 |
2 |
3 |
7 |
6 |
1 |
8 |
3 |
9 |
| Ratea |
2.18 (0.80–4.75) |
0.94 (0.19–2.74) |
0.82 (0.17–2.41) |
0.77 (0.16–2.25) |
0.48 (0.06–1.73) |
0.69 (0.14–2.03) |
1.58 (0.64–3.26) |
1.31 (0.48–2.84) |
0.20 (0.01–1.14) |
1.68 (0.72–3.30) |
0.61 (0.12–1.77) |
1.90 (0.87–3.60) |
| SH | ||||||||||||
| N |
6 |
4 |
7 |
3 |
4 |
5 |
4 |
4 |
6 |
9 |
3 |
6 |
| Ratea |
2.18 (0.80–4.75) |
1.25 (0.34–3.21) |
1.92 (0.77–3.96) |
0.77 (0.16–2.25) |
0.96 (0.26–2.45) |
1.16 (0.38–2.70) |
0.90 (0.25–2.31) |
0.87 (0.24–2.23) |
1.22 (0.45–2.67) |
1.89 (0.86–3.58) |
0.61 (0.12–1.77) |
1.27 (0.46–2.76) |
| Patient-years at risk | 275 | 319.5 | 364.5 | 389 | 417.5 | 432.5 | 443 | 459.5 | 490 | 477 | 495.5 | 474 |
Diabetic ketoacidosis (DKA) was defined as an event requiring hospitalization and intravenous therapy. Severe hypoglycemia (SH) was defined as an event with a loss of consciousness and/or seizures requiring hospitalization and intramuscular and/or intravenous therapy.
Rates are the estimated rate per 100 patient-years (95% confidence interval).
N, total number of events.
Association between HbA1c and patient characteristics
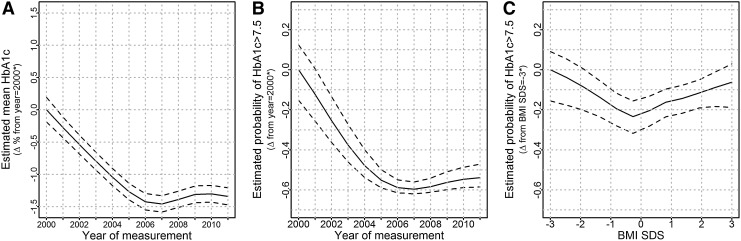
The (log-transformed) HbA1c level significantly and nonlinearly (P<0.001) decreased in the observational period. The change between year 2000 and 2011 remained significant, when the multivariable regression analysis was adjusted for possible differences in patient characteristics (age, gender, treatment modality, BMI SDS, and duration of diabetes) over time (Fig. 3A and B and Table 2): on average, the estimated HbA1c was 1.12 times higher (95% CI 1.11–1.14) in the year 2001 compared with 2011. The probability of suboptimal (HbA1c >7.5%) and poor (HbA1c >9%) metabolic control was significantly higher in the year 2001 compared with 2011 (OR=9, 95% CI 6–12; and OR=25, 95% CI 17–33, respectively) (Table 2 and Fig. 3B). Females had on average 1.02 times higher values of HbA1c compared with males (P=0.009, 95% CI 1.00–1.04) and had a higher probability of suboptimal metabolic control (OR=1.45, 95% CI 1.1–2.0, P=0.02) and a statistically nonsignificant higher probability of poor metabolic control (OR=1.3, 95% CI 0.9–1.9, P=0.20). Age and duration of diabetes were significantly and nonlinearly (P<0.001) associated with log2-HbA1c (Table 2). The HbA1c level initially decreased as age increased. However, the relationship was inverted during adolescence as larger values of HbA1c were estimated for older patients. After the end of adolescence, the HbA1c level decreased with age again. The HbA1c level increased for patients who had a longer duration of diabetes until about the sixth year of duration; after that, the value of HbA1c remained constant. Similar results were observed for suboptimal and poor metabolic control (Table 2).
FIG. 3.
Multiple adjusted estimates of (A) average hemoglobin A1c (HbA1c) by year of measurement and probabilities of suboptimal metabolic control (HbA1c >7.5%) by (B) year of measurement and (C) body mass index (BMI) SD score (SDS). A–C were rescaled, setting the starting point on the y-axes to zero; therefore, the values on the y-axes can be interpreted as estimated differences from the starting point.
Table 2.
Predictors of Metabolic Control, Using Multiple Adjusted Estimated Median Hemoglobin A1c (HbA1c), Ratio of Log-HbA1c, and Proportion of Patients with HbA1c >7.5% or >9% by Patient Characteristics and Aspects of Diabetes Management
| Variable | Median HbA1c (IQR) | HbA1c mean ratioa | P | HbA1c >7.5% | Odds ratio HbA1c >7.5% (CI)a | P | HbA1c >9% | Odds ratio HbA1c >9% (CI)a | P |
|---|---|---|---|---|---|---|---|---|---|
| Gender | |||||||||
| Male |
7.80 (7.20–8.60) |
1.00 |
0.01 |
62.47 |
1.00 |
0.019 |
16.84 |
1.00 |
0.204 |
| Female |
7.96 (7.30–8.80) |
1.02 (1.00–1.04) |
|
67.12 |
1.45 (1.06–1.98) |
|
20.13 |
1.28 (0.87–1.90) |
|
| Age at measurement (years)b | |||||||||
| 1 |
7.60 (6.90–8.30) |
1.00 |
<0.001 |
52.94 |
1.00 |
<0.001 |
5.88 |
1.00 |
<0.001 |
| 5 |
7.70 (7.20–8.20) |
0.97 (0.95–0.98) |
|
62.13 |
0.72 (0.53–0.96) |
|
4.14 |
0.86 (0.52–1.42) |
|
| 10 |
7.70 (7.10–8.30) |
0.94 (0.91–0.97) |
|
57.11 |
0.51 (0.27–0.94) |
|
10.02 |
0.84 (0.29–2.39) |
|
| 15 |
8.10 (7.40–9.00) |
0.96 (0.93–1.00) |
|
71.20 |
0.64 (0.32–1.25) |
|
24.63 |
2.72 (0.91–8.08) |
|
| 20 |
7.90 (7.20–8.85) |
0.95 (0.91–0.99) |
|
66.30 |
0.35 (0.16–0.77) |
|
21.68 |
2.76 (0.83–9.20) |
|
| Duration of diabetes (years)b | |||||||||
| 1 |
7.40 (6.80–8.14) |
1.00 |
<0.001 |
47.75 |
1.00 |
<0.001 |
8.92 |
1.00 |
<0.001 |
| 5 |
8.00 (7.30–8.80 |
1.11 (1.10–1.13) |
|
68.26 |
7.89 (5.87–10.60) |
|
21.80 |
6.27 (4.09–9.59) |
|
| 10 |
8.05 (7.50–8.90) |
1.15 (1.12–1.18) |
|
73.60 |
15.07 (9.31–24.38) |
|
21.25 |
9.49 (4.99–18.05) |
|
| 15 |
8.05 (7.40–8.90 |
1.17 (1.13–1.21) |
|
71.14 |
22.63 (11.09–46.18) |
|
22.82 |
9.77 (3.75–25.46) |
|
| Year of measurementb | |||||||||
| 2001 |
9.10 (8.20–10.15) |
1.00 |
<0.001 |
89.05 |
1.00 |
<0.001 |
52.06 |
1.00 |
<0.001 |
| 2006 |
7.65 (7.10–8.20) |
0.89 (0.88–0.90) |
|
54.70 |
0.11 (0.08–0.14) |
|
10.57 |
0.06 (0.04–0.08) |
|
| 2011 |
7.80 (7.20–8.32) |
0.89 (0.87–0.90) |
|
62.78 |
0.11 (0.08–0.16) |
|
9.36 |
0.04 (0.03–0.06) |
|
| BMI SDSb | |||||||||
| 0 |
7.75 (7.10–8.56) |
1.00 |
0.053 |
58.92 |
1.00 |
0.001 |
15.50 |
1.00 |
0.039 |
| −3 |
8.18 (8.06–8.46) |
1.03 (1.00–1.07) |
|
53.45 |
3.22 (1.39–7.49) |
|
3.45 |
4.89 (1.49–16.04) |
|
| −1 |
7.70 (7.10–8.55) |
1.01 (1.00–1.02) |
|
57.45 |
1.26 (1.02–1.56) |
|
17.60 |
1.35 (1.01–1.82) |
|
| 1 |
7.95 (7.32–8.80) |
1.00 (0.99–1.01) |
|
67.39 |
1.35 (1.10–1.65) |
|
20.52 |
1.29 (0.99–1.67) |
|
| 3 |
7.75 (7.10–8.20) |
0.99 (0.96–1.01) |
|
63.04 |
1.97 (1.13–3.43) |
|
13.04 |
1.45 (0.72–2.93) |
|
| Treatment modality | |||||||||
| MDI |
8.40 (7.50–9.50) |
1.00 |
<0.001 |
74.58 |
1.00 |
0.004 |
33.43 |
1.00 |
<0.001 |
| CSII | 7.80 (7.20–8.40) | 0.98 (0.97–0.99) | 61.25 | 0.72 (0.57–0.91) | 13.19 | 0.61 (0.46–0.80) | |||
Estimates are derived from multiple linear and logistic mixed models including gender, age at measurement, year of measurement, diabetes duration, body mass index (BMI) SD score (SDS), and treatment modality (multiple daily injections [MDI] or continuous subcutaneous insulin infusion [CSII]) as fixed effects. Values for age, duration of diabetes, and year of measurement, which were flexibly modeled using restricted cubic splines, present estimated associations between the variables and the outcomes.
Each estimate is adjusted for all the other variables in the table; a value of 1.00 indicates the reference values.
Restricted cubic splines were used to flexibly model the relationship between the covariate and the outcome. The variables were not categorized, and the values of the covariates do not represent categories. The descriptive statistics are based on the subset of patients with the chosen value of the covariate (±0.5 for BMI SDS).
CI, confidence interval; IQR, interquartile range.
The association between BMI SDS and HbA1c was small and not significant (P=0.053) (Table 2), in that patients with both high and low BMI SDS were more likely to have suboptimal or poor metabolic control (Fig. 3C) (P=0.001 and P=0.039, respectively).
On average, the patients treated with MDI had 1.02 times higher HbA1c compared with those treated with CSII (P<0.001) and a higher probability of suboptimal (OR=1.4, P<0.001) or poor (OR=1.6, P<0.001) metabolic control. The difference between the two treatments was more pronounced when the outcome was suboptimal and poor metabolic control (Table 2).
Discussion
For more than two decades the entire population of Slovene children with T1D has been treated centrally at the tertiary institution with a multidisciplinary team including a pediatric endocrinologist, certified nurse educators, psychologists, dietitians, and a social worker.20 Support and consultations are offered via an emergency 24/7 telephone line for patients, families, caregivers, and primary healthcare pediatricians, regardless of the treatment modality. The same structured education and management plan, emphasizing frequent self-monitoring of blood glucose and continuous glucose monitoring when possible and consultation via 24/7 telephone line, is provided for parents as well as for professional caregivers taking care of children with T1D in either kindergarten or school or during sport activities.21 Psychological support together with regular dietetic consultations is an important part of regular follow-up in the whole patient population. A patients' organization is providing regular annual educational meetings, publications, and summer camps with information available on a Web site (www.sladkorcki.si). CSII was introduced as a standard treatment modality with public reimbursement in the year 2000.
The observed decrease in median HbA1c level of 16.4 mmol/mol (1.5%) in our cohort differs from the finding of the Hvidoere study group, which reported stable center differences in metabolic control with only two out of 21 pediatric centers from 19 countries having a decrease in HbA1c level significantly of roughly 6.6 mmol/mol (0.6%) from 1998 until 2005.13 However, another study reported a pronounced decrease of HbA1c over a period of several years.14 It is interesting that the small but significant difference in the decrease of HbA1c level between genders is also reported by some11,14 studies but not others.13
The present study demonstrated a clear shift to younger age at onset of T1D with a median decrease from 12.68 to 7.53 years. A similar decrease was previously shown in other studies,22 also including our region.15,16
The BMI SDS of the entire group increased nonsignificantly in the first 5 years of diabetes and stabilized thereafter. Intensive treatment is associated with an increase in weight in several studies,1,13 but the increase in BMI SDS in the present study was comparable to the secular increase of BMI SDS during the same period in the general population.23 BMI SDS was found to be significantly associated with suboptimal metabolic control (HbA1c >7.5%), suggesting that patients with higher or lower BMI SDS are prone to poorer metabolic control. Because of the observational design the present study could not investigate the causality of this association.
The concomitant small but significant decrease in the daily insulin dose may be related to the frequent use of CSII, commonly associated with a lower daily insulin dose.6,7,13,24
The use of CSII increased in the investigated cohort to an overall level of use in 74% of patients. This proportion of pediatric patients using CSII is comparable to some academic institutions in the United States and Israel and was higher than in some European countries.9,13 The CSII discontinuation rate of 4.3% is comparable with other pediatric reports25 and typically lower compared with the adult CSII population.26
Recent studies show that CSII as an initial treatment at T1D diagnosis is safe and effective even in the youngest children25,27 with25 or without28 better long-term metabolic control and/or lower risk of SH. The time of CSII initiation after T1D onset in our study decreased significantly from 8.81 to 0.59 years. Long-term studies are needed to evaluate the benefit of early initialization of CSII therapy in the pediatric population.28
In the current study the insulin delivery regimen was slightly but significantly associated with HbA1c, favoring CSII, with stronger association in patients with poor metabolic control. This differs from a recently published study,14 where only the treatment modality from the last year of observation was analyzed. Greater impact of CSII on patients with poorer metabolic control was reported previously.11 The percentage of patients treated with CSII increased steadily during the study, with the CSII initiation time significantly closer to the disease onset, which likely contributed to the continuous improvement in HbA1c. However, uniform diabetes education with the emphasis on regular self-monitoring of blood glucose and continuous glucose monitoring, when possible, carbohydrate counting, telephone communication for dose adjustments, and problem solving with psychological support remained paramount.
The rate of severe acute complications requiring medical intervention was comparable to those in some other reports.14,29 Unfortunately, the national register did not include data on less SH.
The present study has several limitations. Because of the observational design, the study could not control for several factors influencing metabolic control and acute complications. Therefore, observed associations could be biased or may reflect only random effects. However, the attrition from the register was 4.2% as 37 patients were lost to follow-up before the age of 18 years. The treatment modality selection was performed according to the International Society for Pediatric and Adolescent Diabetes guidelines,2 introducing a selection bias especially in the first half of our observational period when CSII use was less frequent. The rate of severe acute complications events must be interpreted along with their definition including only cases where the intramuscular or/and intravenous therapy was administered by healthcare professionals. The national register does not include data on less SH or DKA because significant important numbers of these less severe acute complications routinely resolved without medical intervention.
In conclusion, the analysis of 886 pediatric patients from a national childhood-onset diabetes register demonstrated a significant and clinically meaningful decrease of HbA1c level over a 12-year observation period. This improvement was associated with treatment modality. Additional efforts and solutions are necessary to further improve metabolic control and the quality of life of young people with T1D.
Acknowledgments
The work was supported in part by the Slovenian National Research Agency grants J3–4116 and P3–0343. We thank the certified diabetes educators Ivica Zupancic, RNS, Tadeja Logar Dolinšek, RNS, Ana Gianini, RNS, and Barbara Murn-Berkopec, RNS, the dietician Andreja Sirca Campa, and the psychologists Simona Klemencic and Miha Rutar for their devoted patient care.
Author Disclosure Statement
No competing financial interests exist.
K.D. and S.S.T. collected data, participated in data analysis and interpretation, and drafted and reviewed the manuscript. Na.B. contributed to the study concept and design, supervised the study, participated in data analysis and interpretation, and reviewed/edited the manuscript. L.L. contributed to the study concept and design, performed the statistical analysis, and reviewed/edited the manuscript. Ni.B., M.Z.-T., P.K., and M.A.S. participated in data analysis and interpretation and reviewed/edited the manuscript. T.B. drafted the study concept and design, supervised the study, participated in data analysis and interpretation, and reviewed/edited the manuscript.
References
- 1.Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial The Diabetes Control and Complications Trial Research Group. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 2.Rewers M, Pihoker C, Donaghue K, Hanas R, Swift P, Klingensmith GJ: Assessment of glycemic control and adolescents with diabetes. Pediatr Diabetes 2009;10(Suppl 12):71–81 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association: Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eeg-Olofsson K, Cederholm J, Nilsson PM, Gudbjörnsdóttir S, Eliasson B; Steering Committee of the Swedish National Diabetes Register: Glycemic and risk factor control in type 1 diabetes: results from 13,612 patients in a national diabetes register. Diabetes Care 2007;30:496–502 [DOI] [PubMed] [Google Scholar]
- 5.Phillip M, Danne T, Shalitin S, Buckingham B, Laffel L, Tamborlane W, Battelino T; Consensus Forum Participants: Use of continuous glucose monitoring in children and adolescents. Pediatr Diabetes 2012;13:215–228 [DOI] [PubMed] [Google Scholar]
- 6.Phillip M, Battelino T, Rodriguez H, Danne T, Kaufman F; European Society for Paediatric Endocrinology; the Lawson Wilkins Pediatric Endocrine Society; International Society for Pediatric and Adolescent Diabetes; American Diabetes Association; European Association for the Study of Diabetes: Use of insulin pump therapy in the pediatric age-group: Consensus statement from European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by American Diabetes Association and the European Association for the Study of diabetes. Diabetes Care 2007;30:1653–1662 [DOI] [PubMed] [Google Scholar]
- 7.Pańkowska E, Błazik M, Dziechciarz P, Szypowska A, Szajewska H: Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: a systematic review and meta-analysis of randomized control trials. Pediatr Diabetes 2009;10:52–58 [DOI] [PubMed] [Google Scholar]
- 8.Misso ML, Egberts KJ, Page M, O'Connor D, Shaw J: Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev 2010;(1):CD005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickup JC, Sutton AJ: Severe hypoglycaemia and glycaemic control in Type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008;25:765–774 [DOI] [PubMed] [Google Scholar]
- 10.Renard E: Insulin pump use in Europe. Diabetes Technol Ther 2010;12(Suppl 1):S-29–S-32 [DOI] [PubMed] [Google Scholar]
- 11.Nimri R, Weintrob N, Benzaquen H, Ofan R, Fayman G, Phillip M: Insulin pump therapy in youth with type 1 diabetes: a retrospective paired study. Pediatrics 2006;117:2126–2131 [DOI] [PubMed] [Google Scholar]
- 12.Danne T, Battelino T, Jarosz-Chobot P, Kordonouri O, Pánkowska E, Ludvigsson J, Schober E, Kaprio E, Saukkonen T, Nicolino M, Tubiana-Rufi N, Klinkert C, Haberland H, Vazeou A, Madacsy L, Zangen D, Cherubini V, Rabbone I, Toni S, de Beaufort C, Bakker-van Waarde W, van den Berg N, Volkov I, Barrio R, Hanas R, Zumsteg U, Kuhlmann B, Aebi C, Schumacher U, Gschwend S, Hindmarsh P, Torres M, Shehadeh N, Phillip M; PedPump Study Group: Establishing glycaemic control with continuous subcutaneous insulin infusion in children and adolescents with type 1 diabetes: experience of the PedPump Study in 17 countries. Diabetologia 2008;51:1594–1601 [DOI] [PubMed] [Google Scholar]
- 13.de Beaufort CE, Swift PG, Skinner CT, Aanstoot HJ, Aman J, Cameron F, Martul P, Chiarelli F, Daneman D, Danne T, Dorchy H, Hoey H, Kaprio EA, Kaufman F, Kocova M, Mortensen HB, Njølstad PR, Phillip M, Robertson KJ, Schoenle EJ, Urakami T, Vanelli M; Hvidoere Study Group on Childhood Diabetes 2005: Continuing stability of center differences in pediatric diabetes care: do advances in diabetes treatment improve outcome? The Hvidøre Study Group on Childhood Diabetes. Diabetes Care 2007;30:2245–2250 [DOI] [PubMed] [Google Scholar]
- 14.Rosenbauer J, Dost A, Karges B, Hungele A, Stahl A, Bächle C, Gerstl EM, Kastendieck C, Hofer SE, Holl RW; DPV Initiative and the German BMBF Competence Network Diabetes Mellitus: Improved metabolic control in children and adolescents with type 1 diabetes: a trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care 2012;35:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radosevic B, Bukara-Radujkovic G, Miljkovic V, Pejicic S, Bratina N, Battelino T: The incidence of type 1 diabetes in Republic of Srpska (Bosnia and Herzegovina) and Slovenia in the period 1998–2010. Pediatr Diabetes 2012;14:273–279 [DOI] [PubMed] [Google Scholar]
- 16.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G; EURODIAB Study Group: Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 17.Clarke W, Jones T, Rewers A, Dunger D, Klingensmith GJ: Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes 2009;10(Suppl 12):134–145 [DOI] [PubMed] [Google Scholar]
- 18.Harrell FE, Jr, Lee KL, Pollock BG: Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 1988;80:1199–1202 [DOI] [PubMed] [Google Scholar]
- 19.R Development Core Team: R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing. 2009. www.R-project.org (accessed May27, 2013)
- 20.Cinek O, Sumník Z, de Beaufort C, Rurik I, Vazeou A, Madácsy L, Papo NL, Danne T; SWEET Group: Heterogeneity in the systems of pediatric diabetes care across the European Union. Pediatr Diabetes 2012;13(Suppl 16):5–14 [DOI] [PubMed] [Google Scholar]
- 21.Bratina N, Battelino T: Insulin pumps and continuous glucose monitoring (CGM) in preschool and school-age children: how schools can integrate technology. Pediatr Endocrinol Rev 2010;7(Suppl 3):417–421 [PubMed] [Google Scholar]
- 22.Dahlquist GG, Nyström L, Patterson CC; Swedish Childhood Diabetes Study Group; Diabetes Incidence in Sweden Study Group: Incidence of type 1 diabetes in Sweden among individuals aged 0–34 years, 1983–2007: an analysis of time trends. Diabetes Care 2011;34:1754–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blüher S, Meigen C, Gausche R, Keller E, Pfäffle R, Sabin M, Werther G, Odeh R, Kiess W: Age-specific stabilization in obesity prevalence in German children: a cross-sectional study from 1999 to 2008. Int J Pediatr Obes 2011;6:199–206 [DOI] [PubMed] [Google Scholar]
- 24.Sulli N, Shashaj B: Long term benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes: a 4-year follow-up. Diabet Med 2006;23:900–906 [DOI] [PubMed] [Google Scholar]
- 25.Sulmont V, Souchon PF, Gouillard-Darnaud C, Fartura A, Salmon-Musial AS, Lambrecht E, Mauran P, Abely M: Metabolic control in children with diabetes mellitus who are younger than 6 years at diagnosis: continuous subcutaneous insulin infusion as a first line treatment? J Pediatr 2010;157:103–107 [DOI] [PubMed] [Google Scholar]
- 26.Ronsin O, Jannot-Lamotte MF, Vague P, Lassman-Vague V: Factors related to CSII compliance. Diabetes Metab 2005;31:90–95 [DOI] [PubMed] [Google Scholar]
- 27.Berghaeuser MA, Kapellen T, Heidtmann B, Haberland H, Klinkert C, Holl RW; German Working Group for Insulin Pump Treatment in Paediatric Patients: Continuous subcutaneous insulin infusion in toddlers starting at diagnosis of type 1 diabetes mellitus A multicenter analysis of 104 patients from 63 centres in Germany and Austria. Pediatr Diabetes 2008;9:590–595 [DOI] [PubMed] [Google Scholar]
- 28.Shalitin S, Lahav-Ritte T, Lebenthal Y, Devries L, Phillip M: Does the timing of insulin pump therapy initiation after type 1 diabetes onset have an impact on glycemic control? Diabetes Technol Ther 2012;14:389–397 [DOI] [PubMed] [Google Scholar]
- 29.Svensson J, Johannesen J, Mortensen HB, Nordly S; Danish Childhood Diabetes Registry: Improved metabolic outcome in a Danish diabetic paediatric population aged 0–18 yr: results from a nationwide continuous registration. Pediatr Diabetes 2009;10:461–467 [DOI] [PubMed] [Google Scholar]