Abstract
Significance: Mitochondrial and cellular reactive oxygen species (ROS) play important roles in both physiological and pathological processes. Different ROS, such as superoxide (O2•−), hydrogen peroxide, and peroxynitrite (ONOO•−), stimulate distinct cell-signaling pathways and lead to diverse outcomes depending on their amount and subcellular localization. A variety of methods have been developed for ROS detection; however, many of these methods are not specific, do not allow subcellular localization, and can produce artifacts. In this review, we will critically analyze ROS detection and present advantages and the shortcomings of several available methods. Recent Advances: In the past decade, a number of new fluorescent probes, electron-spin resonance approaches, and immunoassays have been developed. These new state-of-the-art methods provide improved selectivity and subcellular resolution for ROS detection. Critical Issues: Although new methods for HPLC superoxide detection, application of fluorescent boronate-containing probes, use of cell-targeted hydroxylamine spin probes, and immunospin trapping have been available for several years, there has been lack of translation of these into biomedical research, limiting their widespread use. Future Directions: Additional studies to translate these new technologies from the test tube to physiological applications are needed and could lead to a wider application of these approaches to study mitochondrial and cellular ROS. Antioxid. Redox Signal. 20, 372–382.
Introduction
Reactive oxygen species (ROS) are highly reactive metabolites of oxygen, such as superoxide (O2•−), hydrogen peroxide (H2O2), and peroxynitrite (ONOO•−). Their lifetime in biological systems ranges from nanoseconds to seconds depending on their reactivity and the level of cellular antioxidants. Given their high reactivity and numerous clearance mechanisms, ROS exist in vivo in either picomolar or very low nanomolar steady-state concentrations (7). ROS detection in biological systems therefore requires probes that very rapidly react with ROS to compete with antioxidants and produce stable products, which can be quantified. This method is elegantly illustrated by the spin-trapping technique, in which spin traps covalently bind free radicals, producing adducts that can be detected by electron-spin resonance (ESR) (23). There are many other examples of probes that form detectable products reflecting the footprint of ROS formation, which are covered in this review. It is important that these are specific and sufficiently reactive with ROS to provide sensitivity. Many such probes have only recently become available, which we will cover and compare with older methods for ROS detection.
Older Technologies for ROS Detection
Spin trapping
One of the key ROS in biological systems is O2•−. This free radical has a pre-eminent role in biology and pathophysiology, because it is formed by many mammalian enzymes, has significant biological reactivity, and serves as a progenitor for formation of many other ROS, including H2O2, ONOO•−, and lipid peroxides. One of the earliest methods for O2•− detection was spin trapping with 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) (23). It is important to distinct spin traps and spin probes. Spin traps form covalent bond with the radical by addition reaction, while spin probes are oxidized by ROS without binding (14). While DMPO and similar nitrone spin traps are very useful in studies of isolated enzymes and in chemical solutions, they react with O2•− at very slow rate constants, between 30 and 70 M−1s−1, and have difficulty detecting O2•− in most biological systems due to competition with superoxide dismutase (SOD) and ascorbate (55). These slow rate constants also require that DMPO and similar spin traps be used at concentrations ranging from 20 to 100 mM. These high concentrations may have off-target effects and can affect cell viability. Moreover, intracellular reductants such as ascorbate can react with the O2•− adduct of DMPO (DMPO-OOH) and reduce it to a spin-inactive state, severely limiting the use of nitrones such as DMPO in biological systems (66). Recently, one cyclic nitrone, 5-diethoxyphosphoryl-5-methyl-1-pyrroline N-oxide (DEPMPO), has been modified by addition of a triphenylphosphonium group, promoting its selective uptake by mitochondria (27). Unfortunately, the triphenylphosphonium conjugate of DEPMPO (mito-DEPMPO) must also be used at high concentrations (50 mM), which may cause inhibition of mitochondrial ATP synthesis due to the accumulation of large amounts of the lipophilic cation in the mitochondrial matrix and alteration of the mitochondrial membrane potential (36). The use of this mito-DEPMPO spin trap is therefore likely limited by its slow rate constant for reaction with O2•− (33), potential toxicity (34), and nonspecific effects (1), and its use in biological systems is therefore unlikely. Because of these limitations, other approaches have been developed to detect ROS in intact cells and mitochondria. These include chemiluminescence methods, fluorescent probes, and more recently, antibody-based methods that have promise. These will be covered in the following sections.
Chemiluminescent probes
A commonly used chemiluminescence technique for measurement of O2•− is lucigenin-enhanced chemiluminescence (14). The validity of this technique has been questioned on the grounds that O2•− production might be artificially overestimated because of a phenomenon known as redox cycling, in which the lucigenin radical reacts with oxygen to generate O2•− (51). Other chemiluminescent probes include luminol, MCLA, and coelenterazine. Fluorescent probes are also commonly called dyes, and we have applied this term where appropriate. These likely react with a variety of ROS, and are therefore nonspecific. The lucigenin-derived redox cycling is particularly significant in artificial conditions with purified flavin proteins and NADH, but this problem may be overestimated in intact cells and tissues without NADH/NADPH supplementation. Meanwhile, questions persist about redox cycling with these agents, and they are being supplanted by other methods. None of these are specific for the mitochondria.
Fluorescent probes
Dihydroethidium and mitoSOX fluorescence
During the past decade, many research groups used dihydroethidium (DHE) and its mitochondrion-targeted form mitoSOX for cellular and mitochondrial O2•− detection (5, 58). This approach is limited, because DHE can form two fluorescent products. One is ethidium, which is formed by nonspecific redox reactions, while the other is 2-hydroxyethidium (2-OH-E+), a specific adduct of O2•− (58). The fluorescent spectra of ethidium and 2-OH-E+ overlap, and it is therefore difficult to use simple fluorescence detection with methods such as confocal microscopy or other fluorescence-based microscopic assays to accurately measure only 2-OH-E+. We and others have adapted high-pressure liquid chromatography (HPLC) to directly quantify 2-OH-E+ and have extensively validated this approach as a way to quantify O2•− in biological systems (58). Careful use of specific wavelengths of excitation might allows separation of these signals, so that confocal imaging or other fluorescence-based approaches could be used (45).
Dichlorodihydrofluorescein fluorescence
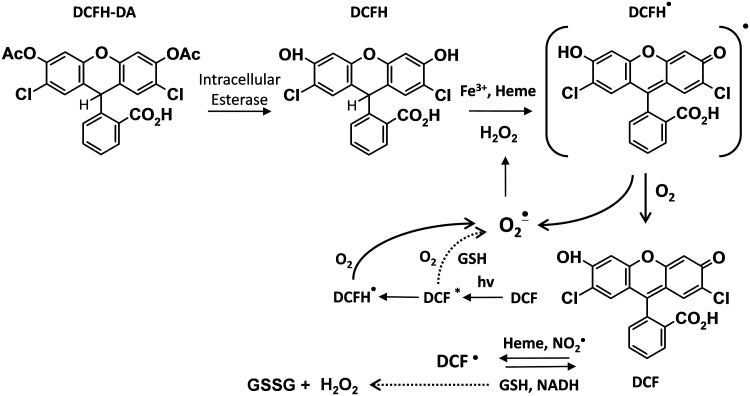
Dichlorodihydrofluorescein diacetate (DCFH-DA) is commonly used for detecting intracellular H2O2 (28). DCFH-DA is a cell-permeable ester and is hydrolyzed inside the cell to the dihydroxy-DCFH, which is retained (Fig. 1). Despite the popularity of this assay, it cannot be reliably used to measure intracellular H2O2 and other ROS for the following reasons: (i) DCFH does not directly react with H2O2; (ii) several one-electron oxidizing species will oxidize DCFH to DCF; (iii) DCF can actually produce O2•− and H2O2 via reaction of DCF radical with the oxygen, thus artificially elevating the very ROS that it is attempting to quantify; (iv) transition metals, cytochrome c, and heme peroxidases can catalyze DCFH oxidation (32). For these reasons, the editorial board of Free Radicals in the Biology and Medicine journal stated that this agent should not be used as a reliable measure of H2O2.
FIG. 1.
Dichlorodihydrofluorescein diacetate (DCFH-DA) intracellular reactions and redox cycling of 2,7-dichlorodihydrofluorescein (DCF).
Dihydrorhodamine fluorescence
Dihydrorhodamine (DHR) is commonly used for detection of ONOO•− (30). This assay is based on the oxidative conversion of DHR to its corresponding two-electron oxidized fluorescent product, rhodamine. DHR oxidation to rhodamine is not only caused by ONOO•−. The oxidative conversion of DHR to rhodamine is mediated by an intermediate DHR radical that can be reduced by thiols and ascorbic acid, leading to false-negative data. It is therefore concluded that DHR can only be used as a nonspecific indicator of intracellular ONOO•− and HOCl or other one-electron oxidant (52).
Detection of extracellular H2O2 by Amplex Red
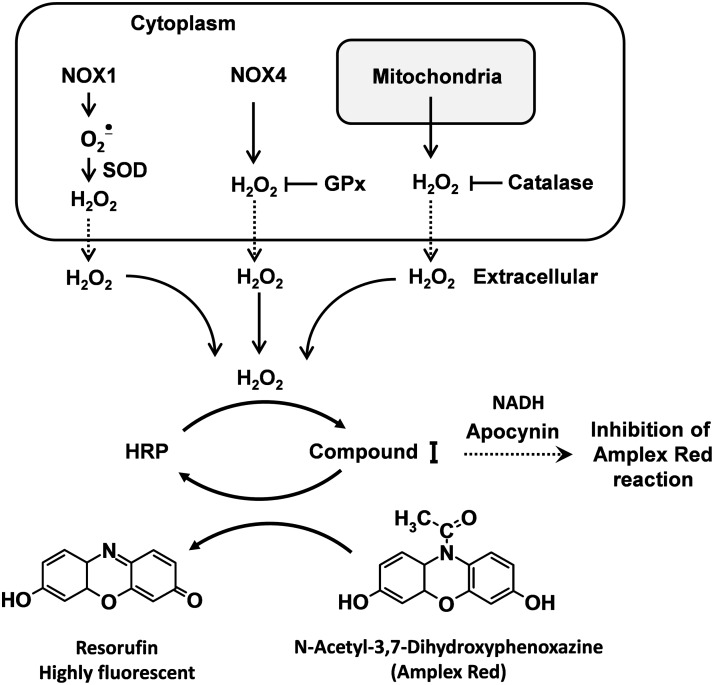
The N-acetyl-3,7-dihydroxyphenoxazine (Amplex Red) assay, developed by Molecular Probes, is based on the horseradish peroxidase-catalyzed oxidation of the nonfluorescent molecule Amplex Red to resorufin, which when excited at 530 nm emits light at 590 nm (57). Horseradish peroxidase reacts with H2O2, generating peroxidase compound I, which produces resorufin on an equimolar basis (Fig. 2). This assay is highly specific and sensitive (63). We have used it extensively in isolated vessels and cells.
FIG. 2.
Detection of hydrogen peroxide (H2O2) with N-acetyl-3,7-dihydroxyphenoxazine (Amplex Red).
Amplex Red can be used to detect H2O2 released from isolated mitochondria (43). Superoxide produced in the mitochondrial matrix is quickly dismutated by SOD2 to H2O2, which readily diffuses through mitochondrial membranes to the surrounding medium.
A caveat for use of Amplex Red is that O2•−can react with peroxidase either in its basal state or when the peroxidase is in the compound I or II state, and thus can lead to formation of compound III. These reactions can alter the stoichiometry of H2O2 detection. It is therefore not possible to quantitatively measure H2O2 by Amplex Red in the presence of substantial fluxes of O2•−. This problem can be remedied by addition of Cu,Zn-SOD to the assay. This eliminates this problem and likely minimally affects the ultimate result, as O2•− spontaneously dismutes to H2O2 even in the absence of SOD at a slightly slower rate.
The Amplex Red method may be affected by potential interference with reducing agents such as apocynin and NADH (Fig. 2), auto-oxidation of Amplex Red dye, light sensitivity (54), and inability to directly assess the intracellular H2O2 (14). The Amplex red dye is somewhat unstable. At high concentrations (50 μM), it can be auto-oxidized and produce O2•− and H2O2. Low concentrations of Amplex Red (10 μM) minimize this problem. Amplex red does not detect intracellular H2O2. Because H2O2 is diffusible, it reaches the equilibrium with the tissue's surrounding buffer, so that values measured in the buffer should provide an index of what was originally produced by the tissue.
Superoxide detection by cytochrome c
Ferricytochrome c reduction is a time-honored and accurate method for detecting large amounts of O2•− released by cells into the extracellular space, by isolated enzymes or by various chemical reactions. This assay however has limitations that prohibit its use in many physiological preparations. It is based on reduction of ferricytochrome c by O2•− to ferrocytochrome c. This reaction can be followed by the spectrophotometric absorbance at 550 nm. The absorptions at the neighboring wavelengths of 540 and 560 nm are not altered, and therefore are used as isosbestic points to normalize the signal at 550 nm. Electrons donated from enzymes and other molecules can directly reduce ferricytochrome c, and the resultant change in absorbance is not specific for O2•−. For this reason, the reduction due to O2•− must be calculated by performing simultaneous assays with SOD added and determining the SOD-inhibitable signal. Cytochrome c reduction is suitable for quantifying O2•− released during the respiratory burst of neutrophils or by isolated enzymes; however, it is difficult to detect the smaller quantities of O2•− generated by nonphagocytic cells such as vascular smooth muscle cells and endothelial cells. Ferricytochrome c, being a large protein, does not gain access to the intracellular space, and therefore cannot be used to detect O2•− released in the cytoplasm or by the mitochondria of intact cells. Finally, reactions of cytochrome c with H2O2 and various commonly used drugs such as oxypurinol, apocynin, and L-NAME can attenuate its reduction and cause artifacts in O2•− detection.
New Methods for ROS Detection
Detection of cellular and mitochondrial O2•− using DHE and mitochondrion-targeted probe mitoSOX
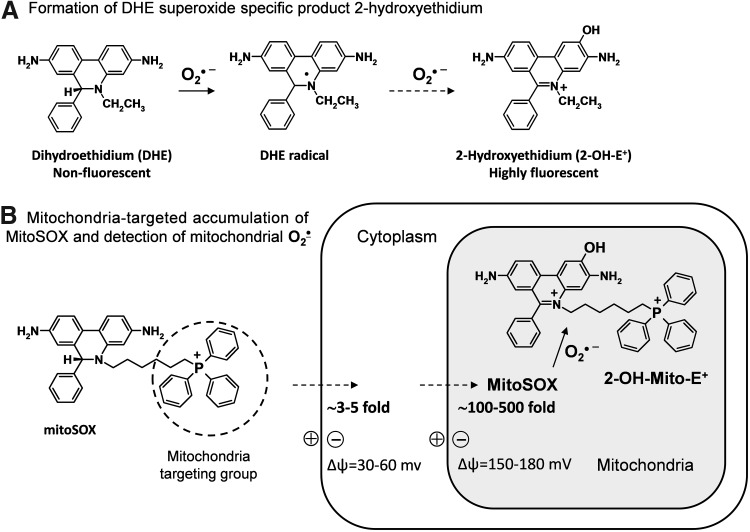
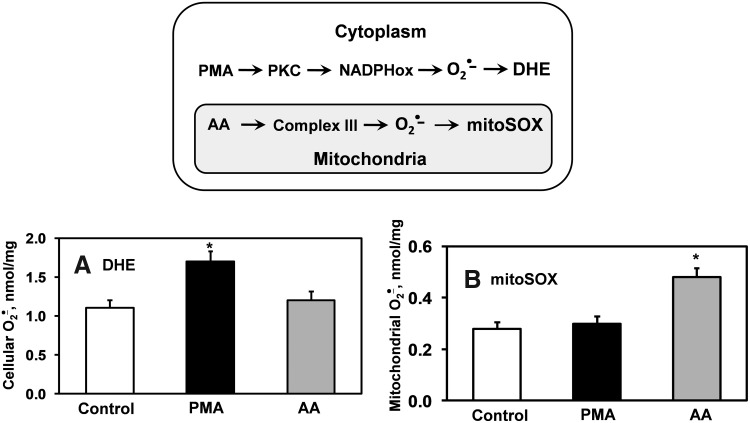
In 2003, Kalyanaraman and colleagues reported formation of a superoxide-specific product from the reaction of DHE with O2•− (Fig. 3A) (56). Following this original report, DHE has been modified to allow detection of O2•− in the mitochondria by addition of a triphenylphosphonium group, which promotes its accumulation in the mitochondria (14). This modified DHE analog is referred to as mitoSOX, and has become commonly used for detection of O2•− within the mitochondria. Analogous to DHE, mitoSOX reacts with O2•− to form 2-hydroxy-mito-ethidium (2-OH-Mito-E+) (Fig. 3B), which can be detected and quantified using HPLC (60). As an example of its specificity for mitochondrial-derived O2•−, previous studies have shown that blockade of mitochondrial complex 3 with antimycin A increases the conversion of mitoSOX to 2-OH-Mito-E, but has no effect on conversion of DHE to 2-OH-E+. In contrast, stimulation of cytoplasmic O2•− using phorbol-12-myristate-13-acetate (PMA) increases 2-OH-E+ formation from DHE, but does not increase formation 2-OH-Mito-E+ from mitoSOX (Fig. 4) (19). These data confirm the specificity of mitochondrial O2•− detection by mitoSOX and illustrate that DHE detects cytoplasmic O2•−, but not mitochondrial O2•−. Separation of O2•− produced in either the mitochondria or the cytoplasm is therefore possible by HPLC measurements of 2-OH-E+ or 2-OH-Mito-E+.
FIG. 3.
Detection of O2•−-specific products of dihydroethidium (DHE) and mitoSOX. (A) Formation of DHE O2•−-specific product 2-hydroxyethidium, and (B) mitochondrion-targeted accumulation of mitoSOX and detection of mitochondrial O2•−.
FIG. 4.
Selective activation of cytoplasmic NADPH oxidases with PMA or treatment of mitochondria with antimycin A results in site-specific production of O2•−. Site-specific detection of cytoplasmic and mitochondrial O2•− using DHE (A) and mitoSOX (B). *p<0.05 vs control.
A caveat to use of mitoSOX is that high concentrations can overload the mitochondria and impair mitochondrial function (45). Furthermore, concentrations of mitoSOX exceeding 2 μM can lead to substantial cytoplasmic accumulation of mitoSOX and thus can compromise mitochondrial specificity of O2•− detection. We therefore suggest using mitoSOX at concentrations 2 μM or less to avoid these complications.
While measurements of 2-OH-Mito-E+ are most accurately achieved by HPLC (61), Beckman and colleagues have reported that mitochondrial O2•− can be accurately quantified in live cells using selective excitation at 385–405 nm and detection at an emission of 560 nm (45). These parameters seem to reduce signals derived from nonspecific fluorescent products. Thus, optimized fluorescence spectroscopy can be used for rapid and specific measurements of mitochondrial O2•−; however, confirmation with HPLC analysis of mitoSOX samples is advisable.
Limitations of DHE and mitoSOX include instability of the probes and their products, complex chemistry, and potential interference with heme enzymes (60). These probes are light sensitive and are prone to auto-oxidation. They require a two-step reaction to detect O2•− involving a free-radical intermediate (62) that can be potentially a subject for reaction with the antioxidants. Finally, formation of O2•−-specific product can be affected by peroxidase reactions, which could compromise quantification of O2•− measurements (21).
Detection of total cellular and mitochondrial O2•− by cyclic hydroxylamine spin probes
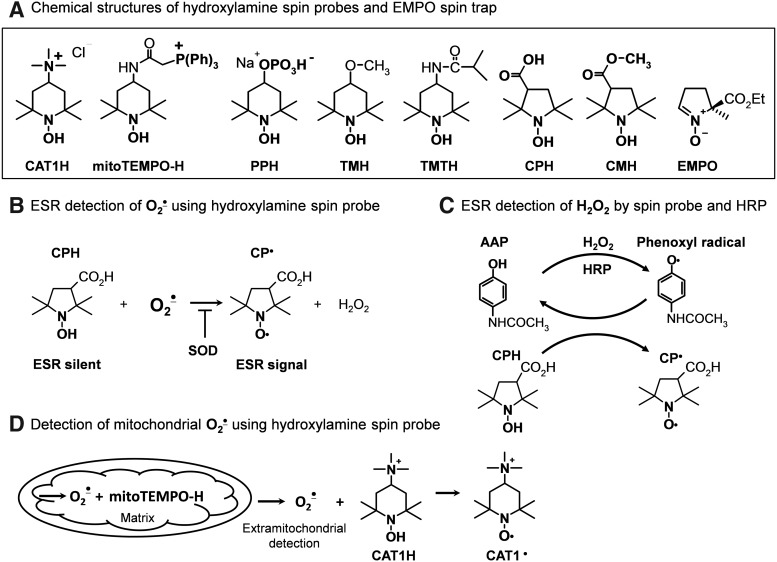
We and others have found that cyclic hydroxylamines (Fig. 5A) can be used for measurement of O2•− in cultured cells, tissues, and in vivo (13, 15, 17). These molecules are not spin traps in that they do not trap radicals, but are oxidized by O2•− and other ROS to form EPR-detectable stable nitroxides with lifetimes of several hours in cell cultures.
FIG. 5.
Chemical structures of hydroxylamine probes (A), ESR detection of cellular superoxide (B), hydrogen peroxide (C) and detection of mitochondrial superoxide (D).
Cyclic hydroxylamines react with O2•− with rate constants of ∼103–104 M−1s−1 (pH=7.4), which is much more rapid that the reaction of O2•− with nitrone spin traps. This favors the competition of nitroxides with cellular antioxidants and enhances the efficiency for detection of intracellular O2•− (Fig. 5B) (17). For this reason, hydroxylamine probes can be used at relatively low concentrations (0.05–1 mM), minimizing potential off-target effects of probes in biological systems. Another advantage of the cyclic hydroxylamines is that they react with O2•− in a single chemical reaction. This minimizes potential artifacts that can occur with multistep redox reactions that occur with other probes (21). Unlike lucigenin or other probes, hydroxylamine is incapable of redox cycling, and therefore has no potential to artificially produce ROS (39).
There are number of cationic, anionic, and neutral hydroxylamine spin probes that have varying degrees of lipophilicity and cell permeability. By careful selection of these probes, one can separately detect intracellular ROS, extracellular ROS, or both. For example, the positively charged 1-hydroxy-2,2,6,6-tetramethylpiperidin-4-yl-trimethylammonium (CAT1H) detects only extracellular O2•− released from intact cells and tissue or extramitochondrial O2•− released from isolated mitochondria. In contrast, 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH) is highly cell permeable and can detect both intracellular and extracellular ROS. The phosphate-containing negatively charged 1-hydroxy-4-phosphono-oxy-2,2,6,6-tetramethylpiperidine (PPH) accumulates inside cells, presumably via active transport. The cell-permeable 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine (CPH) and CMH accumulate in the cytoplasm. CMH also accumulates in mitochondria. Mito-TEMPO-H is a modified nitroxide that is analogous to mitoSOX, in that it contains a triphenylphosphonium group, causing it to be concentrated within the mitochondria.
There is functional evidence to support that these probes partition selectively. Upon stimulation of cytoplasmic O2•− with PMA, both PPH and CMH provide strong signals, while the signal with mito-TEMPO-H is weak. In contrast, stimulation of mitochondrial O2•− with rotenone only increases the CMH and mito-TEMPO-H signals (Fig. 5D). Thus, judicious use of the hydroxylamine spin probes can provide unique information about site-specific production of ROS in the extracellular, intracellular, or mitochondrial compartments.
Despite significant differences in the chemical structures, these probes have comparable rates of oxidation in response to O2•− generated by xanthine and xanthine oxidase (18). It is important to note that conversion of cyclic hydroxylamines to their respective nitroxide radicals is not specific for O2•−, but can be mediated by peroxynitrite and likely other oxidants. Thus, it is necessary to use specific antioxidants to probe the precise nature of the ROS involved in creating the nitroxide radical signal. As an example, overexpression of SOD2, which is targeted to the mitochondria, inhibits the CMH and mito-TEMPO-H signals caused by rotenone (18). In other cases, polyethylene glycol (PEG)-SOD has been found to reduce nitroxide radical production by 70% to 98% (18). Uric acid can be employed as a semiselective scavenger of peroxynitrite (37).
In general, there is no reaction between H2O2 and the cyclic hydroxylamines; however, H2O2 can be detected in isolated mitochondria and other subcellular fractions (Fig. 5C) using horseradish peroxidase mediated co-oxidation of CAT1H (20) or CPH (16).
A distinct advantage of the cyclic hydroxylamines is that they can be used to directly monitor cellular, subcellular, and tissue production of ROS online at room temperature by following the accumulation of nitroxide radical using an ESR spectrometer. For this, we generally run a field scan, and detect the precise g-value of the low field peak, and set the scanner to follow this g-value over time. Parallel experiments using added SOD can confirm that the signal is derived from O2•−. Low-temperature ESR is useful for detection of ROS by intact tissues. For this purpose, samples are incubated in physiological saline containing cyclic hydroxylamines and subsequently frozen in liquid nitrogen, stored at −80°C, and analyzed later at a low temperature in quartz dewar (14).
For detection of ROS using virtually any assay, it is essential that one eliminate signals artificially generated by transition metals that often contaminate physiological buffers. This is particularly true for cyclic hydroxylamines. We have found that premixing of buffer with chelex overnight is very helpful as is the addition of deferoxamine or DTPA.
Detection of cytoplasmic and mitochondrial H2O2 by fluorescent protein-based redox probes
Recently, a revolutionary approach has been employed for detection of ROS and redox status involving the introduction of either plasmids or adenoviruses into cells. The cells then produce chimeric proteins that are capable of detecting ROS or changes in the redox status (4, 25). One such probe is HyPer, which consists of circularly permuted yellow fluorescent protein (cpYFP) inserted into the regulatory domain of the prokaryotic H2O2-sensing protein, OxyR. By molecularly recombining various signal peptides or retention sequences with HyPer, this probe has been successfully targeted to various intracellular organelles, including the nucleus, the cytoplasm, peroxisomes, the mitochondrial intermembrane space, and the mitochondrial matrix. Using these various constructs, Malinouski et al. have provided evidence supporting the presence of low levels of H2O2 in these subcellular compartments. An exception to this is the endoplasmic reticulum, where HyPer was found to be predominantly oxidized (38). This finding suggests that there is a previously unrecognized large flux of H2O2 in the endoplasmic reticulum, and might reflect the need to generate H2O2 to promote disulfide formation and protein folding in this compartment of the cell. A potential advantage of HyPer is that its fluorescence is reversible, and thus once this is loaded into cells, it can be used to follow varying fluxes of peroxide over time. This is in contrast to many commonly used fluorescent dyes, such as DCF, which are permanently activated by a pulse of H2O2. Mitochondrial-targeted redox-sensitive yellow fluorescent protein pHyPer-dMito has been used to analyze H2O2 in the mitochondria of apoptotic HeLa cells (4).
The fluorescence of HyPer can be affected by the cellular redox status and by reduction of the GFP by thioredoxins (26). To avoid these issues, Dick and colleagues have designed new probes in which a H2O2 sensor (Orp1) is conjugated to a redox sensitive reporter (roGFP2) (25). Orp1 is oxidized by H2O2, and the oxidized Orp1 then accepts an electron from cysteine residues on the roGFP2, which then emits fluorescence. This roGFP2-Orp1 construct seems to be a reliable H2O2 sensor that does not depend on the cellular redox status. This roGFP2-Orp1 redox probe may represent one of the most advanced and promising tools for specific, quantitative, dynamic, and compartment-specific H2O2 detection. roGRP2-Orp1 has also been modified to allow mitochondrial targeting.
A second chimeric construct in which roGFP2 is linked to glutathione reductase has been developed for measurement of glutathione redox potential (42). Detailed protocols for the use of these probes in both yeast and mammalian systems have been presented (42).
Although these genetically encoded redox probes can be used for quantitative in vivo mapping of the glutathione redox potential and H2O2 in the cytosol and mitochondria, overexpression of these GPx-like proteins can significantly increase H2O2 scavenging, and thus alter cellular redox signaling on their own (3). Appropriate control experiments are therefore needed to show that antioxidant side effects are not occurring as a result of the use of these probes.
Detection of H2O2 and ONOO•− by boronate-based fluorescent probes
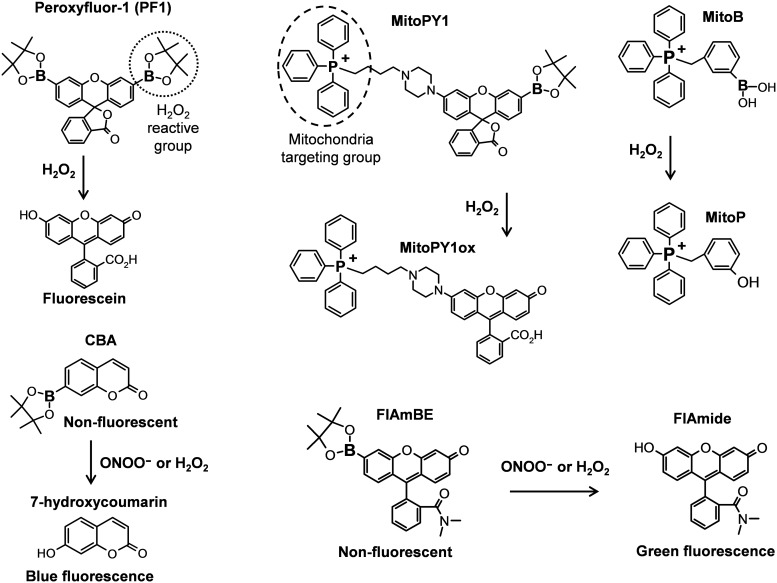
Chang and colleagues have recently synthesized a family of boronate-based probes, including red-fluorescent peroxyresorufin-1, green-fluorescent peroxyfluor-1, and blue-fluorescent peroxyxanthone-1 (9, 41). These fluoresce in the red, green, and blue ranges, respectively, upon exposure to H2O2. These probes possess a fluorophore that is protected by boronate, and upon exposure to H2O2, the boronate undergoes a nucleophilic attack, leading to its removal from the fluorophore and allowing light emission (Fig. 6). These boronate probes are cell permeable and can detect changes in H2O2 in the micromolar range in living cells (41).
FIG. 6.
Molecular structures and reactivity of boronate probes.
These investigators have also synthesized a boronate-based peroxy-yellow 1 probe containing a triphenylphosphonium group, again analogous to mitoSOX, which allows selective detection of H2O2 within the mitochondria (MitoPY1, Fig. 6). This new bifunctional fluorescent probe permits imaging H2O2 within the mitochondria of living cells using imaging techniques such as confocal microscopy and flow cytometry (12).
Intracellular O2•− normally converted to H2O2 by SODs, but under inflammatory condition and oxidative stress, overproduction of O2•− leads to very fast reaction with nitric oxide, producing peroxynitrite (ONOO−). The local ratio between NO and O2•− has a significant impact on the formation, where ONOO•− formation is maximal at the NO/O2•− ratio 1: 1 and is significantly declined when one of the two radicals is present in excess (59). Kalyanaraman and colleagues have recently described boronate probes that react stoichiometrically with ONOO−, yielding corresponding phenols (47, 59). One example, coumarin-7-boronic acid (CBA, rapidly reacts with ONOO•− with a rate constant of 1.1×106 M−1s−1 (Fig. 6). Using CBA and fluorescein dimethylamide boronate (FlAmBE), this group has studied ONOO•− production in endothelial cells and macrophages (63). It seems that the reaction of ONOO•− with boronate leads to a site-specific nitration of an aromatic moiety that can be followed by either HPLC or mass spectrometry (Kalyanaraman, Oxygen Radicals Gordon Conference, 2012). It is quite likely that boronate probes will provide a more reliable approach for detection of OONO− than assays using either dichlorodihydrofluorescein or DHR (31).
As suggested above, there is a potential problem related to the lack of specificity of boronate probes that can detect either ONOO•− or H2O2 (Fig. 6). The use of an ONOO•− scavenger such as uric acid or an H2O2 scavenger such as PEG-catalase might help increase specificity of boronate probes, but further studies are needed to validate and perfect such approaches.
Immuno-spin trapping
During the past decade, a new immunospin-trapping method has been developed based on the concept that DMPO will react with protein radicals, forming epitopes that can be specifically identified immunologically. Mason and colleagues have developed a panel of antibodies that reacts with DMPO–protein radical adducts and have shown that they work well for Western blot analysis, immunostaining, and immunofluorescence and even flow cytometry (44). Immunological techniques can be highly sensitive, and thus this method greatly expands the utility of the spin trapping. Moreover, this approach makes use of DMPO and potentially other spin traps possible without ESR, which, as discussed above, is of limited availability, often lacks sensitivity, and has limited applicability (40, 44). In initial studies, anti-DMPO has been used to detect DMPO–protein adducts of myoglobin and hemoglobin that occurred as a result of self-peroxidation by H2O2 (11). Immunospin trapping has been used to detect free-radical adduct formation in the mitochondria, cells, and tissue samples (24). Of note, DMPO adducts of components of mitochondrial electron transfer have been detected using immunospin trapping (10). It is likely that this approach can be widely used to examine specific targets of oxidative attack within cells and subcellular organelles. A limitation of this approach is that it does not detect free radicals such as O2•− or OH•, but identifies modified protein adducts. It is also unclear if it will detect all radical protein adducts, and perhaps additional antibodies will need to be produced to provide broader sensitivity.
ROS Detection In Vivo Using X- and L-Band ESR Spectroscopy
While there are several approaches to detection of ROS ex vivo, a major challenge has been to detect these in vivo. It has been previously shown that O2•− production can be detected in vivo by infusion of either nitrone spin traps (65) or cyclic hydroxylamines, followed by ex vivo analysis of the blood or tissue samples using X-band (9 GHz) ESR spectroscopy (13, 22, 35). Over the past two decades, substantial effort has been made to visualize radicals using spin probes and L-band ESR spectroscopy. X-band spectroscopy, which is most commonly used, is generally not suitable for this purpose. First, most cavities supplied for X-band will not accommodate experimental animals or even significant amounts of living tissue, but more importantly, the large amounts of water present in such samples absorb most of the microwave energy in the X-band range. L-band (1 GHz) spectroscopy overcomes some of these limitations and can provide special resolution for analysis of living tissues in online experiments (48). L-band ESR can be used for in vivo detection of short-lived free radicals in whole living animals (29). This method has been used to monitor tissue oxygen levels (2), pH, redox status, and glutathione levels (6). A downside of L-band spectroscopy is that these lower-frequency microwave energies reduce sensitivity substantially, and therefore detection of radicals such as O2•− is limited. Efforts are being made to employ nitroxides to detect radicals in vivo with L-band (46, 50); however, these have not been successful in detecting the small amounts of radicals that are important in common pathophysiological conditions.
Antioxidant Activity of ROS Probes
The discussion above has focused principally on the use of various probes to detect ROS; however, reaction of ROS with probes may have two implications: (i) significantly reduce the steady-state levels of O2•−, H2O2 NO, or ONOO•− during the measurements, and (ii) these agents can be of interest as antioxidants (49). Our data showed dependence of ROS detection on DHE or spin probe concentrations; therefore, one should avoid saturation of biological samples with the ROS probe to avoid side effects. Generally, it is a good idea to measure ROS by two independent methods and monitor different species in the same samples. A wide variety of spin traps and spin probes such as DMPO, PBN, TEMPOL, and mito-TEMPO protect the brain, heart, and vascular tissue under conditions of oxidative stress (8, 19, 64). Beneficial responses to these agents have been used to implicate ROS in various pathophysiological conditions.
Conclusions
Historically, studies of ROS and free radicals were strictly confined to the fields of chemistry and physics. It has however become increasingly clear that these fleeting molecules play critical roles in both normal physiology and pathophysiology. This recognition has made it essential that tools are available that allow their detection and measurement in complex biological samples, including cells, tissues, and eventually intact animals. Many initial methods such as cytochrome c reduction and spin trapping were suitable for high level of ROS production (e.g., activated neutrophils) (53); however, studies of low levels of intracellular ROS require new more sensitive methods (14). In the past decade, new methods for site-specific detection of ROS have been developed and validated, which are helping to fulfill this goal. These new methods include use of the boronates, immunospin probes, and mitochondrial-specific probes that can be adapted to other approaches such as confocal imaging, live-cell imaging, Western analysis, flow cytometry, and HPLC methods commonly employed in research laboratories. We therefore view these developments with substantial optimism with the hope that these approaches will continue to be validated and more widely used.
Abbreviations Used
- 2-OH-E+
2-hydroxyethidium
- 2-OH-Mito-E+
2-hydroxy-mito-ethidium
- Amplex Red
N-acetyl-3,7-dihydroxyphenoxazine
- CAT1H
1-hydroxy-2,2,6,6-tetramethylpiperidin-4-yl-trimethylammonium
- CBA
coumarin-7-boronic acid
- CMH
1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine
- CPH
1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine
- DCF
2,7-dichlorodihydrofluorescein
- DCFH-DA
dichlorodihydrofluorescein diacetate
- DEPMPO
5-diethoxyphosphoryl-5-methyl-1-pyrroline N-oxide
- DHE
dihydroethidium
- DHR
dihydrorhodamine
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- ESR
electron-spin resonance
- FlAmBE
fluorescein dimethylamide boronate
- H2O2
hydrogen peroxide
- HPLC
high-pressure liquid chromatography
- MCLA
luciferin analogue, 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazole [1,2-a] pyrazin-3one
- mito-DEPMPO
triphenylphosphonium conjugate of DEPMPO
- MitoPY1
triphenylphosphonium conjugate with boronate-based peroxy-yellow 1
- mitoSOX
mitochondrion-targeted DHE
- mitoTEMPO-H
1-hydroxy-4-[2-triphenylphosphonio)-acetamido]-2,2,6,6-tetramethylpiperidine
- O2•−
superoxide
- ONOO•−
peroxynitrite
- PEG
polyethylene glycol
- PMA
phorbol-12-myristate-13-acetate
- PPH
1-hydroxy-4-phosphono-oxy-2,2,6,6-tetramethylpiperidine
- RET
reverse electron transfer
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Acknowledgments
This work was supported by funding from National Institutes of Health grants PO-1 HL058000 and RO-1 HL094469.
References
- 1.Abou-Khalil S, Abou-Khalil WH, Planas L, Tapiero H, and Lampidis TJ. Interaction of rhodamine 123 with mitochondria isolated from drug-sensitive and -resistant Friend leukemia cells. Biochem Biophys Res Commun 127: 1039–1144, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Ahmad R, Som S, Johnson DH, Zweier JL, Kuppusamy P, and Potter LC. Multisite EPR oximetry from multiple quadrature harmonics. J Magn Reson 214: 135–143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht SC, Barata AG, Grosshans J, Teleman AA, and Dick TP. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab 14: 819–829, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, and Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3: 281–286, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Benov L, Sztejnberg L, and Fridovich I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic Biol Med 25: 826–831, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Bobko AA, Eubank TD, Voorhees JL, Efimova OV, Kirilyuk IA, Petryakov S, Trofimiov DG, Marsh CB, Zweier JL, Grigor'ev IA, Samouilov A, and Khramtsov VV. In vivo monitoring of pH, redox status, and glutathione using L-band EPR for assessment of therapeutic effectiveness in solid tumors. Magn Reson Med 67: 1827–1836, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadenas E. and Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29: 222–230, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Carney JM. and Floyd RA. Protection against oxidative damage to CNS by alpha-phenyl-tert-butyl nitrone (PBN) and other spin-trapping agents: a novel series of nonlipid free radical scavengers. J Mol Neurosci 3: 47–57, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Chang MC, Pralle A, Isacoff EY, and Chang CJ. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J Am Chem Soc 126: 15392–15393, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YR. EPR spin-trapping and nano LC MS/MS techniques for DEPMPO/OOH and immunospin-trapping with anti-DMPO antibody in mitochondrial electron transfer system. Methods Mol Biol 477: 75–88, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Deterding LJ, Ramirez DC, Dubin JR, Mason RP, and Tomer KB. Identification of free radicals on hemoglobin from its self-peroxidation using mass spectrometry and immuno-spin trapping: observation of a histidinyl radical. J Biol Chem 279: 11600–11607, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Dickinson BC. and Chang CJ. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J Am Chem Soc 130: 9638–9639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dikalov S, Fink B, Skatchkov M, and Bassenge E. Comparison of glyceryl trinitrate-induced with pentaerythrityl tetranitrate-induced in vivo formation of superoxide radicals: effect of vitamin C. Free Radic Biol Med 27: 170–176, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Dikalov S, Griendling KK, and Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49: 717–727, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dikalov S, Skatchkov M, Fink B, and Bassenge E. Quantification of superoxide radicals and peroxynitrite in vascular cells using oxidation of sterically hindered hydroxylamines and electron spin resonance. Nitric Oxide 1: 423–431, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, and Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikalov SI, Dikalova AE, and Mason RP. Noninvasive diagnostic tool for inflammation-induced oxidative stress using electron spin resonance spectroscopy and an extracellular cyclic hydroxylamine. Arch Biochem Biophys 402: 218–226, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Dikalov SI, Kirilyuk IA, Voinov M, and Grigor'ev IA. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic Res 45: 417–430, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, and Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doughan AK. and Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal 9: 1825–1836, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Fernandes DC, Wosniak J, Jr, Pescatore LA, Bertoline MA, Liberman M, Laurindo FR, and Santos CX. Analysis of DHE-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am J Physiol Cell Physiol 292: C413–C422, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Fink B, Dikalov S, and Bassenge E. A new approach for extracellular spin trapping of nitroglycerin-induced superoxide radicals both in vitro and in vivo. Free Radic Biol Med 28: 121–128, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Finkelstein E, Rosen GM, and Rauckman EJ. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys 200: 1–16, 1980 [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Mejiba SE, Zhai Z, Akram H, Deterding LJ, Hensley K, Smith N, Towner RA, Tomer KB, Mason RP, and Ramirez DC. Immuno-spin trapping of protein and DNA radicals: “tagging” free radicals to locate and understand the redox process. Free Radic Biol Med 46: 853–865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutscher M, Sobotta MC, Wabnitz GH, Ballikaya S, Meyer AJ, Samstag Y, and Dick TP. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J Biol Chem 284: 31532–31540, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanschmann EM, Lonn ME, Schutte LD, Funke M, Godoy JR, Eitner S, Hudemann C, and Lillig CH. Both thioredoxin 2 and glutaredoxin 2 contribute to the reduction of the mitochondrial 2-cys peroxiredoxin prx3. J Biol Chem 285: 40699–40705, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardy M, Rockenbauer A, Vasquez-Vivar J, Felix C, Lopez M, Srinivasan S, Avadhani N, Tordo P, and Kalyanaraman B. Detection, characterization, and decay kinetics of ROS and thiyl adducts of mito-DEPMPO spin trap. Chem Res Toxicol 20: 1053–1060, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hempel SL, Buettner GR, O'Malley YQ, Wessels DA, and Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med 27: 146–159, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Jiang J, Liu KJ, Shi X, and Swartz HM. Detection of short-lived free radicals by low-frequency electron paramagnetic resonance spin trapping in whole living animals. Arch Biochem Biophys 319: 570–573, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Jourd'heuil D, Jourd'heuil FL, Kutchukian PS, Musah RA, Wink DA, and Grisham MB. Reaction of superoxide and nitric oxide with peroxynitrite. Implications for peroxynitrite-mediated oxidation reactions in vivo. J Biol Chem 276: 28799–28805, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Kalyanaraman B. Oxidative chemistry of fluorescent dyes: implications in the detection of reactive oxygen and nitrogen species. Biochem Soc Trans 39: 1221–1225, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd, and Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keszler A, Kalyanaraman B, and Hogg N. Comparative investigation of superoxide trapping by cyclic nitrone spin traps: the use of singular value decomposition and multiple linear regression analysis. Free Radic Biol Med 35: 1149–1157, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Khan N, Wilmot CM, Rosen GM, Demidenko E, Sun J, Joseph J, O'Hara J, Kalyanaraman B, and Swartz HM. Spin traps: in vitro toxicity and stability of radical adducts. Free Radic Biol Med 34: 1473–1481, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Kozlov AV, Szalay L, Umar F, Fink B, Kropik K, Nohl H, Redl H, and Bahrami S. Epr analysis reveals three tissues responding to endotoxin by increased formation of reactive oxygen and nitrogen species. Free Radic Biol Med 34: 1555–1562, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Kurtoglu M. and Lampidis TJ. From delocalized lipophilic cations to hypoxia: blocking tumor cell mitochondrial function leads to therapeutic gain with glycolytic inhibitors. Mol Nutr Food Res 53: 68–75, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuzkaya N, Weissmann N, Harrison DG, and Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol 70: 343–354, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Malinouski M, Zhou Y, Belousov VV, Hatfield DL, and Gladyshev VN. Hydrogen peroxide probes directed to different cellular compartments. PLoS one 6: e14564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchesi E, Rota C, Fann YC, Chignell CF, and Mason RP. Photoreduction of the fluorescent dye 2′-7′-dichlorofluorescein: a spin trapping and direct electron spin resonance study with implications for oxidative stress measurements. Free Radic Biol Med 26: 148–161, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Mason RP. Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping. Free Radic Biol Med 36: 1214–1223, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Miller EW, Albers AE, Pralle A, Isacoff EY, and Chang CJ. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J Am Chem Soc 127: 16652–16659, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan B, Sobotta MC, and Dick TP. Measuring EGSH and H2O2 with roGFP2-based redox probes. Free Radic Biol Med 51: 1943–1951, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, and Greenamyre JT. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem 280: 42026–42035, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Ramirez DC, Chen YR, and Mason RP. Immunochemical detection of hemoglobin-derived radicals formed by reaction with hydrogen peroxide: involvement of a protein-tyrosyl radical. Free Radic Biol Med 34: 830–839, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, and Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A 103: 15038–15043, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sano T, Umeda F, Hashimoto T, Nawata H, and Utsumi H. Oxidative stress measurement by in vivo electron spin resonance spectroscopy in rats with streptozotocin-induced diabetes. Diabetologia 41: 1355–1360, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Sikora A, Zielonka J, Lopez M, Dybala-Defratyka A, Joseph J, Marcinek A, and Kalyanaraman B. Reaction between peroxynitrite and boronates: EPR spin-trapping, HPLC Analyses, and quantum mechanical study of the free radical pathway. Chem Res Toxicol 24: 687–697, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smirnov AI, Norby SW, Clarkson RB, Walczak T, and Swartz HM. Simultaneous multi-site EPR spectroscopy in vivo. Magn Reson Med 30: 213–220, 1993 [DOI] [PubMed] [Google Scholar]
- 49.Tosaki A, Blasig IE, Pali T, and Ebert B. Heart protection and radical trapping by DMPO during reperfusion in isolated working rat hearts. Free Radic Biol Med 8: 363–372, 1990 [DOI] [PubMed] [Google Scholar]
- 50.Vallyathan V, Leonard S, Kuppusamy P, Pack D, Chzhan M, Sanders SP, and Zweir JL. Oxidative stress in silicosis: evidence for the enhanced clearance of free radicals from whole lungs. Mol Cell Biochem 168: 125–132, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Vasquez-Vivar J, Hogg N, Pritchard KA, Jr, Martasek P, and Kalyanaraman B. Superoxide anion formation from lucigenin: an electron spin resonance spin-trapping study. FEBS Lett 403: 127–130, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Wardman P. Methods to measure the reactivity of peroxynitrite-derived oxidants toward reduced fluoresceins and rhodamines. Methods Enzymol 441: 261–282, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Wyche KE, Wang SS, Griendling KK, Dikalov SI, Austin H, Rao S, Fink B, Harrison DG, and Zafari AM. C242T CYBA polymorphism of the NADPH oxidase is associated with reduced respiratory burst in human neutrophils. Hypertension 43: 1246–1251, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Zhao B, Ranguelova K, Jiang J, and Mason RP. Studies on the photosensitized reduction of resorufin and implications for the detection of oxidative stress with Amplex Red. Free Radic Biol Med 51: 153–159, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, and Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A 102: 5727–5732, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, and Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34: 1359–1368, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Zhou M, Diwu Z, Panchuk-Voloshina N, and Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem 253: 162–168, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Zielonka J. and Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med 48: 983–1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zielonka J, Sikora A, Joseph J, and Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: direct reaction with boronate-based fluorescent probe. J Biol Chem 285: 14210–14216, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez M, Vasquez-Vivar J, Avadhani NG, and Kalyanaraman B. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic Biol Med 44: 835–846, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zielonka J, Vasquez-Vivar J, and Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc 3: 8–21, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Zielonka J, Zhao H, Xu Y, and Kalyanaraman B. Mechanistic similarities between oxidation of hydroethidine by Fremy's salt and superoxide: stopped-flow optical and EPR studies. Free Radic Biol Med 39: 853–863, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Zielonka J, Zielonka M, Sikora A, Adamus J, Joseph J, Hardy M, Ouari O, Dranka BP, and Kalyanaraman B. Global profiling of reactive oxygen and nitrogen species in biological systems: high-throughput real-time analyses. J Biol Chem 287: 2984–2995, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuo L, Chen YR, Reyes LA, Lee HL, Chen CL, Villamena FA, and Zweier JL. The radical trap 5,5-dimethyl-1-pyrroline N-oxide exerts dose-dependent protection against myocardial ischemia-reperfusion injury through preservation of mitochondrial electron transport. J Pharmacol Exp Ther 329: 515–523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem 263: 1353–1357, 1988 [PubMed] [Google Scholar]
- 66.Zwicker K, Dikalov S, Matuschka S, Mainka L, Hofmann M, Khramtsov V, and Zimmer G. Oxygen radical generation and enzymatic properties of mitochondria in hypoxia/reoxygenation. Arzneimittelforschung 48: 629–636, 1998 [PubMed] [Google Scholar]