Abstract
Background: Sporotrichosis is a fungal infection caused by Sporothrix schenckii complex, usually restricted to the skin, subcutaneous cellular tissue, and adjacent lymphatic vessels. Antimicrobial photodynamic therapy (aPDT) could be a good alternative to manage localized, superficial infections. Case report: A 65-year-old African woman was diagnosed with a fixed cutaneous sporotrichosis on her left arm, treated with itraconazol and oral terbinafine with partial improvement. Topical 16% methyl aminolevulinate (MAL, Metvix®)-PDT was used without success. Methods: An in vitro photoinactivation test with the isolated microorganism revealed phenothiazinium salts to be more effective than MAL. Conclusions: PDT with intralesional 1% methylene blue (MB) in combination with intermittent low doses of itraconazole obtained complete microbiological and clinical response.
Introduction
Sporotrichosis is a fungal infection caused by the dimorphic organism Sporothrix schenkii complex. The disease has several clinical manifestations, but is primarily cutaneous with associated lymphadenopathy. Itraconazole (100–200 mg) by day is the treatment of choice in most cases. Potassium iodide and amphotericin B are also effective; however, their adverse effects often weigh against its use.1 Photodynamic therapy (PDT) involves the combination of harmless visible light combined with a photosensitizer (PS) in the presence of oxygen, which generates reactive oxygen species (ROS), which have a cytotoxic effect.2 As some PSs bind rapidly and selectively to microbial cells, PDT has been proposed as an alternative approach for localized infections. PDT may be an effective treatment option for patients with recalcitrant fungal infections3 and subcutaneous mycoses.4 However, the available in vivo data, mostly obtained with aminolevulinic acid (ALA) and its derivate methyl aminolevulinate (MAL),5 and less often with methylene blue (MB),6 are not sufficient to support its routine clinical use. In the case of sporotrichosis, there are no published studies using PDT either in vitro or in vivo.
Here, we report on a patient with recalcitrant cutaneous sporotrichosis successfully treated with PDT combined with low and intermittent doses of itraconazole, based on in vitro photodynamic inactivation studies.
Case Report
A 65-year-old black woman from Guinea (Africa), with a personal history of chronic hepatitis C and fixed cutaneous sporotrichosis on her left arm, presented with five very painful, ulcerated, indurated nodules ∼2–4 cm in diameter. She had received different treatments, such as itraconazole 200 mg/day for 10 weeks, which had to be terminated because of significant increase of her liver enzymes, and also terbinafine daily for 3 months, with partial improvement.
Considering her underlying liver disease, the localized form of her sporotrichosis, and our good experience using MAL-PDT to treat cutaneous and nail mycoses,7,8 we used PDT and added oral itraconazole intermittently, depending upon her liver enzyme levels. After informed consent, we firstly used MAL 16% cream (Metvix, Galderma, La Défense, Cedex, France) applying it on the lesions and 1 cm around, under occlusion and protected from the light for 3 h (Tegaderm, 3M Healthcare, St. Paul, MN). After that, the lesions were illuminated using a 635 nm light-emitting diode (LED) lamp (Aktilite®, Photocure ASA, Oslo, Norway, 37 J/cm2 to each lesion). Treatment was administered for three sessions, one every 2 weeks, but the intense pain associated with the treatment (score 8 on a visual analogue scale from 0 to 10), together with the lack of significant clinical improvement, led us to explore an alternative approach.
In order to explain the lack of efficacy of MAL-PDT in this patient, we studied the in vitro photosensitivity of the S. schenkii fungal cells isolated from the patient.
Materials and Methods
Photosensitizers and chemicals
MAL was purchased from Sigma Aldrich® (St Louis, MO). MB, new methylene blue (NMB) and 1,9-dimethylmethylene blue (DMMB) were purchased from Aldrich® (Gillingham, UK). Culture media were Sabouraud dextrose agar (SB) CM0041 and Columbia blood agar (Oxoid LTD, Hampshire, England). Cloramphenicol was added to the culture as antibacterial agent (Sigma Aldrich, St Louis, MO). Solvents and reagents were ethanol (Alcohocel®, Barcelona, Spain), distilled water, and physiological serum (Fresenius Kabi®, Spain).
Light sources
Fungus was irradiated using an LED-based lamp emitting at 639.8±10 nm with an irradiance of 19.0 mW/cm2.
Microorganisms and growth conditions
The mould-to-yeast form conversion was obtained by transferring the fungus to blood agar and incubating at 35°C in 5–10% CO2. Stock inoculum suspensions of the yeast form were prepared in distilled water. Cell viability was assessed by counting the number of colony-forming units (CFUs) after an incubation period of 1 week at 30°C on SB with chloramphenicol 50 mg/L. The sample optical density McFarland values were adjusted to 0.5.
In vitro photodynamic inactivation of yeasts
Starting from a 7-day-old yeast culture, suspensions of the 0.5 McFarland value were prepared in double-distilled water. Ninety microliters of these suspensions were dispensed into wells of a microtiter plate, and different concentrations of MAL or any of the three phenothiazinium dyes were added. The suspensions were then maintained at 35°C in the dark for 3 h for MAL and 15 min for others. Afterwards, fugal cells were subjected to LED illumination at room temperature using a fluence of 37 J/cm2 (30 min, 5 cm distance). Fungal cultures grown under the same conditions with PS in the dark, or illuminated without PS, served as controls. After the treatments, samples and controls were incubated on SB plates at 35°C for 1 week, and the antifungal effect was determined by calculating the number of CFU per mL in samples and controls.
Results
Under the experimental conditions described, PDT with MAL did not inhibit the growth of the fungus, even using high concentrations (from 0 to 6 M) (Fig. 1). However, all the phenothiazinium dyes (MB, NMB, or DMMB) obtained a 6 log10 fungicidal effect with 1, 1.25, and 1.5 μM of NMB, MB, and DMMB, respectively (Fig. 1).
FIG. 1.
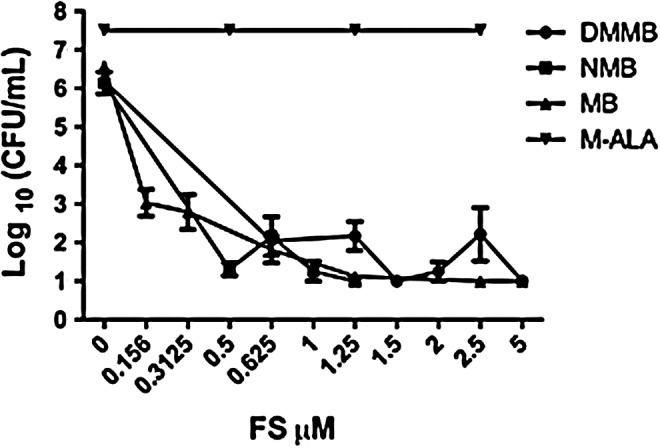
In vitro photosensitivity study of the Sporothrix schenkii fungal cells isolated from the patient after incubation with several concentrations of methyl aminolevulinate (MAL), methylene blue (MB), new methylene blue (NMB), and 1,9-dimethylmethylene blue (DMMB) and irradiating with a light-emitting diode (LED)-based lamp emitting at 639.8±10 nm with an irradiance of 19.0 mW/cm2 using a fluence of 37 J/cm2.
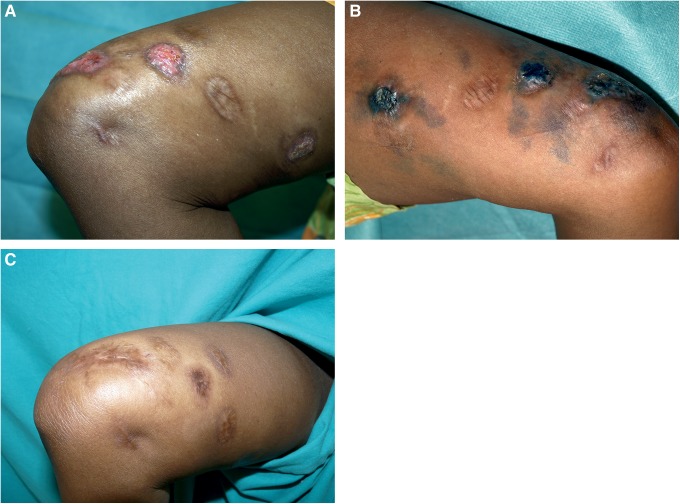
Based on these in vitro results, we decided to try PDT using MB, because out of the three phenothiazinium salts tested, it was the one available in pharmaceutical grade. The clinical protocol used was based on that proposed by Tardivo et al.9: 1% MB solution (Carreras, Barcelona, Spain) was intralesionally injected until the lesion became dark blue (Fig. 2A and B). Twenty minutes after injection, each lesion was irradiated with a fluence of 37 J/cm2 using the Aktilite lamp. Irradiation was painful, but less than with MAL-PDT (score 4). The treatment was repeated every 2 weeks for 3 months. Microbiological cultures were performed after every PDT session, and one histological study was made after the third one. No fungal structures were observed in the cutaneous tissue, and in the cultures only 1 CFU grew after the second PDT session, significantly less than the abundant growth obtained before PDT was started. The small lesion showed complete clinical response after three sessions of MB-PDT, whereas the two larger ones required two additional sessions to ensure complete clinical and microbiological response (Fig. 2C).
FIG. 2.
(A) Ulcerated nodules on the arm after being treated with three sessions of methyl aminolevulinate photodynamic therapy (MAL-PDT). (B) Intralesional injection of 0.1% methylene blue (MB) solution in the nodules until they became dark blue. (C) After four sessions of MB-PDT and intermittent low doses of itraconazole (100 mg/day).
Discussion
The present study has demonstrated for the first time that PDT could be useful in treating local cutaneous sporotrichosis. Additionally, an innovative aspect of this clinical case is the addition of the in vitro photodynamic inactivation test with the isolated microorganism in order, first, to explain the MAL-PDT failure, and, second, to establish fungal susceptibility to an alternative PS.
To our knowledge, the fungicidal effect of PDT against S. schenkii complex has not been previously reported. MAL-PDT has been shown to be effective in different superficial mycoses: dermatophytes,10, yeasts, and non-dermatophyte molds.8 Based on these clinical reports, we chose this photosensitizer as the first option to treat our patient. However, from the in vitro study, we found, at least for this particular Sporothrix strain, that it was more photosensitive to phenothiazinium salts than MAL-Protoporphyrin IX (PpIX). Additionally, the higher clinical efficacy of MB-PDT compared with MAL-PDT in this case could be the result of the method of administration: intralesional for MB versus topical for MAL.
S. schenckii complex is a thermally dimorphic fungus that produces melanoid pigments, such as melanin and pyomelanin, that are considered to be important virulence factors.11 Optical interference by the highly absorbing melanoid pigments together with the antioxidant effect of melanin could limit the efficacy of PDT for this fungus. Light absorption interference with melanin would be expected to be lower for MB (absorption peak 654 nm) than for PpIX (low peak in the 630 nm). Furthermore, MB has a much higher molar absorption coefficient in the red spectrum than PpIX, and would give higher ROS production for the same fluence. It could be possible that the melanin content of Sporothrix spp. could scavenge the ROS produced by MAL-PDT but was insufficient to scavenge higher amount of ROS produced during MB-PDT.
MB-PDT has been proven to be effective against chromoblastomycosis, another subcutaneous mycosis resistant to conventional antifungal drugs, caused by dematiaceous fungal species. It was highly effective in killing the samples of Fonsecaea pedrosoi and Cladophialophora carrionii tested in vitro, and also in treating clinical lesions.12 On the other hand, Yang et al.13 used ALA-PDT to treat a patient with chromoblastomycosis caused by Fonsecaea monophora, obtaining partial improvement.
Sporotrichosis has been also successfully treated using local hyperthermia.1 In the case of PDT, the efficacy cannot be attributed to hyperthermia, because the temperature was controlled during the in vitro studies, and the lamp used for the in vivo studies, Aktilite, was fitted with a fan to maintain the temperature of the treated area.
The use of PDT as an adjuvant treatment combined with conventional antimicrobial therapy can lead to an overall improvement in the efficacy of the antifungal chemotherapy, as well as in reduction in the duration of antifungal administration, and to lower toxicity to the patient.4 It has been shown that the efficacy and safety of either an itraconazole pulse regimen for cutaneous sporotrichosis (200 mg b.i.d. for 1 week and off for 3 weeks, for 48 weeks) or the continuous regimen (100 mg b.i.d. for 48 weeks).14 However, we have to keep in mind that prolonged exposure to itraconazole, administered either continuously or intermittently, may precipitate severe and irreversible hepatotoxic events, either in asymptomatic patients or, especially, in those with risk factors, such as our patient with hepatitis C.15 In the present case, the results of the cumulative effect of itraconazole would be related to the possibility of clinical response, which would have to be considered. Even though itraconazole had to be stopped because of liver toxicity, it had been taken for several weeks at high doses without response. On the other hand, itraconazole-resistant strains of S. schenckii complex have been previously reported.16
One of the advantages of antimicrobial photodynamic therapy (aPDT) is its broad spectrum activity and the nonspecific action, which allows it to be effective reagardless of the etiologic agent.3 However, some PSs are more effective in photoinactivating some microorganisms than others. Our case supports the usefulness of in vitro photosensitivity testing in order to choose the most effective PS for every patient, in the same way that antibiograms select the most effective antibiotic for each bacterial isolate.
Acknowledgments
This study was supported by the Research Groups B65 and B85 from the Department of Science, Technology and University of the Government of Aragón, Spain. Dr. Hamblin was supported by United States National Institutes of Health (NIH) grant R01AI 050875. We thank Galderma for lending us the Aktilite lamp used in the clinical part of the study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kauffman C.A., Hajjeh R., and Chapman S.W. (2000). Practice guidelines for the management of patients with sporotrichosis. For the Mycoses Study Group. Infectious Diseases Society of America. Clin. Infect. Dis. 30, 684–687 [DOI] [PubMed] [Google Scholar]
- 2.Henderson B.W., and Dougherty T.J. (1992). How does photodynamic therapy work? Photochem. Photobiol. 55, 145–157 [DOI] [PubMed] [Google Scholar]
- 3.Dai T., Fuchs B.B., Coleman J.J., Prates R.A., Astrakas C., St. Denis T.G., et al. (2012). Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front. Microbiol. 3, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyon J.P., Pedroso e Silva Azevedo Cde M., Moreira L.M., de Lima C.J., and de Resende M.A. (2011). Photodynamic antifungal therapy against chromoblastomycosis. Mycopathologia 172, 293–297 [DOI] [PubMed] [Google Scholar]
- 5.Qiao J., Li R., Ding Y., and Fang H. (2010). Photodynamic therapy in the treatment of superficial mycoses: an evidence-based evaluation. Mycopathologia 170, 339–343 [DOI] [PubMed] [Google Scholar]
- 6.Scwingel A.R., Barcessat A.R., Nunez S.C., and Ribeiro M.S. (2012). Antimicrobial photodynamic therapy in the treatment of oral candidiasis in HIV-infected patients. Photomed. Laser Surg. 30, 429–432 [DOI] [PubMed] [Google Scholar]
- 7.Aspiroz C., Gilaberte Y., Paz–Cristobal P., and Rezusta A. (2011). Distal onycholysis resolved with photodynamic therapy in an elderly patient on multiple medication. Enferm. Infecc. Microbiol. Clin. 29, 626–628 [DOI] [PubMed] [Google Scholar]
- 8.Gilaberte Y., Aspiroz C., Martes M.P., Alcalde V., Espinel–Ingroff A., and Rezusta A. (2011). Treatment of refractory fingernail onychomycosis caused by nondermatophyte molds with methylaminolevulinate photodynamic therapy. J. Am. Acad. Dermatol. 65, 669–671 [DOI] [PubMed] [Google Scholar]
- 9.Tardivo J.P., Del Giglio A., Paschoal L.H., and Baptista M.S. (2006). New photodynamic therapy protocol to treat AIDS-related Kaposi's sarcoma. Photomed. Laser Surg. 24, 528–531 [DOI] [PubMed] [Google Scholar]
- 10.Calzavara–Pinton P.G., Venturini M., Capezzera R., Sala R., and Zane C. (2004). Photodynamic therapy of interdigital mycoses of the feet with topical application of 5-aminolevulinic acid. Photodermatol. Photoimmunol. Photomed. 20, 144–147 [DOI] [PubMed] [Google Scholar]
- 11.Almeida–Paes R., Frases S., Araujo Gde S., de Oliveira M.M., Gerfen G.J., Nosanchuk J.D., et al. (2012). Biosynthesis and functions of a melanoid pigment produced by species of the sporothrix complex in the presence of L-tyrosine. Appl. Environ. Microbiol. 78, 8623–8630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyon J.P., Moreira L.M., de Carvalho V.S., dos Santos F.V., de Lima C.J., and de Resende M.A. (2013). In vitro photodynamic therapy against Foncecaea pedrosoi and Cladophialophora carrionii. Mycoses 56,157–161 [DOI] [PubMed] [Google Scholar]
- 13.Yang Y., Hu Y., Zhang J., Li X., Lu C., Liang Y., et al. (2012). A refractory case of chromoblastomycosis due to Fonsecaea monophora with improvement by photodynamic therapy. Med. Mycol. 50, 649–653 [DOI] [PubMed] [Google Scholar]
- 14.Song Y., Li S.S., Zhong S.X., Liu Y.Y., Yao L., and Huo S.S. (2011). Report of 457 sporotrichosis cases from Jilin province, northeast China, a serious endemic region. J. Eur. Acad. Dermatol. Venereol. 10.1111/j.1468-3083.2011.04389[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Tuccori M., Bresci F., Guidi B., Blandizzi C., Del Tacca M., and Di Paolo M. (2008). Fatal hepatitis after long-term pulse itraconazole treatment for onychomycosis. Ann. Pharmacother. 42, 1112–1117 [DOI] [PubMed] [Google Scholar]
- 16.Oliveira D.C., Lopes P.G., Spader T.B., Mahl C.D., Tronco–Alves G.R., Lara V.M., et al. (2011). Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J. Clin. Microbiol. 49, 3047–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]