Abstract
Background: This study compared infusion set function for up to 1 week using either a Teflon® (Dupont™, Wilmington, DE) catheter or a steel catheter for insulin pump therapy in type 1 diabetes mellitus.
Subjects and Methods: Twenty subjects participating in a randomized, open-labeled, crossover study were asked to wear two Quick-Set® and two Sure-T® infusion sets (both from Medtronic Minimed, Northridge, CA) until the infusion set failed or was worn for 1 week. All subjects wore a MiniMed continuous glucose monitoring system for the duration of the study.
Results: One subject withdrew from the study. There were 38 weeks of Sure-T wear and 39 weeks of Quick-Set wear with no difference in the survival curves of the infusion sets. There was, however, a 15% initial failure rate with the Teflon infusion set. After 7 days, both types of infusion sets had a 64% failure rate. Overall, 30% failed because of hyperglycemia and a failed correction dose, 13% were removed for pain, 10% were pulled out by accident, 10% had erythema and/or induration of>10 mm, 5% fell out because of loss of adhesion, and 4% were removed for infection. The main predictor of length of wear was the individual subject. There was no increase in hyperglycemia or daily insulin requirements when an infusion set was successfully used for 7 days (n=25 of 77 weeks).
Conclusions: We found no difference between steel and Teflon infusion sets in their function over 7 days, although 15% of Teflon sets failed because of kinking on insertion. The strongest predictor of prolonged 7-day infusion set function was the individual subject, not the type of infusion set.
Introduction
There has been a progressive increase in the number of patients using continuous subcutaneous insulin infusion (CSII) pump therapy since its introduction over 30 years ago. Meta-analyses of CSII therapy compared with multiple daily injections therapy have shown a modest improvement in glycemic control and a lower incidence of hypoglycemia with CSII therapy.1,2 Major disadvantages of pump use include infusion set failure, which can lead to hyperglycemia and diabetic ketoacidosis, and local tissue reactions, scarring, and infections at the infusion sites. The initial guidelines to change an infusion set every 2–3 days were published in 1983 and were based on anecdotal case reports.3 Since then, there has been one published randomized trial assessing length of infusion set wear beyond 3 days.4 This study compared using insulin lispro to insulin aspart for 5 days of infusion set wear. There was no difference between the two insulins, with an average length of infusion set wear of 98 and 96 h, respectively. The researchers reported increased glucose levels after 48 h of wearing an infusion set and recommended changing infusion sets every 2 days.
With the advent of real-time continuous glucose monitoring, there are many groups working on combining CSII with real-time continuous glucose monitoring to achieve partial or full closed-loop control of insulin delivery. Current systems require that a patient wear an infusion set linked to a pump and a continuous glucose sensor inserted at a second site. Wearing multiple devices at separate sites is a deterrent to wide-scale adoption of a closed-loop insulin delivery system. The adhesives associated with both devices can cause tape allergies and denude the superficial layer of the skin when the adhesive is removed. Finding multiple, comfortable sites on which to attach the devices can be challenging, particularly for children. Combining the insulin set with the glucose sensor on a single platform would reduce the number of sites and insertions. Several continuous glucose sensors are approved for 5–7 days of wear, whereas current guidelines for infusion set use is to replace them every 2–3 days. It is therefore of interest to determine if there are different types of infusion sets that may have longer lengths of wear. Although many of our patients feel they are able to wear steel infusion sets longer than Teflon™ (Dupont™, Wilmington, DE) infusion sets, there has not been a systematic study comparing the length of wear using steel compared with Teflon infusion sets. Our primary objective was therefore to compare the length of infusion set wear using a Teflon catheter (Quick-Set®) or a steel needle catheter (Sure-T®) (both from Medtronic Minimed, Northridge, CA) in people with type 1 diabetes.
Research Design and Methods
This was a randomized, open-labeled, single-site crossover study. Subjects were eligible to participate if they were 12–45 years old, had had the clinical diagnosis of type 1 diabetes for at least 1 year, had been using a Medtronic insulin pump for at least 3 months, and had a hemoglobin A1c level of <10%. Females could not be enrolled if they had a positive pregnancy test at the time of enrollment. After signing an informed consent approved by the institutional review board, subjects were randomized to initially wear a steel 6-mm infusion set (Sure-T) or a Teflon 6-mm infusion set (Quick-Set). Both infusion sets are inserted at a 90° angle. All infusion sets were inserted by study staff in non-lipohypertrophied areas. The following week subjects were crossed over to the other infusion set, and this was repeated for a total of 4 weeks, so that they wore each infusion set twice.
All participants were instructed on the use of the Medtronic real-time continuous glucose monitoring system at the time of their enrollment visit. All data from the Medtronic pump were downloaded into CareLink Pro® software (Medtronic) at each clinic visit. They were also provided with a Nova Max® Plus (Nova Diabetes Care, Billerica, MA) glucose meter, a combination blood glucose meter and blood ketone meter. They were instructed to wear each infusion set for 1 week or until set failure. Set failure was determined by (1) the meter blood glucose level not decreasing by at least 50 mg/dL an hour after a correction bolus for a blood glucose level greater than 300 mg/dL, (2) blood ketone levels greater than 0.6 mmol/L, or (3) evidence of infection at the infusion site. In reviewing the data, there were eight events where the glucose level was greater than 250 mg/dL for which the subject gave a correction bolus but 1 h later the glucose level had not decreased by 50 mg/dL. When the glucose level did not improve, the patients changed their infusion sets. Subjects stated that they were uncomfortable waiting for the glucose level to go above 300 mg/dL before giving a correction dose. In the data analysis we considered these eight subjects to have failed correction doses because of an infusion site failure.
The study was powered based on an earlier unpublished study involving 10 subjects who were asked to wear two infusion sets for 7 days. The mean duration of wear was 5.1 days with an SD of 2.0 days. We considered a 1 day difference in infusion set wear to be clinically significant, and using a paired t test with a power of 0.8 and P of 0.05 we would require a sample size of 34 weeks. We therefore planned on 40 weeks of wear for each infusion set to account for a 15% dropout rate. In our previous study we had detected a propensity for some subjects to be able to wear infusion sets longer than other subjects. In our study design we therefore asked each subject to wear each infusion set twice, so there would be 80 insertions to evaluate. We used Kaplan–Meier plots with log rank statistics to assess the significance of the type of infusion set on infusion set survival. We used two-way analysis of variance to assess the relative importance of the infusion set compared with the individual subject on infusion set survival.
Results
Twenty participants were enrolled in this study; 19 completed all scheduled visits. Participants ranged in age from 12 to 41 years old (mean, 22 years old), had a mean duration of diabetes of 11 years (range, 1–26 years), and had a mean hemoglobin A1c level of 7.8% (range, 6.5–9.6%). The mean body mass index (BMI) was 23.5±5.6 kg/m2 (range, 15.8–33.1 kg/m2). One subject withdrew after 1 week. There were data for 38 weeks of Sure-T wear (38 insertions) and 39 weeks of Quick-Set wear (39 insertions).
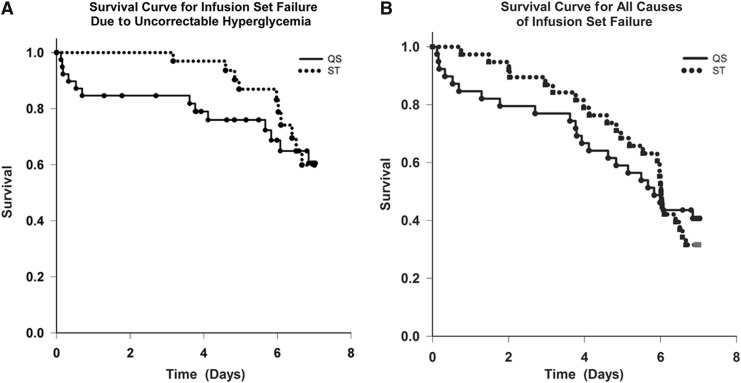
Eighty-seven percent of the steel sets and 77% of the Teflon sets were functioning after 3 days (this number includes the 15% that failed on the first day because of kinking), after 5 days 68% of the steel and 59% of the Teflon sets were functioning, after 6 days 53% of the steel and 46% of the Teflon sets were functioning, and at the end of 7 days 32% of the steel and 33% of the Teflon sets were functioning. Using Kaplan–Meier survival curve analysis (Fig. 1), there was no overall difference in the failure rate of the steel versus Teflon infusion sets. There was a 15% failure rate on initial insertion of the Teflon catheter because of kinking that was not recognized by the investigator or subject until there was hyperglycemia and/or high pressure alarms hours later. There was no significant difference in the BMI between those who experienced kinking and those who did not. The mean BMI of the four subjects with kinking was 24.2 kg/m2 (range, 18.4–30.0 kg/m2), and the mean BMI for those who did not have kinking was 25.8 kg/m2 (range, 15.8–33.1 kg/m2). Overall, 30% failed with each type of infusion set owing to hyperglycemia and a failed correction dose, 13% were removed because of pain at the infusion site, 13% were accidentally pulled out, 6% “fell out” because of adhesive tape issues (generally with bathing), 4% were removed because of infection, and in 18% there was more than 10 mm of erythema and/or induration at the infusion site when it was removed. Infusion site infection was given as the reason for removing the infusion set three times; all occurred in a single subject after 3.8–5.5 days of infusion set wear, and in one instance oral antibiotics were prescribed. There could be overlap of infusion site issues with subjects having several findings at the same time. For example, there were 14 instances in which erythema and/or induration of greater than 10 mm was recorded: four were associated with hyperglycemia and a failure to correct; five were associated with pain, itching, or infection at the site; one instance occurred when the infusion set was accidently pulled out; and on four occasions the erythema and induration were noted at the time the set was removed after 7 days of successful wear. In all causes of infusion set failure, there was no difference between the steel and Teflon infusion sets. The order of infusion set wear had no effect on the study outcome.
FIG. 1.
Survival curves for infusion sets: (A) infusion set failure due to uncorrectable hyperglycemia (when the end point was hyperglycemia [>250 mg/dL] and the meter blood glucose level did not decrease by at least 50 mg/dL an hour after a correction bolus and/or blood ketone levels were greater than 0.6 mmol/L) and (B) for all causes of infusion set failure (uncorrectable hyperglycemia with or without ketonemia, pain, infusion set fell out [loss of adhesion], pulled out accidentally, erythema and induration, and infection). The solid line is the Teflon catheter (Quick-Set [QS]), and the dotted line is the steel needle catheter (Sure-T [ST]).
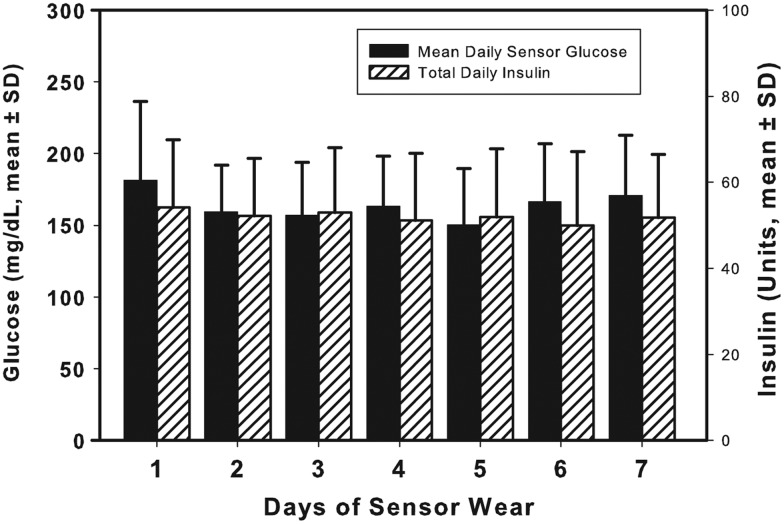
The strongest determinant of infusion set survival was the individual subject. The mean and SD of days of infusion set wear per individual is given in Figure 2. The individuals were ordered based on their mean days of set wear for the purposes of this graph. Twenty-one percent had a mean length of infusion set wear of <4 days, and 42% had a mean length of infusion set wear of >6 days. Overall, the median duration of infusion set wear was 6.06 days (25th–75th percentiles, 4.35–6.98 days), with 25 of the 77 insertions lasting the full 7 days (32%). Of interest is that there was no deterioration in glucose levels or a need for increased insulin requirements over those 7 days (Fig. 3). Four of the 19 subjects were able to wear each set 7 days, and 12 of the 19 subjects (63%) wore an infusion set for more than 6 days 50% of the time. When comparing the effect of the type of infusion (steel vs. Teflon) and the effect of the individual subject on infusion set wear, it was the individual (P=0.002) and not the type of infusion set (P=not significant) that was most significant in determining infusion set survival (two-way analysis of variance).
FIG. 2.
Length of wear in days for all infusions sets worn per subject. Teflon (Quick-Set) sets that failed shortly after insertion because of kinking were excluded. Data are mean±SD values.
FIG. 3.
Sensor glucose and total daily insulin doses for each of the 7 days of infusion set wear when either a Teflon (n=13) or steel (n=12) infusion set was worn for 7 days. Data are mean±SD values. P<0.001 refers to the linear correlation between mean length of wear with the ordered subjects.
Discussion
Our initial hypothesis was that steel infusion sets would perform better than Teflon. Højbjerre et al.5 demonstrated that the mean perfusion pressure increased significantly with wear time of 48 h in Teflon but not steel catheters. A higher maximal pressure was required to deliver a bolus infusion through Teflon than through steel catheters. The researchers also observed a significant increase in adipose tissue blood flow with Teflon but not with steel infusion sets. They hypothesized that steel was more biocompatible than Teflon. Although these studies were only for 48 h, they suggested that steel catheters would have an advantage over Teflon. On the other hand, in a survey of 90 pump users, Johansson et al.6 reported that Teflon infusion sets were changed on a mean of 4.8 days, whereas steel infusion sets were changed on a mean of 3.8 days (P=0.001). In our trial subjects were asked to wear each infusion set for up to 7 days, and there was no significant difference in the overall length of wear between the steel or Teflon infusion sets. Of note is that Teflon infusion sets had an early 15% failure rate because of kinking on insertion. This failure rate is comparable to the 9% rate of initial insertion set failures for another vertically inserted Teflon catheter.7 It is possible the kinking was due to striking muscle fascia. Ultrasound measurements have determined that 6% of all 6-mm 90° insertions will be intramuscular in adults8; however, these measurements also included arms and thighs, which on average had 2–6 mm less subcutaneous tissue compared with the abdomen or buttocks. In a pediatric study9 the mean±SD thickness of skin and subcutaneous tissue measured by ultrasound over the arm was 6.3±1.9 mm, over the thigh was 7.5±2.1, over the abdomen was 8.0±3.4 mm, and over the buttocks was 8.1±2.8 mm in children 14–17 years old. Using measurements inclusive of all sites, the researchers estimated that 35% of injections using a 6-mm needle would be intramuscular in 14–17 year olds and that 38% of injections would be intramuscular in 7–13 year olds. We were concerned that an intramuscular insertion might occur and result in kinking, so all of our insertions were by research staff, and all insertions were in the abdomen or buttocks in areas where the researcher determined there was adequate subcutaneous tissue. There was no difference in BMI between those with and without kinking.
One of the common reasons for discontinuing pump therapy in adults and children is inflammation at the infusion sites. Mecklenburg et al.10 reported that 53% of adult patients discontinuing CSII therapy gave inflammation at the infusion site as their reason for quitting; infected infusion sites occurred in 29% of patients with a frequency of one infection every 27 patient-months, and there was a subgroup of patients who experienced repeated episodes of infection.11 In the pediatric age group, Wood et al.12 reported that 21% of the children discontinuing CSII therapy cited infusion site issues as their reason for quitting. In studies assessing dermatologic complications of CSII therapy in children (predominately using Teflon catheters), scars >3 mm occurred in 12–37% of patients, subcutaneous erythematous nodules in 21–42%, erythema without nodules in 25–66%, and abscesses in 8–12% (abscesses were counted from onset of CSII therapy).13,14 In the present study there was a 14% incidence of erythema and induration of >10 mm when sets were worn up to 7 days, all occurring after 3 days of set wear, and there were three site infections, all occurring in the same patient.
Another common problem with CSII therapy is the development of unexplained hyperglycemia that resolves with changing the infusion set. In a multicenter, 13-week study with 256 subjects wearing Teflon infusion sets for a mean duration of 71 h, unexpected hyperglycemia and/or infusion set occlusion occurred in 61% of subjects using insulin lispro, 62% of those using insulin aspart, and 68% of subjects using insulin glulisine.15 In a double-blind crossover study comparing insulin lispro with insulin aspart, subjects were asked to wear the infusion set for 5 days while wearing a continuous glucose monitoring device. The authors described an increase in average glucose levels from 123 to 164 mg/dL (P<0.05) from Day 2 to 5 with a concomitant increase in total daily insulin dose from 49±12 to 55±18 U (P=0.05).4 In another study of 12 subjects asked to wear a Teflon catheter for up to 5 days, the authors reported that the mean daily glucose levels increased from a mean of 135±68 mg/dL on Day 1 to 162±72 mg/dL on Day 5 (P=difference not significant).16 We did not see this increase in glucose levels or insulin requirements during the 25 weeks that subjects wore an infusion set for 7 days. Our interpretation of the difference between their study results and ours is that some of their subjects were experiencing infusion site failure, whereas in our study these subjects were excluded when we analyzed those who wore an infusion set successfully for 7 days. This indicates that the length of wear of an infusion set needs to be individualized because 42% of our subjects could consistently wear an infusion set for 6–7 days without a deterioration of their glucose levels or a need for increased insulin. The underlying reason for this individual variability in infusion set length of function is currently not understood. There is a liquid interface between the interstitial fluid and the insulin being intermittently pumped through an infusion set. It will be important to determine if there are macrophages and an inflammatory response occurring within and around the infusion set catheters and to determine if there is insulin precipitation within the tubing in the sets with early failure. Insulin precipitation within the infusion set catheters has previously been described.17 The etiology for infusion set failure is probably multifactorial, and there are clear individual differences in the biologic response to the foreign body that is inserted to infuse insulin, phenol, and m-cresol.
In our study, we were not able to show that one infusion set was superior in terms of infusion set survival, as both had a failure rate at 7 days of 68%. If the infusion set and a continuous glucose sensor were combined in a single platform, the infusion set would last more than 6 days over 50% of the time, and there would be a significant advantage in using a steel infusion set to avoid the early 15% failure rate. Of concern is that 20% of the infusion sets lasted less than 4 days. The early failure seen with 15% of Teflon catheters was secondary to kinking and probably related to the flexibility of Teflon, the insertion angle, and/or the insertion device. The insertion device may play a role because different insertion devices are associated with higher failure rates, as was seen when a high failure rate of the Accu-Chek® FlexLink Plus (Roche Diagnostics, Indianapolis, IN) triggered a product recall in 2011.18 It is our experience that there is a much lower rate of kinking when the Teflon catheter is manually inserted at a 30° angle (Silhouette® infusion set [Minimed]).
Limitations of our study include self-measurement of infusion site induration and erythema by the subject, except for measurements obtained by study staff at the 7-day visit. Infusion sets were inserted by research personnel to avoid lipohypertrophied areas; however, many patients may use these sites in their daily management of diabetes, which may be another factor affecting duration of infusion set wear.
It is our conclusion that the time for changing infusion sets can be individualized, with some patients requiring a change every 2–3 days, whereas others can change a set less frequently, at least in this short-term study. Current infusion sets will need to be improved in order to have a combination sensor and infusion set platform that will last for 7 days. When considering such a platform, there is an advantage to using a steel infusion set because it would avoid the initial 15% failure rate due to Teflon kinking on insertion.
Acknowledgments
This study was funded by an unrestricted grant from Medtronic Minimed for a Principal Investigator-initiated study. Infusion sets were donated by Unomedical. P.J.P. was on a fellowship grant from Genentech. We want to thank our altruistic study subjects who made this study possible.
Author Disclosure Statement
All authors were employees or students at Stanford at the time the study was conducted. B.A.B. is on the medical advisory board for Medtronic Minimed and Unomedical and has received research funding from Medtronic Minimed for both Principal Investigator-initiated and Medtronic-funded studies. Medtronic has also supplied sensors and pumps at a research discount to NIH- and JDRF-funded research studies on which B.A.B. has been an investigator. P.J.P., K.B., G.F., M.G.E., S.S., and D.M.W. declare no competing financial interests exist.
P.J.P., K.B., and B.A.B. were responsible for study design, acquisition of data, data analysis, and writing the manuscript. G.F. and M.G.E. assisted with data collection, analysis of the data, and made revisions to the manuscript. S.S. and D.M.W. reviewed and edited the manuscript.
References
- 1.Pickup JC, Sutton AJ: Severe hypoglycaemia and glycaemic control in Type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008;25:765–774 [DOI] [PubMed] [Google Scholar]
- 2.Pickup J, Mattock M, Kerry S: Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ 2002;324:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control: Toxic-shock syndrome in a patient using a continuous subcutaneous insulin infusion pump—Idaho. MMWR Morb Mortal Wkly Rep 1983;32:404–406, 412. [PubMed] [Google Scholar]
- 4.Thethi TK, Rao A, Kawji H, Mallik T, Yau CL, Christians U, Fonseca V: Consequences of delayed pump infusion line change in patients with type 1 diabetes mellitus treated with continuous subcutaneous insulin infusion. J Diabetes Complications 2010;24:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Højbjerre L, Skov-Jensen C, Kaastrup P, Pedersen PE, Stallknecht B: Effect of steel and Teflon infusion catheters on subcutaneous adipose tissue blood flow and infusion counter pressure in humans. Diabetes Technol Ther 2009;11:301–306 [DOI] [PubMed] [Google Scholar]
- 6.Johansson UB, Adamson U, Lins PE, Wredling R: Patient management of long-term continuous subcutaneous insulin infusion. J Adv Nurs 2005;51:112–118 [DOI] [PubMed] [Google Scholar]
- 7.Renard E, Guerci B, Leguerrier AM, Boizel R: Lower rate of initial failures and reduced occurrence of adverse events with a new catheter model for continuous subcutaneous insulin infusion: prospective, two-period, observational, multicenter study. Diabetes Technol Ther 2010;12:769–773 [DOI] [PubMed] [Google Scholar]
- 8.Gibney MA, Arce CH, Byron KJ, Hirsch LJ: Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin 2010;26:1519–1530 [DOI] [PubMed] [Google Scholar]
- 9.Lo Presti D, Ingegnosi C, Strauss K: Skin and subcutaneous thickness at injecting sites in children with diabetes: ultrasound findings and recommendations for giving injection. Pediatr Diabetes 2012;13:525–533 [DOI] [PubMed] [Google Scholar]
- 10.Mecklenburg RS, Benson EA, Benson JW, Jr, Blumenstein BA, Fredlund PN, Quinn TS, Metz RJ, Nielsen RL: Long-term metabolic control with insulin pump therapy. Report of experience with 127 patients. N Engl J Med 1985;313:465–468 [DOI] [PubMed] [Google Scholar]
- 11.Mecklenburg RS, Benson EA, Benson JW, Jr, Fredlund PA, Quinn T, Metz RJ, Nielsen RL, Sanner CA: Acute complications associated with insulin infusion pump therapy. Report of experience with 161 patients. JAMA 1984;252:3265–3269 [PubMed] [Google Scholar]
- 12.Wood JR, Moreland EC, Volkening LK, Svoren BM, Butler DA, Laffel LM: Durability of insulin pump use in pediatric patients with type 1 diabetes. Diabetes Care 2006;29:2355–2360 [DOI] [PubMed] [Google Scholar]
- 13.Schober E, Rami B: Dermatological side effects and complications of continuous subcutaneous insulin infusion in preschool-age and school-age children. Pediatr Diabetes 2009;10:198–201 [DOI] [PubMed] [Google Scholar]
- 14.Conwell LS, Pope E, Artiles AM, Mohanta A, Daneman A, Daneman D: Dermatological complications of continuous subcutaneous insulin infusion in children and adolescents. J Pediatr 2008;152:622–628 [DOI] [PubMed] [Google Scholar]
- 15.van Bon AC, Bode BW, Sert-Langeron C, DeVries JH, Charpentier G: Insulin glulisine compared to insulin aspart and to insulin lispro administered by continuous subcutaneous insulin infusion in patients with type 1 diabetes: a randomized controlled trial. Diabetes Technol Ther 2011;13:607–614 [DOI] [PubMed] [Google Scholar]
- 16.Schmid V, Hohberg C, Borchert M, Forst T, Pfutzner A: Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy—trouble starts on day 3. J Diabetes Sci Technol 2010;4:976–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolpert HA, Faradji RN, Bonner-Weir S, Lipes MA: Metabolic decompensation in pump users due to lispro insulin precipitation. BMJ 2002;324:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roche ACCU-CHEK FlexLink Plus infusion set: Class I recall—potential for under-delivery of insulin. 2011. www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm248784.htm (accessed September10, 2013)