Abstract
In this study, we sought to investigate the putative association of the oxidized metabolites derived from linoleic acid (OXFAs) with pediatric nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes (T2D). We studied 80 obese adolescents (age 13.3±3.31 years; body mass index 33.0±6.79 kg/m2), who underwent an oral glucose tolerance test, a magnetic resonance imaging (MRI) to measure the hepatic fat content, and the measurement of OXFAs and caspase-cleaved Citokeratin18 fragment (CK-18), a robust biomarker of liver injury. In this study, we show that only in subjects with hepatic steatosis, the OXFAs are associated with the CK-18 and that this association is modulated by the PNPLA3 rs738409 variant. We also show that most of the OXFAs are associated with a lower insulin secretion and that adolescents with T2D have higher levels of OXFAs than subjects with impaired or normal glucose tolerance. These observations lead to the hypothesis that the OXFAs may be the pathogenic link between liver injury and T2D and that the novel therapeutic opportunities targeting the OXFAs are possible in adolescents with early-onset NAFLD and T2D. Antioxid. Redox Signal. 20, 383–389.
Introduction
The increase of childhood obesity in the last decades has been accompanied by an increased prevalence, within the pediatric age, of nonalcoholic fatty liver disease (NAFLD), a complex condition ranging from isolated hepatic steatosis to steatohepatitis, cirrhosis, and liver failure (1). Studies in obese children and adolescents have shown that NAFLD is associated with several metabolic conditions, such as insulin resistance and type 2 diabetes (T2D) (1), and for this reason, it may be considered the hepatic component of the metabolic syndrome and a prelude to T2D (1).
Innovation.
We show for the first time the association between the linoleic acid-derived oxidized compounds with pediatric steatohepatitis and type 2 diabetes (T2D). Moreover, we show a novel interaction between the PNPLA3 rs738409 single-nucleotide polymorphism and the oxidized lipids (OXFAs) in modulating liver injury. Based on these findings, we hypothesize that the OXFAs maybe represent novel pathogenic mediators of liver injury and T2D in obese children and adolescents. In addition, these findings suggest that the novel therapeutic opportunities targeting the OXFAs may be possible in adolescents with early-onset non-alcoholic steatohepatitis (NASH) and T2D.
Recent studies indicate that the excess of dietary imbalance between omega-6 (n-6) and omega-3 (n-3) polyunsaturated fatty acids (PUFA) leads to an adverse cardiovascular and metabolic profile, thereby contributing to the pathogenesis of NAFLD (2). Linoleic acid (LA) is the most abundant PUFA in human diets and the direct precursor of the oxidized LA metabolites (OXLAMs): 9- and 13-hydroxy-octadecadienoic acid (9- and 13-HODE) and 9- and 13-oxo-octadecadienoic acid (9- and 13-oxoODE) (2). Because humans cannot synthesize LA de novo, diet is the sole source of LA in blood and other tissues, where LA is converted into arachidonic acid (AA), which in turn may be oxidized in 5-, 8-, 9-, 11-, 12-, 15-hydroxy-eicosatetraenoic acid (HETE) (2). Although oxidative stress is being recognized as a pivotal mechanism contributing to hepatocyte injury, the role of products of free-radical oxidation in pediatric NAFLD remains unexplored.
In this study, we aimed to assess whether the oxidized lipids (OXFA) derived directly from the oxidation of LA or from the oxidation of AA (OXAAM) might play a role in hepatic steatosis and liver injury in a cohort of obese adolescents (Table 1) and whether these compounds might be related to the derangement of glucose metabolism frequently observed in patients with NAFLD. In addition, expanding on our recent finding of an interaction between the rs738409 variant in the patatin-like phospholipase domain–containing protein 3 (PNPLA3) gene and n-6/n-3 PUFA ratio in modulating alanine aminotransferase (ALT) levels (7), we explored whether this variant might lead to liver injury by interacting with these compounds.
Table 1.
Clinical Features of the Study Population
| Nonsteatosis (24) | Steatosis (56) | p-value | |
|---|---|---|---|
| Age (years) |
15.2±3.6 |
13.3±3.0 |
0.01 |
| Sex% (M/F) |
42/58 |
47/53 |
0.20 |
| Ethnicity% (C/AA/H/A) |
46/33/17/4 |
35/9/52/4 |
0.008 |
| z-score BMI |
1.73±1.21 |
2.38±0.35 |
0.0006 |
|
Glucose metabolism and lipids | |||
| GT% (NGT/IGT/T2D) |
75/21/4 |
64/32/4 |
0.59 |
| Fasting glucose (mg/dl) |
91.8±9.9 |
95.5±11.0 |
0.16 |
| 2-h glucose (mg/dl) |
118.0±33.4 |
132.6±32.0 |
0.04 |
| WBISI |
2.58±1.89 |
1.34±1.10 |
0.0006 |
| IGI |
4.65±2.94 |
4.88±2.88 |
0.45 |
| DI |
8.27±4.65 |
5.90±5.39 |
0.009 |
| Triglycerides (mg/dl) |
94.3±63.0 |
152.6±112.6 |
0.001 |
| Cholesterol (mg/dl) |
159.0±40.0 |
162.3±34.4 |
0.56 |
| HDL (mg/dl) |
47.3±12.2 |
39.9±8.9 |
0.007 |
| LDL (mg/dl) |
93.5±36.8 |
90.4±28.5 |
0.74 |
| Adiponectin (μg/mL) |
10.9±6.2 |
6.6±2.8 |
0.0009 |
|
Liver function | |||
| HFF% |
1.57±0.89 |
22.4±11.2 |
<0.001 |
| ALT (mU/L) |
18.7±9.4 |
57.3±49.5 |
<0.001 |
| AST (mU/L) |
22.4±5.1 |
39.9±30.4 |
<0.001 |
| CK-18 (U/L) | 109.4±33.5 | 204.4±134.2 | <0.001 |
Continuous variables are shown as mean and standard deviation.
M, male; F, female; C, Caucasians; AA, African Americans; H, Hispanics; A, Asians; BMI, body mass index; GT, glucose tolerance; NGT, normal glucose tolerance; IGT, impaired glucose tolerance; T2D, type 2 diabetes; WBISI, whole-body insulin sensitivity index; IGI, insulinogenic index; DI, disposition index; HDL, high density lipoprotein; LDL, low density lipoprotein; HFF%, hepatic fat content; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK-18, caspase-generated CK-18 fragments.
OXFAs and Liver Injury in Obese Adolescents
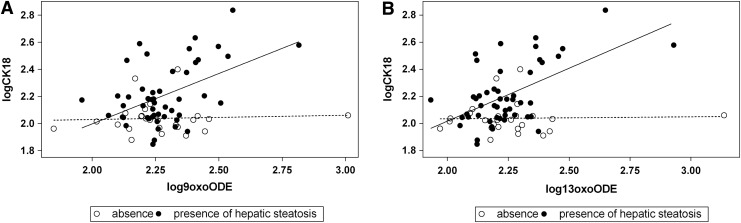
We observed that oxidized lipids, except the 9-HETE, are associated with caspase-cleaved Citokeratin18 fragment (CK-18) (Table 2) and that there is an interaction between hepatic steatosis and OXLAMs, including 9-HODE (p=0.050), 13-HODE (p=0.026), 9-oxo-ODE (p=0.022), and 13-oxo-ODE (p=0.0016), independent of age, sex, z-score body mass index (BMI), and ethnicity. In fact, the association between these compounds and the CK-18 is only present in subjects with steatosis (Fig. 1), suggesting that these species may represent a second hit leading to NASH. This finding is consistent with previous data in adults showing that only the four compounds, which, in our study, interact with steatosis to determine liver injury (9-HODE, 13-HODE, 9-oxo-ODE, and 13-oxo-ODE), are associated with NASH (2). In addition, a recent study has shown that intrahepatic fat in subjects with steatohepatitis is composed of an excess n-6 PUFA (5) with a progressive decrease in the product/precursor ratio for the n-6 pathway from controls to NASH subjects (5), suggesting that subjects with NAFL and NASH convert LA into its derived compounds more than the healthy subjects. The mechanism linking these compounds to liver damage may be related to their ability to activate peroxisome proliferator-activated receptor (PPAR)-alpha, a potent modulator of lipid transport and oxidation. In fact, PUFA metabolites, such as OXLAMs, have one to two orders of magnitude greater affinity for PPAR-alpha and are more potent transcriptional activators of PPAR-alpha-dependent genes than their ancestry compounds (3). These evidence generate the hypothesis that in a liver rich in n-6 PUFA, the continuous production of the OXLAM may perpetuate the intracellular oxidative stress leading to hepatic inflammation.
Table 2.
Association Between Linoleic Acid, Arachidonic Acid, and Their Oxidized Metabolites and Markers of Steatosis, Liver Injury, Insulin Sensitivity, and Secretion
| CK-18 Beta; SE | p-value | ALT Beta; SE | p-value | HFF% Beta; SE | p-value | |
|---|---|---|---|---|---|---|
| LA |
0.12; 0.11 |
0.25 |
0.12; 0.07 |
0.10 |
0.03; 0.01 |
0.012 |
| AA |
0.07; 0.09 |
0.46 |
0.08; 0.06 |
0.21 |
0.01; 0.01 |
0.15 |
| 5-HETE |
0.50; 0.21 |
0.021 |
0.29; 0.14 |
0.048 |
−0.009; 0.02 |
0.70 |
| 8-HETE |
0.46; 0.18 |
0.016 |
0.27; 0.12 |
0.032 |
−0.002; 0.02 |
0.91 |
| 9-HETE |
0.43; 0.22 |
0.05 |
0.27; 0.14 |
0.072 |
−0.01; 0.02 |
0.66 |
| 11-HETE |
0.44; 0.21 |
0.038 |
0.27; 0.14 |
0.066 |
−0.005; 0.02 |
0.82 |
| 12-HETE |
0.49; 0.18 |
0.009 |
0.28; 0.12 |
0.031 |
0.02; 0.02 |
0.43 |
| 15-HETE |
0.49; 0.20 |
0.017 |
0.29; 0.13 |
0.035 |
0.005; 0.02 |
0.87 |
| 9-HODE |
0.32; 0.17 |
0.060 |
0.23; 0.11 |
0.046 |
0.01; 0.02 |
0.36 |
| 13-HODE |
0.45; 0.16 |
0.0089 |
0.24; 0.11 |
0.042 |
0.02; 0.02 |
0.18 |
| 9-oxo-ODE |
0.18; 0.07 |
0.019 |
0.07; 0.05 |
0.21 |
0.01; 0.01 |
0.15 |
| 13-oxo-ODE | 0.23; 0.08 | 0.0018 | 0.08; 0.05 | 0.17 | 0.01; 0.01 | 0.27 |
| WBISI Beta; SE | p-value | IGI Beta; SE | p-value | DI Beta; SE | p-value | |
|---|---|---|---|---|---|---|
| LA |
−0.18; 0.08 |
0.018 |
0.0001; 0.07 |
0.99 |
−0.32; 0.18 |
0.078 |
| AA |
−0.11; 0.06 |
0.083 |
−0.07; 0.06 |
0.23 |
−0.46; 0.22 |
0.030 |
| 5-HETE |
−0.23; 0.15 |
0.13 |
−0.18; 0.17 |
0.28 |
−0.21; 0.08 |
0.0004 |
| 8-HETE |
−0.17; 0.13 |
0.21 |
−0.16; 0.15 |
0.29 |
−0.21; 0.09 |
0.011 |
| 9-HETE |
−0.18; 0.15 |
0.24 |
−0.15; 0.17 |
0.37 |
−0.17; 0.08 |
0.029 |
| 11-HETE |
−0.18; 0.15 |
0.23 |
−0.18; 0.17 |
0.28 |
−0.19; 0.08 |
0.011 |
| 12-HETE |
−0.28; 0.13 |
0.032 |
−0.01; 0.15 |
0.91 |
−0.22; 0.09 |
0.016 |
| 15-HETE |
−0.25; 0.14 |
0.080 |
−0.08; 0.16 |
0.61 |
−0.20; 0.08 |
0.016 |
| 9-HODE |
−0.16; 0.12 |
0.19 |
−0.24; 0.13 |
0.44 |
−0.30; 0.09 |
0.0026 |
| 13-HODE |
−0.18; 0.12 |
0.13 |
−0.10; 0.13 |
0.08 |
−0.24; 0.10 |
0.02 |
| 9-oxo-ODE |
−0.04; 0.05 |
0.47 |
−0.01; 0.06 |
0.76 |
−0.11; 0.23 |
0.61 |
| 13-oxo-ODE | −0.05; 0.06 | 0.31 | −0.05; 0.6 | 0.39 | −0.27; 0.20 | 0.27 |
Beta estimate (Beta) and the standard error (SE) are shown.
LA, linoleic acid; AA, arachidonic acid; HETE, hydroxy-eicosatetraenoic acid; HODE, hydroxy-octadecadienoic acid; oxo-ODE, oxo-octadecadienoic acid.
FIG. 1.
Interaction between OXLAMs and steatosis in modulating liver injury. The figure shows the interaction between the presence/absence of hepatic steatosis 9-oxo-octadecadienoic acid (oxo-ODE) (A) and 13-oxo-ODE (B) in modulating caspase-cleaved Citokeratin18 fragment (CK-18) levels (r2=0.16; p=0.002 and r2=0.25; p<0.0001, respectively). The correlation between these compounds and the CK-18 in subjects with steatosis is shown by the continuous line, whereas in subjects without steatosis is shown by the dotted line.
Is the Association Between Liver Injury and OXFAs Mediated by the PNPLA3 rs738409 Single-Nucleotide Polymorphism?
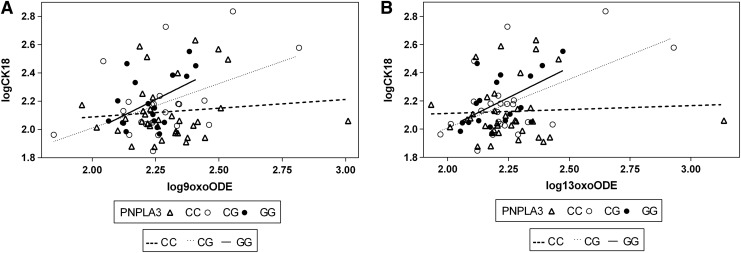
The PNPLA3 is a gene coding for a protein known as adiponutrin, and the rs738409 is a nonsynonymous single-nucleotide polymorphism (SNP) characterized by a C to G substitution encoding an isoleucine to methionine substitution at the amino acid position 148, known to be associated with hepatic fat content (HFF%) and liver injury in adults and children (7). The adiponutrin is a lipid droplet protein and shows both a lipogenic and lipolytic activity. We have previously shown that this variant interacts with the dietary n-6/n-3 PUFA ratio in modulating liver injury (7); herein, we show evidence suggesting that the effect of the PNPLA3 variant might be modulated by the oxidized species derived from the LA. In fact, we observed a significant interaction between the rs738109 and 13-oxo-ODE (p=0.04), whereas the 9-oxo-ODE was approaching the significance (p=0.09) (Fig. 2). The two interactions became more significant after adjusting for age, gender, z-score BMI, and ethnicity (p=0.004 and p=0.02, respectively). There was, in fact, a positive association between the 13-oxo-ODE and 9-oxo-ODE with CK-18 only in subjects homozygous (p=0.01 and p=0.03, respectively) and heterozygous for the risk allele (p=0.006 and p=0.02, respectively), but not within the group of subjects homozygous for the common allele (p=0.77 and p=0.54, respectively) (Fig. 2). These data lead to the hypothesis that the association between OXFAs and liver injury might be driven by the PNPLA3 rs738409 SNP.
FIG. 2.
Interaction between OXLAMs and PNPLA3 rs738409 in modulating liver injury. The figure shows the interaction between the PNPLA3 rs738409 single-nucleotide polymorphism and the 9-oxo-ODE (A) and 13-oxo-ODE (B) in modulating CK-18 levels. The continuous line represents the GG, the dotted line the CG, and the dashed line the CC. There was a positive association between the 13-oxo-ODE and 9-oxo-ODE with CK-18 only in subjects homozygous (p=0.01 and p=0.03, respectively) and heterozygous for the risk allele (p=0.006 and p=0.02, respectively), but not within the group of subjects homozygous for the common allele (p=0.77 and p=0.54, respectively).
OXFAs as a Potential Link Between Liver Injury and T2D in Obese Adolescents
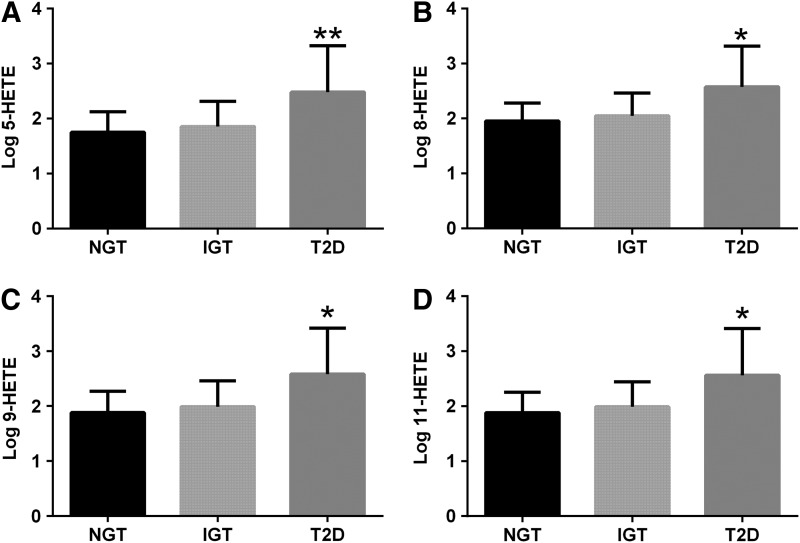
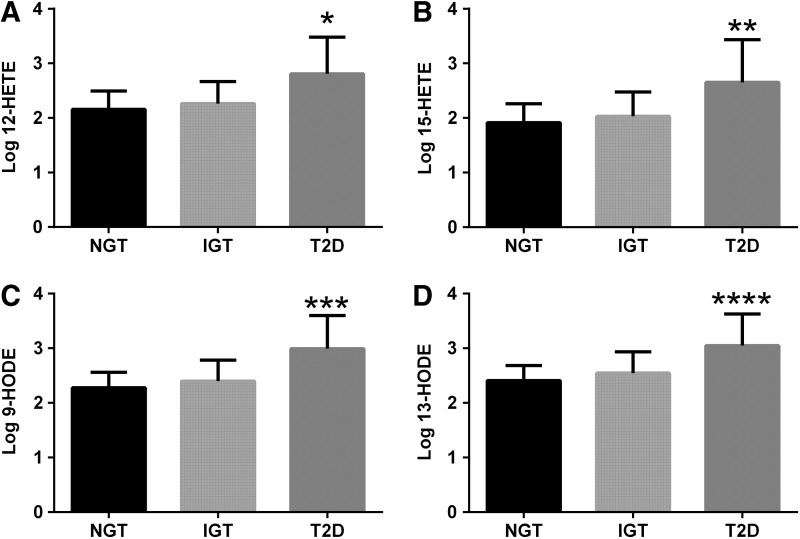
Another important novel finding of our study is the association between the OXFAs and impaired insulin secretion. The LA, AA, and all of the oxidized fatty acids, but the 9-oxo-ODE and 13-oxo-ODE, were associated with disposition index (DI) (Table 2). In addition, there was an increase in OXFA levels across the spectrum of glucose tolerance, with T2D patients showing higher levels than impaired glucose tolerance (IGT) and normal glucose tolerance (NGT) (Figs. 3 and 4).
FIG. 3.
OXFAs across the spectrum of glucose tolerance. The figure shows the differences in 5-hydroxy-eicosatetraenoic acid (HETE) (A), 8-HETE (B), 9-HETE (C), and 11-HETE (D) levels among the glucose tolerance groups. Obese adolescents with type 2 diabetes (T2D) showed higher 5-HETE (p=0.006), 8-HETE (p=0.01), 9-HETE (p=0.01), and 11-HETE (p=0.01) levels compared with subjects with impaired glucose tolerance (IGT) or normal glucose tolerance (NGT). *p=0.01; **p=0.006.
FIG. 4.
OXFAs across the spectrum of glucose tolerance. The figure shows the differences in 12-HETE (A), 15-HETE (B), 9-hydroxy-octadecadienoic acids (HODE) (C), and 13-HODE (D) levels among the glucose tolerance groups. Obese adolescents with T2D showed higher 12-HETE (p=0.005) and 15-HETE (p=0.01), and 9-HODE (p=0.0004) and 13-HODE (p=0.02) levels compared with subjects with IGT or NGT. *p=0.005; **p=0.01; ***p=0.0004; ****p=0.02.
This suggests that the OXFAs play a deleterious role on insulin secretion. Although the effect of these species on the beta cell is largely unknown, a recent study has shown the beneficial effect of a low n-6/n-3 PUFA ratio on the beta-cell function (8). In particular, Wei et al. observed that the change in the intracellular composition of PUFA characterized by an increased n-3 and a decreased n-6 enhances glucose, amino acids, and glucagon-like peptide-1-stimulated insulin secretion and renders the beta cell strongly resistant to cytokine-induced cell death (8). In addition, the fact that the OXAA were associated with insulin secretion more than the OXLAM is consistent with recent observations showing that some HETE reduce insulin secretion and increase cell death in human islets (4). Based on those findings, we hypothesize that OXFAs may be the pathogenic link between NASH and T2D.
Strengths and Limitations
This study has several strengths, such as (i) the use of novel mass spectrometry assays to measure for the first time in obese youth the plasma profile of oxidized fatty acids, (ii) the young age of the patients, and thus, the absence of risk factors linked to alcohol consumption and aging, (iii) the use of magnetic resonance imaging (MRI) measurement to assess HFF%, and (iv) the extensive characterization of the metabolic phenotype of our patients. The major limitation of this study is the lack of liver biopsy, but these observations can be done only in cohorts showing a wide spectrum of the disease, and thus, only enrolling subjects with and without NASH in whom performing an invasive liver biopsy associated with potentially serious morbidity is precluded.
Concluding Remarks and Future Directions
Our findings are novel and may have a great translational potential since those species are solely derived from LA, which cannot be synthesized de novo and therefore the diet represents its only source. Recent studies, in fact, have shown that lowering dietary n-6 causes a reduction of OXFAs levels (6). In addition, it has been shown that the reduction of the OXFAs can be obtained pharmacologically (9) and that this reduction correlates with an improvement in the histological features of NASH (9). Our observations raise the intriguing hypothesis that these therapeutic approaches might be useful also in obese children and adolescents with NASH and T2D.
Notes
Study population
We studied 80 obese children and adolescents (32 Caucasians, 13 African Americans, 32 Hispanics, and 3 Asians of Chinese descents; mean age 13.3±3.31 years; mean BMI 33.0±6.79). Twenty subjects showed IGT (5 Caucasians, 6 African Americans, 8 Hispanics, and 1 Asian) and four subjects showed T2D (1 Caucasian, 2 Hispanics, and 1 Asian). The study was conducted according to the principles of the Declaration of Helsinki and approved by the Yale University Human Investigation Committee. Written parental informed consent and written child assent were obtained from all participants. The clinical features of the study population according to the presence of hepatic steatosis, as defined by a cutoff of HFF% of 5.5%, are shown in Table 1.
Metabolic studies
All metabolic studies were done at the Yale Center for Clinical Investigation (YCCI) at 8.00 am following a 10–12-h overnight fast. A standard oral glucose tolerance test (OGTT) (1.75 g/kg body weight, up to 75 g) was performed.
Indices of insulin sensitivity
Insulin sensitivity was assessed by the whole-body insulin sensitivity index (WBISI).
Beta-cell function
The insulinogenic index (IGI), which is a commonly used index of beta-cell function, was calculated from the OGTT data: IGI=Δinsulin (0–30 min) in microunits per milliliter divided by the glucose (0–30 min) in milligrams per deciliter. The DI was calculated as the product of the IGI and the WBISI (7).
Imaging studies
Fast–MRI to assess HFF% was performed as previously described (7). Hepatic steatosis was defined as the HFF% higher than 5.5%.
Biochemical analyses
Plasma glucose was determined using a glucose analyzer by the glucose oxidase method (Beckman Instruments, Brea, CA). Plasma insulin was measured by the Linco RIA, lipid levels using an Auto-Analyzer (model 747–200), and liver enzymes using standard automated kinetic enzymatic assays.
Measurement of the CK-18
The apoptosis-associated neoepitope in the C-terminal domain of the CK-18 was measured by the M30-Apoptosense ELISA kit (PEVIVA, Bromma, Sweden). All assays were performed in duplicate, and the absorbance was determined using a microplate reader (molecular Devices M2, Sunnyvale, CA).
Lipid extraction from human plasma
Lipid extractions and protein hydrolyses were performed using disposable threaded borosilicate glass test tubes with PTFE-lined caps. Before use, all glassware tubes, caps, and pipette tips were washed with nitric acid to remove trace transition metals, extensively rinsed with Chelex-treated water containing 1 μM diethylenetriamine pentaacetic acid (DTPA; pH 7.0 in H2O), and then rinsed with pure Chelex-treated water. Plastic tips were further rinsed in methanol and air-dried before use. Test tubes were also baked at 500°C overnight to remove residual potential organics. All plasma samples for analyses contained antioxidant cocktail (DTPA [2 mM final] and butylated hydroxytoluene [500 μM final]) with head space overlaid with argon. Samples were thawed in ice/water bath immediately before sample handling for LC/MS/MS analysis. Fatty acids and oxidized fatty acids in plasma were extracted as previously described (6–9). Briefly, plasma (50 μl), internal standard (synthetic 15(S)-HETE-d8), and potassium hydroxide were added to the glass test tubes, overlaid with argon, and sealed. Lipids were hydrolyzed at 60°C under argon atmosphere for 2 h, and then, the released fatty acids were extracted into the hexane layer twice by liquid/liquid extraction. With each extraction, argon was used to purge the head space of the tube before sealing and vortexing/centrifugation. The combined hexane layers were dried under nitrogen gas and then resuspended in 200 μl of 85% methanol/water (v/v).
Liquid chromatography online electrospray ionization tandem mass spectrometry
The levels of multiple fatty acid oxidation products (free plus esterified) in plasma were quantified using liquid chromatography online electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS) (18). Briefly, lipid extract was injected onto a high performance liquid chromatography (Waters 2690 Separations Module, Franklin, MA), and the oxidized fatty acids and their precursors were separated through a C18 column (Phenomenex ODS (2), 2×150 mm, 5 μm, Rancho Palos Verdes, CA) using a gradient starting from 85% methanol containing 0.2% acetic acid over 10 min and then to 100% methanol containing 0.2% acetic acid over 2 min, following by 100% methanol containing 0.2% acetic acid for 15 min. The oxidized fatty acids and their precursors were quantified on a triple quadrupole mass spectrometer (Quattro Ultima; Micromass, Manchester, UK) using ESI in negative ion mode and multiple reaction monitoring using characteristic parent→daughter ion transitions for the specific molecular species monitored (6–9). The lipid peroxidation products analyzed included structurally specific species of HETEs (5, 8, 9, 11, 12, and 15), HODEs (9 and 13), oxo-octadecadienoic acids (HODEs 9 and 13), and their precursor's AA and LA. The sample preparation and the quantitation of oxidized fatty acids by LC/ESI/MS/MS were performed by an investigator who was blinded to the liver histology and other clinical data. 15-HETE-d8 (Cayman Chemicals, Ann Arbor, MI) was used as internal standard for the calibration of oxidized fatty acids in plasma, as previously described (6–9).
Genotyping
Genomic DNA was extracted from peripheral blood leukocytes. The PNPLA3 rs738409 variant was genotyped as previously reported (7).
Statistics
Before analyzing the data, all the variables were tested for normality, with non-normally distributed variables log transformed to be better approximated by normality, except for HFF%, for which, a square root transformation was used. All continuous variables were compared among the groups using the analysis of variance. Adjusted comparisons were performed using a general linear model, adjusting for age, gender, ethnicity, and z-score BMI. Prevalence among groups was compared using the chi-square statistic. A linear regression was used to test the association between the oxidized lipids and CK-18, ALT, HFF%, WBISI, IGI, and DI. To evaluate the interaction between steatosis or the PNPLA3 rs738409 variant and the oxidized lipids on the CK-18 levels, a general linear model, including the single terms and an interaction term (e.g., oxidized lipids X steatosis), was run. Unless otherwise specified, the data are shown as raw means and standard deviations.
Abbreviations Used
- AA
arachidonic acid
- ALT
alanine aminotransferase
- CK-18
caspase-cleaved Citokeratin18 fragment
- DI
disposition index
- HFF%
hepatic fat content
- HETE
hydroxy-eicosatetraenoic acid
- HODE
hydroxy-octadecadienoic acid
- IGI
insulinogenic index
- IGT
impaired glucose tolerance
- LA
linoleic acid
- NAFLD
nonalcoholic fatty liver disease
- n-6
omega-6
- n-3
omega-3
- OGTT
oral glucose tolerance test
- OXAAM
oxidized arachidonic acid metabolites
- OXFA
oxidized lipids
- OXLAM
oxidized LA metabolites
- oxo-ODE
oxo-octadecadienoic acid
- PNPLA3
patatin-like phospholipase domain–containing protein 3
- PUFA
polyunsaturated fatty acids
- SNP
single-nucleotide polymorphism
- T2D
type 2 diabetes
Acknowledgments
The authors are grateful to the patients and their families as well as to the Yale Center for Genome Analyses (YCGA) and YCCI and Hospital Research Unit (HRU) personnel. This work was supported by the American Heart Association (AHA) (13SDG14640038) and 2012 YCCI scholar award to N.S., National Institutes of Health (NIH) (grants R01-HD-40787, R01-HD-28016, and K24-HD-01464 to S.C.; grants DK076852 and DK082451 to A.E.F.). This publication was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Advancing Translational Science (NCATS), a component of National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
References
- 1.D'Adamo E, Santoro N, and Caprio S. Metabolic syndrome in pediatrics: old concepts revised, new concepts discussed. Pediatr Clin North Am 58: 1241–1255, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, Zhang R, McIntyre TM, and Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res 51: 3046–3054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krey G, Braissant O, L'Horset F, Kalkhoven E, Perroud M, Parker MG, and Wahli W. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol 11: 779–791, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, and Nadler JL. 12-Lipoxygenase products reduce insulin secretion and {beta}-cell viability in human islets. J Clin Endocrinol Metab 95: 887–893, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, Sterling RK, Fuchs M, Zhou H, Watkins SM, and Sanyal AJ. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology 50: 1827–1838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, Majchrzak-Hong SF, et al. . Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids 87: 135–141, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santoro N, Savoye M, Kim G, Marotto K, Shaw MM, Pierpont B, and Caprio S. Hepatic fat accumulation is modulated by the interaction between the rs738409 variant in the PNPLA3 gene and the dietary omega6/omega3 PUFA intake. PLoS One 7: e37827, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei D, Li J, Shen M, Jia W, Chen N, Chen T, Su D, Tian H, Zheng S, Dai Y, and Zhao A. Cellular production of n-3 PUFAs and reduction of n-6-to-n-3 ratios in the pancreatic beta-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes 59: 471–478, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, and McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology 54: 1610–1619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]