Abstract
Background: We showed previously that subclinical low-risk papillary thyroid microcarcinoma (PTMC) could be observed without immediate surgery. Patient age is an important prognostic factor of clinical papillary thyroid carcinoma (PTC). In this study, we investigated how patient age influences the observation of low-risk PTMC.
Methods: Between 1993 and 2011, 1235 patients with low-risk PTMC chose observation without immediate surgery. They were followed periodically with ultrasound examinations. These patients were enrolled in this study. We divided them into three subsets based on age at the beginning of observation: young (<40 years), middle-aged (40–59 years), and old patients (≥60 years). Observation periods ranged from 18 to 227 months (average 75 months).
Results: We set three parameters for the evaluation of PTMC progression: (i) size enlargement, (ii) novel appearance of lymph-node metastasis, and (iii) progression to clinical disease (tumor size reaching 12 mm or larger, or novel appearance of nodal metastasis). The proportion of patients with PTMC progression was lowest in the old patients and highest in the young patients. On multivariate analysis, young age was an independent predictor of PTMC progression. However, none of the 1235 patients showed distant metastasis or died of PTC during observation. Although only 51 patients (4%) underwent thyrotropin (TSH) suppression based on physician preference, the PTMC of all patients enrolled in this TSH suppression study, except one, were clinically stable. To date, 191 patients underwent surgery for various reasons after observation. None showed recurrence except for one in the residual thyroid, and none died of PTC after surgery.
Conclusions: Old patients with subclinical low-risk PTMC may be the best candidates for observation. Although PTMC in young patients may be more progressive than in older patients, it might not be too late to perform surgery after subclinical PTMC has progressed to clinical disease, regardless of patient age.
Introduction
Papillary thyroid carcinoma (PTC) is the most common malignancy of the thyroid. To date, several clinicopathological features predicting a poor prognosis of PTC patients have been identified, including old age, extrathyroid and/or extranodal extension, clinical lymph-node and/or distant metastasis, and large tumor size (1–4). In 2011, Miyauchi et al. showed that, in thyroglobulin antibody (TgAb)-negative patients who underwent total thyroidectomy, short Tg-doubling time (≤2 years) more strongly influenced cause-specific survival (CSS) than the above clinicopathological features (5), and Tsushima et al. recently demonstrated that TgAb-positive patients with no postoperative decrease in the TgAb level showed a poorer disease-free survival (DFS) rate (6). PTC without these features, however, is generally indolent with an excellent prognosis.
PTC measuring 10 mm or less is defined as papillary microcarcinoma (PTMC), and the prevalence of ultrasonography and ultrasonography-guided fine-needle aspiration biopsy (FNAB) has made it possible to detect PTMC measuring 3 mm or more. Most PTMC are very indolent unless they have one or more of the aggressive features indicated above, and a high incidence of PTMC has been confirmed in autopsies and screenings by ultrasonography.
Autopsy studies showed that the detection rate of PTMC measuring 3–9.9 mm ranged from 0.5% to 5.2% (7–10). Takebe et al. performed a mass screening for thyroid cancer using ultrasound and ultrasound-guided FNAB, and they found thyroid carcinoma in 3.5% of otherwise healthy women aged 30 years or older, and 75% of the carcinomas measured 15 mm or less (11). The prevalence of subclinical PTMC was thus found to be about 1000 times higher than that of clinical thyroid carcinoma (1.9–11.7 per 100,000 females) (7,11).
These findings strongly suggest that most low-risk PTMC lacking aggressive features are harmless, and immediate surgery for all of them is definitely an overtreatment. It can be assumed that all advanced thyroid PTC were PTMC in the past. To date, however, only periodical observation can distinguish progressive PTMC from harmless PTMC accounting for their majority. Based on this concept, our coauthor (Akira Miyauchi) proposed an observation trial without immediate surgery for low-risk PTMC as an alternative to immediate surgery at a meeting at Kuma Hospital in 1993, which was approved. We reported promising results of this trial in 2003 and 2010 (12,13).
In the present study, we focused on the relationship between PTMC progression and patient age. Age is an important prognostic factor of thyroid papillary and follicular carcinomas and has been adopted in prominent classification systems such as TNM, AMES, and MACIS (14–16). Mazzaferri et al. demonstrated that recurrences of papillary and follicular carcinomas were most frequent at the extremes of age (<20 and >59 years), but their mortality rates successively increased with patient age older than 40 years (17). In 2013, Miyauchi et al. divided TgAb-negative PTC patients into three subsets based on age—young (<40 years), middle-aged (40–59 years), and old patients (≥60 years)—and the results showed that biochemical persistent disease (BPD; Tg detectable after total thyroidectomy under thyrotropin [TSH] suppression) was more frequent in young and old patients than in middle-aged patients (18). Miyauchi et al. also showed that the incidence of a short Tg-doubling time was much higher among old patients than the other two subsets (18). These findings strongly suggested that, in clinical PTC, CSS becomes poor with old age, but DFS shows a biphasic pattern for patient age, which prompted us to investigate how patient age influences the progression of low-risk PTMC during observation. In this study, we show the differences and similarities between subclinical PTMC on observation and clinical PTC in relation to patient age.
Patients and Methods
Diagnosis and observation of papillary microcarcinoma patients
The diagnosis of PTC was based on ultrasonography-guided FNAB. When the PTC measured 10 mm or less, we presented two management options to the patient: observation alone or surgical treatment. Exclusion criteria for observation were: (i) the presence of regional lymph-node metastasis or distant metastasis, (ii) signs or symptoms of invasion to the recurrent laryngeal nerve or trachea, (iii) FNAB findings suggesting high-grade malignancy, and (iv) tumors located adjacent to the recurrent laryngeal nerve or trachea. For patients with these features, immediate surgery without observation was recommended.
Patients who chose observation underwent periodical follow-up on ultrasonography once or twice per year as described (12,13). Fifty-one patients (4%) patients underwent TSH suppression with levothyroxine based on physician preference. Of these, 2, 24, and 25 patients were aged younger than 40 years, 40–59 years, and 60 years or older respectively. Patients whose PTMC showed signs of progression such as significant size enlargement and novel appearance of lymph-node metastasis were also advised to undergo surgery at that point.
Patient series
Between 1993 and 2011, 1235 patients at Kuma Hospital were diagnosed as having PTMC and were under observation for 18 months or longer. This series included 340 patients enrolled in our last study (13). The backgrounds and clinicopathological features of the 1235 patients are summarized in Table 1. Sixty-one patients (5%) had one or more first-degree relatives with differentiated thyroid carcinoma and were diagnosed as having familial nonmedullary thyroid carcinoma (FNMTC) in the present study. The criteria of FNMTC were the same as those established in previous reports by Uchino et al. and by our own group (19,20). The average period of observation was 60 months (18–227 months). To date, 191 patients (16%) underwent surgery after observation for various reasons such as carcinoma progression (size enlargement or novel appearance of lymph-node metastasis), enlargement of a coexisting follicular tumor or multinodular goiter, difficulty in controlling coexisting Graves' disease, changes in the preference of the patient, and decision of a different attending physician. The average postoperative follow-up period was 75 months (1–246 months). We postoperatively followed the patients as described (1–4).
Table 1.
Backgrounds and Clinicopathological Features of 1235 Patients
| Sex | |
| Male |
124 (10%) |
| Female |
1111 (90%) |
| Age | |
| ≥60 years |
496 (40%) |
| 40–59 years |
570 (46%) |
| <40 years |
169 (14%) |
| FNMTC | |
| Yes |
61 (5%) |
| No |
1174 (95%) |
| Thyroid antibody | |
| Yes |
383 (32%) |
| No |
806 (68%) |
| Unknown |
46 |
| Tumor size | |
| ≤5 mm |
324 (26%) |
| >5 mm and ≤8 mm |
686 (56%) |
| >8 mm and ≤10 mm |
225 (18%) |
| Thyrotropin (TSH) suppression | |
| Yes |
51 (4%) |
| No |
1184 (96%) |
| Suspected of or diagnosed as multiplicity | |
| Yes |
147 (12%) |
| No |
1088 (88%) |
| Coexisting diseases | |
| Nodules |
596 (48%) |
| Graves' disease | 80 (6%) |
Values are presented as number (percent), with the percentages rounded down to whole numbers.
FNMTC, familial nonmedullary thyroid carcinoma.
Evaluation of carcinoma progression on observation
On observation, we set three parameters for the evaluation of PTMC progression in this study: (i) size enlargement, (ii) novel appearance of lymph-node metastasis, and (iii) progression to clinical disease. We defined tumor enlargement as when the tumor's size increased by 3 mm or more compared with that at the beginning of the observation.
Regional lymph-node metastasis was diagnosed on ultrasonography based on criteria described previously (12,13). When a suspicious node was detected on ultrasonography, we performed FNAB for the node and measured the Tg in the washout of the needles used for the FNAB (21) to diagnose whether the lymph node was metastatic or reactive.
We defined progression to clinical disease as the tumor size reaching 12 mm or larger, or the novel appearance of lymph-node metastasis. The appearance of lymph-node metastasis is thought to be a sign of an aggressive nature of the cancer. We thought that increase in size by 3 mm for very small PTMC without other unfavorable signs might not be critically important, and these patients could be kept on observation. This is based on our experience that the PTMC often fluctuate in size as described previously (22). We tentatively set the upper limit of tumor size for observation at 12 mm This parameter included all patients with novel appearance of node metastasis and some patients with size enlargement to 12 mm or larger.
Surgery after observation
We generally perform hemithyroidectomy (lobectomy with isthmectomy) when a carcinoma lesion is solitary and there is no evidence of nodal involvement. We generally perform a total thyroidectomy if: (i) carcinoma lesions are multiple, (ii) other pathological lesions such as follicular tumor and multinodular goiter are present in the contralateral lobe, or the patient has Graves' disease, or (iii) clinical lymph-node metastasis is detected. We perform therapeutic lymph-node dissection for patients with novel appearance of node metastasis. For prophylactic node dissection, our policy is to perform ipsilateral and bilateral central node dissection in cases of hemithyroidectomy and total thyroidectomy respectively. In this retrospective study, however, other thyroidectomies such as subtotal thyroidectomy and isthmectomy and prophylactic lateral compartment dissection had been performed for some patients for various reasons.
Statistical analyses
The chi-square test was employed to compare variables. The Kaplan–Meier method and log-rank test were adopted to analyze time-dependent variables. The Cox regression model was also adopted for the multivariate analysis. These analyses were performed using StatView-J v5.0. A p-value of less than 0.05 was regarded as significant, and that between 0.05 and 0.1 was considered marginal.
Results
Of the 1235 patients, 58 (4.6%) showed size enlargement, 19 (1.5%) showed a novel appearance of lymph-node metastasis (4 in the central and 15 in the lateral compartments), and 43 (3.5%) showed progression to clinical disease. We then divided the patients into three subsets based on their age at the initiation of their observation: young (<40 years, 169 patients), middle-aged (40–59 years, 570 patients), and old patients (≥60 years, 496 patients) in accordance with the study by Miyauchi et al. (18). Table 2 indicates the relationship between carcinoma progression and the three subsets of patients. The incidence of size enlargement, novel appearance of lymph-node metastasis, and progression to clinical disease were each significantly inversely related to patient age.
Table 2.
Relationship Between Carcinoma Progression and Age of Patients
| |
Age of patients |
|
|
||
|---|---|---|---|---|---|
| |
<40 years (young) |
40–59 years (middle-aged) |
≥60 years (old) |
Total |
|
| (n=169) | (n=570) | (n=496) | (n=1235) | p-Value | |
| Size enlargement [n (%)] |
14 (5.9%) |
33 (5.7%) |
11 (2.2%) |
58 |
=0.0014 |
| Novel appearance of node metastasis [n (%)] |
9 (5.3%) |
8 (1.4%) |
2 (0.4%) |
19 |
<0.0001 |
| Progression to clinical disease [n (%)] | 15 (8.9%) | 20 (3.5%) | 8 (1.6%) | 43 | <0.0001 |
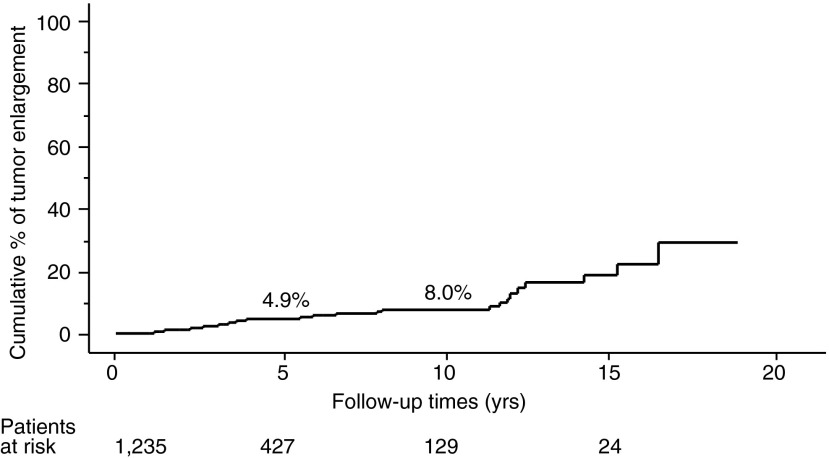
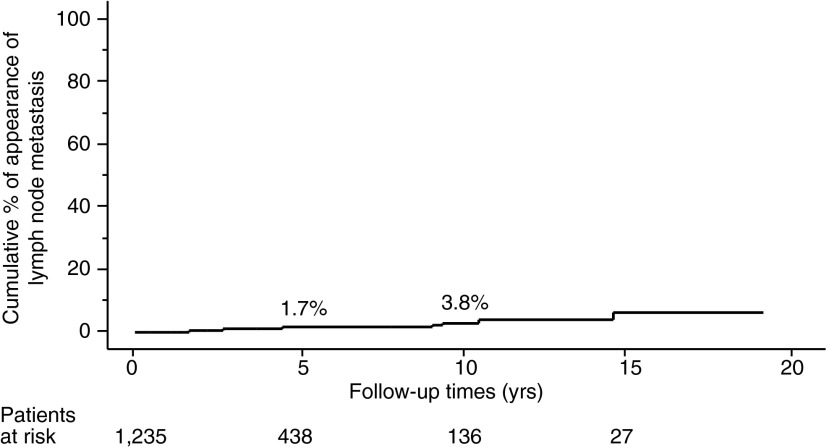
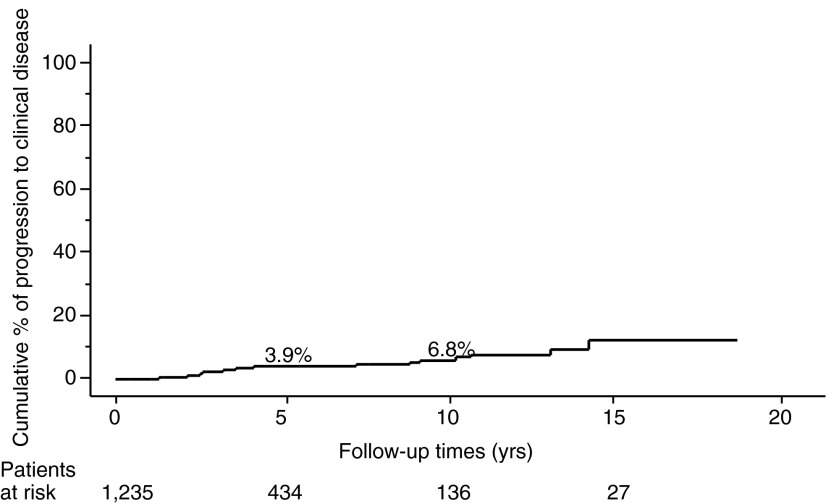
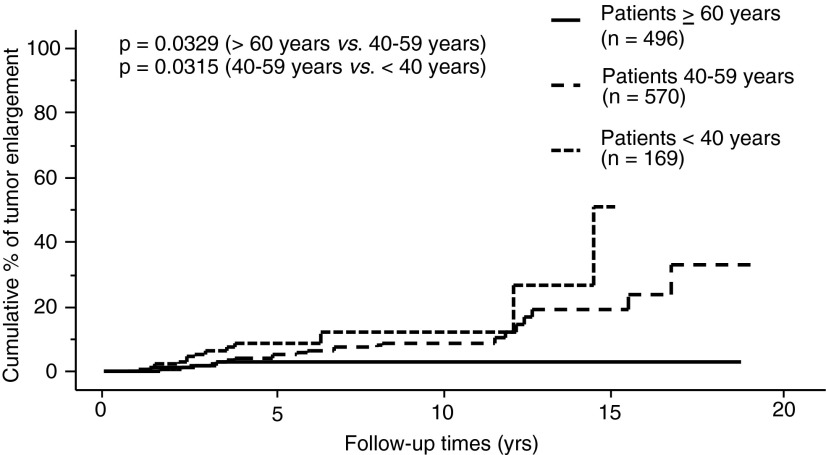
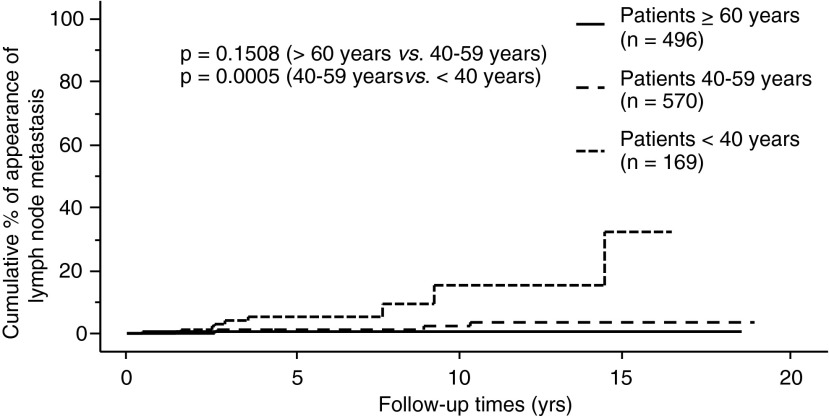
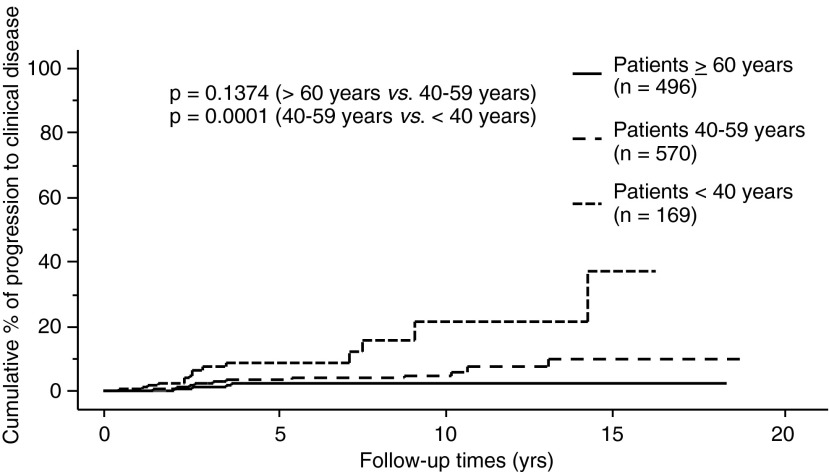
The proportion of PTMC progression in our entire series, as assessed by the Kaplan–Meier method, is shown in Figures 1–3. The incidence rates of size enlargement, novel appearance of node metastasis, and progression to clinical disease were 8.0, 3.8, and 6.8% on 10-year observation respectively. The proportions of PTMC progression in the three subsets of patients are shown in Figures 4–6; the proportions were lowest and highest in old and young patients respectively. Table 3 summarizes the number of patients at risk and the incidence rates at the 5-year and 10-year observations in each subset.
FIG. 1.
Proportion of patients in our entire series whose papillary thyroid microcarcinoma (PTMC) showed enlargement by 3 mm or more.
FIG. 2.
Proportion of patients in the entire series whose PTMC showed novel appearance of lymph-node metastasis.
FIG. 3.
Proportion of patients in the entire series whose PTMC developed into clinical disease.
FIG. 4.
Relationship between age and proportion of patients whose PTMC showed enlargement by 3 mm or more.
FIG. 5.
Relationship between age and proportion of patients whose PTMC showed novel appearance of lymph-node metastasis.
FIG. 6.
Relationship between age and proportion of patients whose PTMC developed into clinical disease.
Table 3.
The Number of Patients at Risk and Incidence Rates of the Three Events in Each Subset
| Observation periods | 5-year | 10-year | 15-year |
|---|---|---|---|
| Size enlargement | |||
| Patients at risk (n) |
157/225/45 |
36/82/11 |
6/18/0 |
| Incidence rates (%) |
4.0/5.0/9.1 |
4.0/9.1/12.1 |
|
| Novel appearance of node metastasis | |||
| Patients at risk (n) |
160/229/49 |
36/87/13 |
5/20/2 |
| Incidence rates (%) |
0.5/1.4/5.2 |
0.5/2.3/16.1 |
|
| Progression to clinical disease | |||
| Patients at risk (n) |
157/229/48 |
36/87/13 |
6/19/2 |
| Incidence rates (%) | 2.2/4.0/9.5 | 2.5/4.9/22.5 | |
Data are presented as number or percent of patients in each age group (old/middle-aged/young).
The multivariate analysis revealed that young age (<40 years) independently reflected PTMC progression (Tables 4–6). Tumor size 9 mm or larger was also an independent predictor of progression to clinical disease (Table 6). Although only 51 patients underwent TSH suppression, 50 of them (98.0%) were clinically stable. This incidence is larger than that in patients without TSH suppression (95.2%; 1130 of 1187 patients), although the difference did not reach significance. Since none of the patients who underwent TSH suppression showed novel node metastasis or progression to clinical disease, we did not include TSH suppression in the variables for multivariate analysis (Tables 5 and 6).
Table 4.
Multivariate Analysis of Predictors of Size Enlargement of Low-Risk Papillary Thyroid Microcarcinoma in All Patients
| Variables | p-Value | Odds ratio [CI] |
|---|---|---|
| Male gender |
0.6211 |
1.274 [0.524–2.950] |
| TSH suppression |
0.2335 |
0.315 [0.041–2.183] |
| Family history |
0.1919 |
1.855 [0.733–4.695] |
| Suspected of or diagnosed as multiplicity |
0.4656 |
0.683 [0.245–1.901] |
| Young age (<40 years) |
0.0033 |
2.500 [1.357–4.608] |
| T >5 mm and T ≤8 mm |
0.2488 |
1.475 [0.762–2.857] |
| T ≥9 mm | 0.1331 | 1.862 [0.827–4.202] |
CI, confidence interval; T, tumor size.
Table 5.
Multivariate Analysis of Predictors of Novel Lymph-Node Appearance of Low-Risk Papillary Thyroid Microcarcinoma in All Patients
| Variables | p-Value | Odds ratio [CI] |
|---|---|---|
| Male gender |
0.6019 |
0.583 [0.077–4.425] |
| Family history |
0.9932 |
0.991 [0.131–7.462] |
| Suspected of or diagnosed as multiplicity |
0.3127 |
1.908 [0.544–6.711] |
| Young age (<40 years) |
<0.0001 |
6.757 [2.725–16.949] |
| T>5 mm and T ≤8 mm |
0.4404 |
0.658 [0.228–1.985] |
| T ≥9 mm | 0.4721 | 1.548 [0.470–5.102] |
None of the patients who underwent TSH suppression showed novel node metastasis.
Table 6.
Multivariate Analysis of Predictors of Becoming Clinical Disease of Low-Risk Papillary Thyroid Microcarcinoma in All Patients
| Variables | p-Value | Odds ratio [CI] |
|---|---|---|
| Male gender |
0.8800 |
0.923 [0.324–2.625] |
| Family history |
0.1564 |
2.123 [0.750–6.024] |
| Suspected of or diagnosed as multiplicity |
0.7186 |
1.189 [0.463–3.049] |
| Young age (<40 years) |
<0.0001 |
4.348 [2.293–8.196] |
| T >5 mm and T ≤8 mm |
0.5817 |
1.278 [0.534–3.068] |
| T ≥9 mm | 0.0005 | 4.717 [1.961–11.364] |
None of the patients who underwent TSH suppression became clinical.
Tables 7–9 indicate multivariate analysis of predictors of size enlargement, novel lymph node appearance, and becoming clinical disease in the subsets of middle-aged patients and old patients. Middle age had a significant and marginal predictive value for size enlargement (Table 7) and progression to clinical disease (Table 9) respectively. Tumor size ≥9 mm and tumor size 6–8 mm were independent and marginal predictors of progression to clinical disease respectively (Table 9). None of the patients who underwent TSH suppression showed carcinoma progression (Tables 7–9).
Table 7.
Multivariate Analysis of Predictors of Size Enlargement of Low-Risk Papillary Thyroid Microcarcinoma in the Subgroups of Middle-Aged and Old Patients
| Variables | p-Value | Odds ratio [CI] |
|---|---|---|
| Male gender |
0.1593 |
1.901 [0.777–4.651] |
| Family history |
0.3289 |
1.678 [0.593–4.762] |
| Suspected of or diagnosed as multiplicity |
0.7346 |
0.836 [0.296–2.358] |
| Middle-aged (40–59 years) |
0.0280 |
2.174 [1.087–4.348] |
| T >5 mm and T ≤8 mm |
0.1884 |
1.664 [0.779–3.552] |
| T ≥9 mm | 0.5361 | 1.379 [0.498–3.817] |
None of the patients who underwent TSH suppression showed carcinoma enlargement.
Table 8.
Multivariate Analysis of Predictors of Novel Lymph-Node Appearance of Low-Risk Papillary Thyroid Microcarcinoma in the Subgroups of Middle-Aged and Old Patients
| Variables | p-Value | Odds ratio [CI] |
|---|---|---|
| Male gender |
0.9594 |
1.056 [0.123–8.627] |
| Suspected of or diagnosed as multiplicity |
0.9055 |
0.882 [0.111–7.042] |
| Middle-aged (40–59 years) |
0.1595 |
3.067 [0.644–14.706] |
| T >5 mm and T ≤8 mm |
0.6175 |
1.722 [0.235–12.630] |
| T ≥9 mm | 0.5929 | 1.506 [0.302–7.506] |
None of the patients who underwent TSH suppression or has family history showed novel node metastasis.
Table 9.
Multivariate Analysis of Predictors of Becoming Clinical Disease of Low-Risk Papillary Thyroid Microcarcinoma in the Subgroups of Middle-Aged and Old Patients
| Variables | p-Value | Odds ratio [CI] |
|---|---|---|
| Male gender |
0.6770 |
1.259 [0.425–3.731] |
| Family history |
0.7767 |
1.233 [0.290–5.236] |
| Suspected of or diagnosed as multiplicity |
0.8733 |
0.907 [0.425–3.021] |
| Middle-aged (40–59 years) |
0.0708 |
2.146 [0.936–4.926] |
| T >5 mm and T ≤8 mm |
0.0794 |
3.759 [0.856–16.390] |
| T ≥9 mm | 0.0042 | 9.259 [2.020–41.667] |
None of the patients who underwent TSH suppression became clinical.
The surgery of 5 of the 191 patients who underwent surgery after observation was performed in other hospitals. Table 10 indicates the extent of surgery and pathological findings of the remaining 186 patients. Total thyroidectomy was performed in 93 patients (50%), and the remaining patients underwent limited thyroidectomy such as lobectomy with isthmectomy (44%), subtotal thyroidectomy (4%), and isthmectomy (3%). As described in Patients and Methods, we routinely dissect the central compartment. In the present patient series, we dissected the central compartment in all 186 patients, including four who showed a novel appearance of central node metastasis.
Table 10.
Extent of Surgery and Pathological Findings of 186 Patients Who Underwent Surgery After Observation
| Thyroidectomy | |
| Total |
93 (50) |
| Limited |
93 (50) |
| Lymph-node dissection | |
| Central only |
152 (82) |
| Central and lateral |
34 (18) |
| Multiplicity | |
| Yes |
128 (69) |
| No |
58 (31) |
| Significant extrathyroid extensiona | |
| Yes |
2 (1)b |
| No |
184 (99) |
| Central node metastasis | |
| Yes |
50 (27) |
| No |
136 (73) |
| Lateral node metastasis | |
| Yes |
20 (59) |
| No | 14 (41) |
Details of the remaining five patients are unknown. Values are presented as number (percent), with the percentages rounded down to whole numbers.
Intraoperative findings.
Both minimally extended to the muscular layer of the esophagus.
Thirteen patients underwent therapeutic lateral node dissection and 21 underwent prophylactic lateral node dissection. The remaining two who showed lateral node metastasis during observation underwent surgery at other hospitals and are not listed in Table 10. To date, only one of these 186 patients had PTC recurrence. This patient was one of the 93 patients (1.1%) who underwent limited thyroidectomy; the PTC recurred in the remnant thyroid 130 months after surgery. This patient is, however, still under observation because the recurred lesion is small and has been stable for 12 months after the detection of the recurred lesion.
To date, none of the 1235 patients in our series who underwent observation, including the 191 who underwent surgery after observation, showed distant metastasis or died of PTC. By questionnaire, we confirmed that six patients, who had undergone observation, died of other diseases such as breast carcinoma, lung carcinoma, and brain hemorrhage without undergoing surgery for PTC.
Discussion
In this study, we demonstrate that in 1235 patients with low-risk PTMC on observation, (i) the incidence rates of size enlargement, novel appearance of lymph-node metastasis, and progression to clinical disease were 8.0%, 3.8%, and 6.8% at their 10-year observation respectively; (ii) the proportion of PTMC progression was lowest in old (≥60 years) patients and highest in the young patients (<40 years); and (iii) in the multivariate analysis, young age was an independent predictor of PTMC progression, and in the subsets of middle-aged and old patients (≥40 years), middle age was an independent or marginal predictor of size enlargement and progression to clinical disease.
The incidence rates of size enlargement at the 5- and 10-year observations in this study were 4.9% and 8.0% respectively, which were lower than those in our previous study (6.4% and 15.9%) (13). This might be because of the increase in the number of patients at risk at the 5- and 10-year observations.
Sugitani et al. showed that the average age of patients who had PTMC with enlargement was younger than the average age of patients with PTMC without enlargement (50.6 years vs. 54.6 years) (23), but they did not perform a longitudinal analysis. In the present study, we divided the patient series into three subsets based on age: young (<40 years), middle-aged (40–59 years), and old patients (≥60 years), and compared the proportions of PTMC progression among these subsets in univariate and multivariate analyses. Interestingly, the PTMCs in the old patients were the most likely to stay small and stable, which is very discrepant with the repeated finding in clinical PTC that old age is the strongest predictor of cancer-specific death (1–4) and significantly related to short Tg-doubling time (18). We emphasize the significant differences in tumor biology between subclinical PTMC and clinically advanced PTC in elderly patients. Our data indicate that old patients with subclinical low-risk PTMC are the best candidates for observation.
Conversely, the PTMCs of the young patients were significantly more progressive than those of middle-aged or old patients (Figs. 4–6). This finding was consistent with the high recurrence rate of clinical PTC among young patients (17,24). However, the CSS of young patients has been reported to be excellent (17,24) and their incidence of short Tg-doubling time was described as very low (18). To date, none of the 1235 patients in our series who underwent observation, including the 191 who underwent surgery after observation, showed distant metastasis or died of PTC. Therefore, young patients with PTMC can also be candidates for observation, since it would not be too late for surgery if their subclinical PTMC turned into clinical disease.
In the present study, we found that only a small portion of the PTMCs showed progression and required surgery; the vast majority remained subclinical. Another very important finding is that the activity of PTMC progression was inversely related to patient age. Autopsy studies demonstrated that the incidence of latent carcinoma was lower in childhood than in adulthood (25), but did not increase with age in adulthood (7,25–27). These findings indicate that the majority of PTMCs arise while patients are young and lose their growth activity with age, staying small for the rest of the patient's life. If PTMCs arise at any stage of adulthood, the incidence of latent PTMC would increase with age in a linear fashion. We therefore speculate that only a small portion of PTMCs regain their growth activity with aging and become clinical PTC with a poor prognosis.
TSH suppression in addition to observation may be effective to prevent the progression of PTMC, because only 1 of 51 patients (2%), who underwent TSH suppression, showed size enlargement in comparison to 57 of 1184 patients (4.8%) without TSH suppression. None of the patients with TSH suppression showed novel appearance of nodal metastasis or developed clinical disease. However, in our series, only 4% of the patients underwent TSH suppression, and further prospective studies of large numbers of patients are therefore needed to draw any definitive conclusions.
Although larger tumor size (≥9 mm) was not an independent predictor of size enlargement or novel appearance of lymph-node metastasis, it was significantly related to progression to clinical disease in the entire patient series and the subsets of middle-aged and old patients (Table 9). This may be due to our definition of progression to clinical disease. Tumors that are large from the beginning naturally reach 12 mm more easily.
In the series of patients who underwent surgery after observation, 1 of the 93 patients (1.1%) who underwent limited thyroidectomy had a recurrence in the remnant thyroid. This is consistent with our previous finding that, in solitary T1N0M0 patients who underwent limited thyroidectomy, the recurrence rate for the remnant thyroid was 1% (28). The prognosis of solitary T1N0M0 patients was excellent (28), indicating that total thyroidectomy is not always necessary for PTMC patients who undergo surgery after observation, unless they became node-positive due to a novel appearance of lymph-node metastasis, or they have multiple carcinoma lesions, a positive family history, or other pathological lesions in the contralateral lobe. The present data also suggest that thyroid ablation may not be necessary in patients who underwent total thyroidectomy and central node dissection for PTMC.
We routinely perform prophylactic central node dissection for surgery for PTMC after observation. Central node metastasis is difficult to detect in preoperative imaging studies (29), and our previous study demonstrated that 9.8% of PTMC located in one lobe had node metastasis in the contralateral central compartment (30). The Guidelines by the Japanese Society of Thyroid Surgeons and the Japan Association of Endocrine Surgeons also recommend routine central node dissection to prevent reoperation for recurrence to this compartment that may induce severe complications such as recurrent laryngeal nerve injury and persistent hypoparathyroidism, although it is not evident that routine central compartment dissection improves the survival of patients (31).
In summary, we demonstrate that subclinical low-risk PTMC can be observed without immediate surgery, and especially, old patients with PTMC might be the best candidates for observation. Although PTMC in young patients may be more progressive than PTMC in older patients, it might not be too late to perform surgery after subclinical PTMC has progressed to clinical disease.
Author Disclosure Statement
All of the authors declare no conflicts of interest. No competing financial interests exist.
References
- 1.Ito Y, Miyauchi A.2009Prognostic factors and therapeutic strategies for differentiated carcinoma of the thyroid. Endocr J 56:177–192 [DOI] [PubMed] [Google Scholar]
- 2.Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A.2012Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung and bone: analysis of 5768 patients with average 10-year follow-up. World J Surg 36:1274–1278 [DOI] [PubMed] [Google Scholar]
- 3.Ito Y, Ichihara K, Masuoka H, Fukushima M, Inoue H, Kihara M, Tomoda C, Higashiyama T, Takamura Y, Kobayashi K, Miya A, Miyauchi A.2010Establishment of an intraoperative staging system (iStage) by improving UICC TNM classification system for papillary thyroid carcinoma. World J Surg 34:2570–2580 [DOI] [PubMed] [Google Scholar]
- 4.Ito Y, Hirokawa M, Jikuzono T, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Kuma K, Miyauchi A.2007Extranodal tumor extension to adjacent organs predicts a worse cause-specific survival of patients with papillary thyroid carcinoma. World J Surg 31:1194–1201 [DOI] [PubMed] [Google Scholar]
- 5.Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Takamura Y, Higashiyama T, Fukushima M, Kihara M, Kihara M, Inoue H, Tomoda C, Yabuta T, Masuoka H.2011Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid 21:707–716 [DOI] [PubMed] [Google Scholar]
- 6.Tsushima Y, Miyauchi A, Ito Y, Kudo T, Masuoka H, Yabuta T, Fukushima M, Kihara M, Higashiyama T, Takamura Y, Kobayashi K, Miya A, Kikumori T, Kimai T, Kikuchi T.2013Prognostic significance of changes in serum thyroglobulin antibody levels of pre- and post-total thyroidectomy in thyroglobulin antibody-positive papillary thyroid carcinoma patients. Endocr J 60:871–876 [DOI] [PubMed] [Google Scholar]
- 7.Harach HR, Franssila KO, Wasenlus VM.1985Occult papillary carcinoma of the thyroid: a “normal” finding in Finland. A systematic autopsy study. Cancer 56:531–538 [DOI] [PubMed] [Google Scholar]
- 8.Fukunaga FH, Yatani R.1975Geographic pathology of occult thyroid carcinomas. Cancer 36:1095–1099 [DOI] [PubMed] [Google Scholar]
- 9.Samson RJ.1977Prevalence and significant of occult thyroid cancer. In: DeGroot LJ. (ed) Radiation-Associated Thyroid Carcinoma. Grune & Stratton, New York, pp 137–153 [Google Scholar]
- 10.Thorvaldsson SE, Tulimius H, Bjornsson J, Bjamason O.1992Latent thyroid carcinoma in Iceland at autopsy. Pathol Res Pract 188:747–750 [DOI] [PubMed] [Google Scholar]
- 11.Takebe K, Date M, Yamamoto Y.1994. [Mass screening for thyroid cancer with ultrasonography]. KARKINOS 7:309–317 [Google Scholar]
- 12.Ito Y, Uruno R, Nakano K, Takamura Y, Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, Kuma S, Kuma K, Miyauchi A.2003An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 13:381–388 [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, Tomoda C, Takamura Y, Kobayashi K, Miya A.2010An observation trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 34:28–35 [DOI] [PubMed] [Google Scholar]
- 14.Sobin LH, Gospodarowicz M, Wittekind C, eds 2010UICC: TNM Classification of Malignant Tumors. Seventh edition. Wiley-Blackwell, New York [Google Scholar]
- 15.Cady B, Rosai R.1988An expanded view of risk group definition in differentiated thyroid carcinoma. Surgery 104:947–953 [PubMed] [Google Scholar]
- 16.Hay ID, Bergstrahl EJ, Goellner JR, Ebersold JR, Grant CS.1993Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1058 [PubMed] [Google Scholar]
- 17.Mazzaferri EL, Jhiang SM.1994Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 97:418–428 [DOI] [PubMed] [Google Scholar]
- 18.Miyauchi A, Kudo T, Kihara M, Higashiyama T, Ito Y, Kobayashi K, Miya A.2013Relationship of biochemically persistent disease and thyroglobulin-doubling time to age at surgery in patients with papillary thyroid carcinoma. Endocr J 60:415–421 [PubMed] [Google Scholar]
- 19.Uchino S, Noguchi S, Kawamoto H, Yamashita H, Watanabe S, Yamashita H, Shuto S.2002Familial nonmedullary thyroid carcinoma characterized by multifocality and a high recurrence rate in a large study population. World J Surg 26:897–902 [DOI] [PubMed] [Google Scholar]
- 20.Ito Y, Kakudo K, Hirokawa M, Fukushima M, Yabuta T, Tomoda C, Inoue H, Kihara M, Higashiyama T, Uruno T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A.2009Biological behaviour and prognosis of familial papillary thyroid carcinoma. Surgery 145:100–105 [DOI] [PubMed] [Google Scholar]
- 21.Uruno T, Miyauchi A, Shimizu K, Tomoda C, Takamura Y, Ito Y, Miya A, Kobayashi K, Matsuzuka F, Amino N, Kuma K.2005Usefulness of thyroglobulin measurement in fine-needle aspiration biopsy specimens for diagnosing cervical lymph node metastasis in patients with papillary thyroid cancer. World J Surg 29:483–485 [DOI] [PubMed] [Google Scholar]
- 22.Ito Y, Miyauchi A.2007A therapeutic strategy for incidentally detected papillary microcarcinoma of the thyroid. Nat Clin Pract Endocrinol Metab 3:240–248 [DOI] [PubMed] [Google Scholar]
- 23.Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y.2010Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: Our treatment strategies and outcomes. World J Surg 34:1222–1231 [DOI] [PubMed] [Google Scholar]
- 24.Ito Y, Miyauchi A, Kihara M, Takamura Y, Kobayashi K, Miya A.2012Relationship between prognosis of papillary thyroid carcinoma patient and age: a retrospective single-institution study. Endocr J 59:399–405 [DOI] [PubMed] [Google Scholar]
- 25.Franssila KO, Harach HR.1986Occult papillary carcinoma of the thyroid in children and young adults. A systemic autopsy study in Finland. Cancer 58:715–719 [DOI] [PubMed] [Google Scholar]
- 26.Lang W, Borrusch H, Bauer L.1988Occult carcinomas of the thyroid. Evaluation of 1020 sequential autopsies. Am J Clin Pathol 90:72–76 [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Tello FJ, Martinez-Cabruia R, Fernandez-Martin J, Lasso-Oria C, Ballestin-Carcavilla C.1993Occult carcinoma of the thyroid. A systematic study from Spain of two series performed with two different methods. Cancer 71:4022–4029 [DOI] [PubMed] [Google Scholar]
- 28.Ito Y, Masuoka H, Fukushima M, Inoue H, Kihara M, Tomoda C, Higashiyama T, Takamura Y, Kobayashi K, Miya A, Miyauchi A.2010Excellent prognosis of patients with solitary T1N0M0 papillary thyroid carcinoma who underwent thyroidectomy and elective lymph node dissection without radioiodine therapy. World J Surg 34:1285–1290 [DOI] [PubMed] [Google Scholar]
- 29.Ito Y, Miyauchi A.2009Prognostic factors and therapeutic strategies for differentiated carcinomas of the thyroid. Endocr J 56:177–192 [DOI] [PubMed] [Google Scholar]
- 30.Ito Y, Jikuzono T, Higashiyama T, Asahi S, Tomoda C, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Kuma K, Miyauchi A.2006Clinical significance of lymph node metastasis of the thyroid papillary carcinoma located in one lobe. World J Surg 30:1821–1828 [DOI] [PubMed] [Google Scholar]
- 31.Takami H, Ito Y, Okamoto T, Yoshida A.2011Therapeutic strategy for differentiated thyroid carcinoma in Japan based on a newly established guideline managed by Japanese Society of Thyroid Surgeons and Japanese Association of Endocrine Surgeons. World J Surg 35:111–121 [DOI] [PubMed] [Google Scholar]