Abstract
Background: Thyroid hormones are important determinants of energy expenditure, and in rodents, adipose tissue affects thyroid hormone homeostasis via leptin signaling. The relationship between thyroid hormones and nutritional status in humans has been assessed primarily in drastic dietary or bariatric surgery interventions, while limited information is available on serial assessment of this axis during moderate, prolonged dietary restriction.
Methods: To evaluate the effects of moderate dietary restriction on thyroid hormone homeostasis, 47 subjects with a body mass index (BMI) of 25–45 kg/m2 were enrolled in a longitudinal intervention study; 30 nonoverweight volunteers were also enrolled as controls. Overweight and obese subjects underwent a 12-month individualized dietary intervention aimed at achieving a 5–10% weight loss.
Results: The intervention resulted in a 6.3±0.9 kg (6.5±1.0%) weight loss. At baseline, thyrotropin (TSH) and T3 concentrations correlated significantly with fat mass (R=0.257, p=0.024 and R=0.318, p=0.005, respectively). After weight loss, T3 decreased significantly (from 112.7±3.1 to 101.8±2.6 ng/dL, p<0.001) in the absence of significant changes in TSH or free T4 (fT4). The decrease in serum T3 correlated with the decrease in weight (R=0.294, p<0.001). The T3:fT4 ratio decreased significantly (p=0.02) in individuals who lost >5% body weight.
Conclusions: T3 concentration closely correlates with individual nutritional status, and moderate weight loss results in a decrease in T3 with minimal changes in other thyroid hormone homeostasis parameters. The data suggest that a decrease in peripheral conversion of the prohormone T4 into its hormonally active metabolite T3 is at least in part responsible for the observed changes in thyroid hormone homeostasis.
Introduction
Obesity is the result of a sustained imbalance between energy intake and energy expenditure (EE) (1). This condition has reached epidemic proportions, and its health and economic consequences are enormous (2,3). The common first-line treatment modality is behavior modification, which includes dietary restriction and increased physical activity, aimed at generating a sustained negative energy balance. Most hypocaloric diets, irrespective of the macronutrient composition, are efficacious, but over time, the rate of weight loss tends to decrease and relapse is the rule rather than the exception (4–7). Various observations in experimental animal models and humans suggest that diet-induced weight loss in turn generates a series of compensatory mechanisms, including an increase in appetite and decrease in EE, which ultimately play a major role in preventing further weight loss and promote weight regain (8–10).
The thyroid hormone (TH) has a primary role in regulating total EE, particularly by stimulating resting EE (REE), and modulating adaptive thermogenesis and muscle metabolism (11–14). In response to peripheral and central stimuli, thyrotropin-releasing hormone (TRH) is released from the hypothalamus and stimulates the secretion of thyrotropin (TSH) in the pituitary, which in turn drives the secretion of T4 and T3 from the thyroid gland. The action of TH in the target tissues is the result of a multilevel control system that allows the precise time- and tissue-specific delivery of the hormonal signal via the interaction of T3, the active form of TH, with its nuclear receptor isoforms (15). The pool of T3 is the net result of its secretion from the thyroid gland, the peripheral conversion of the prohormone T4 into T3, and its degradation. Peripheral conversion of T4 into T3, achieved by the combined actions of the deiodinases type 1 and type 2 (DIO1 and DIO2) is responsible in humans for a substantial component of the circulating and tissue pool of T3, and plays a critical role in the pre-receptor modulation of the hormonal signal (16). In rodents, changes in energy stores are signaled to the TRH neurons in the hypothalamus via leptin (17), which acts as an important afferent signal in the peripheral control of thyroid function. Through this pathway, dietary restriction exerts an inhibitory role on the hypothalamic–pituitary–thyroid (HPT) axis, resulting in a net decrease in circulating TSH and TH, thus contributing to reduce the EE to compensate for the reduction in energy intake. It is not clear whether this pathway plays a relevant role in the maintenance of energy homeostasis in humans.
Given the key role of TH in EE regulation, substantial research efforts have been devoted to understanding HPT activity during weight loss. Most human data on the relationship between TH parameters and nutritional status are derived from drastic interventions such as bariatric surgery, very low calorie diets, or manipulation of macronutrient composition (18–23). Limited information is available on serial assessment of this axis and peripheral TH metabolism during moderate weight loss achieved by dietary restriction and behavior modification.
A better understanding of the interaction between moderate calorie restriction, weight loss, and TH homeostasis could produce valuable information on the adaptive (or maladaptive) response to dietary intervention, and provide an insight into more effective behavioral and pharmacologic interventions to achieve optimal weight loss and prevent weight gain recidivism. Here, we report the results of a study aimed at characterizing the response of the HPT axis and peripheral metabolism of TH to a moderate dietary weight-loss intervention.
Materials and Methods
Subjects study design
Data were collected from a single-center prospective cohort study of weight loss through behavioral interventions in overweight humans (ClinicalTrials.gov ID# NCT00344266). The study was approved by the NIDDK-NIAMS Institutional Review Board, and conducted at the National Institutes of Health Clinical Research Center in Bethesda, Maryland.
All volunteers provided written informed consent for participation in the study. Study participants with a body mass index (BMI) of 25–45 kg/m2 were enrolled in a weight-loss intervention (intervention group) with a maximum of six inpatient visits for comprehensive metabolic measurements. Volunteers with a BMI of 19–24.9 kg/m2 (nonoverweight group) were enrolled in a single inpatient visit to allow for the cross-sectional analysis of the relationship between thyroid hormone and metabolic parameters. Exclusion criteria, common to both groups, included a history of diabetes or fasting glucose >126 mg/dL, cardiovascular disease, hypertension, hypo- or hyperthyroidism, Cushing's Syndrome, pregnancy or use of hormonal contraceptives, and use of tobacco products.
Weight-loss intervention
Overweight and obese study participants underwent a 12-month weight-loss intervention. All study subjects received education/counseling by registered dietitians emphasizing lifestyle change through diet and behavioral modification. Individualized diets were planned to create a deficit of ∼500–1000 kcal/day, aiming to achieve a 5–10% weight loss over the 12-month study period (24). Subjects were encouraged to participate in regular physical activity, consisting of at least 150 minutes of moderate intensity aerobic physical activity each week (25). During each inpatient visit, a registered dietitian met with the study volunteers to review food logs, weight loss achieved, and to readjust the dietary intervention as needed. Individual diet-planning sessions during the initial two to four weeks were held to adjust or reinforce dietary plans. Weight-loss subjects also participated in education/support group sessions with the dietitians biweekly during the first three months and monthly for the remainder of the study.
Dietary analysis
Prior to each study visit, study participants were instructed to record three consecutive days (one weekend day and two weekdays) of dietary intake. Food records were then reviewed with the participant by the registered dietitians using three-dimensional food models and other visual aids to obtain as much detail as possible regarding the foods consumed. Food records were analyzed for energy and macronutrient content in Nutrition Data Systems for Research (NDSR, Minneapolis, MN).
Inpatient visit study procedures
Study participants were admitted to the NIH Clinical Center and, after an overnight fast, underwent the following procedures: blood sampling, anthropometrics, indirect calorimetry, dual-energy X-ray absorptiometry (DXA; Lunar iDXA; GE Healthcare, Madison, WI) scan, and (limited to the weight-loss intervention group) nutrition counseling with registered dietitians. Volunteers in the nonoverweight group underwent a single inpatient visit, while weight-loss group subjects came for follow-up visits at 1.5, 3, 6, 9, and 12 months.
Body-composition analysis
Body composition was measured by DXA. Scan analysis was performed using GE Encore v11.10 software.
Biochemical assays
All blood samples were drawn in the morning after a 12-hour overnight fast; serum and plasma aliquots were stored at −80°C. Routine urine analysis, chemistry and hormonal assays, and CBC with differential were performed in the Department of Laboratory Medicine of the NIH Clinical Center. TSH, free T4 (fT4), and T3 were analyzed by the Department of Laboratory Medicine by immunoassay on a Siemens Immulite 2500 analyzer platform. Reverse T3 (rT3) was measured with the use of a radioimmunoassay (ALPCO Diagnostics, Salem, NH) by the NIDDK Core Laboratory. Intra- and interassay variability was 6.5% and 7.6% respectively. T3:fT4 and T3:rT3 ratios were also assessed as indexes of peripheral conversion of TH (26).
Indirect calorimetry
After a 12-hour overnight fast, REE was measured at 7:00 a.m. by indirect calorimetry using the ventilated hood technique (ParvoMedics TrueOne 2400, Sandy, UT). Recordings were obtained for at least 30 minutes, of which the last 20 minutes were analyzed. REE data are expressed as kcal/24 h.
Statistical analysis
Statistical analysis was performed on data from participants in the intervention group that completed at least three admissions, and the nonoverweight group. Primary endpoints of this study were the serial changes in TH homeostasis parameters during weight loss and the cross-sectional relationship between TH axis parameters and nutritional status. A subgroup analysis was also performed on individuals who had lost ≥5% of their total body weight. Intention-to-treat analysis with the last measure carried forward was used for volunteers who withdrew from the study. Data were expressed as mean±SEM. Differences between groups and within the weight-loss intervention group were assessed by the Student's t-test and the Wilcoxon rank-sum test where appropriate. Linear regression analysis was used to analyze association between T3, TSH, fT4, fat mass, and REE. REE and T3 were also adjusted for changes in body composition (27). Longitudinal changes in thyroid hormone parameters as well as fat mass were analyzed among groups by repeated measures analysis of variance (ANOVA). All analyses were two-tailed, and an α error of 0.05 was considered the threshold for statistical significance. No correction for multiple comparisons was applied in the statistical analysis. All analyses were performed using SPSS v19 Statistics (IBM Corp., Armonk, NY) and Prism 5 (GraphPad, La Jolla, CA).
Results
Study participants
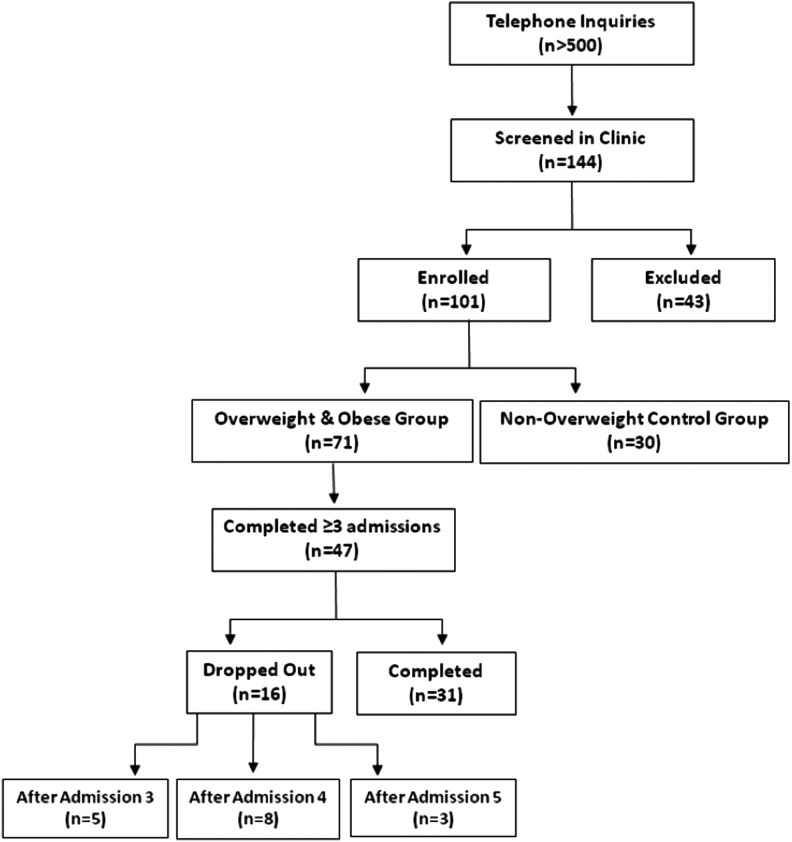
A total of 144 volunteers were screened for participation in the study, and 101 subjects (71 overweight and obese, and 30 nonoverweight controls) were enrolled between August 2007 and March 2011. In the weight-loss intervention group, 47 subjects completed three or more admissions, and 31 completed the entire study (Fig. 1) for an average (SD) follow-up of 9.2±3.8 months. Baseline characteristics of the study groups are shown in Table 1. At baseline, there were no significant differences between those who did and those who did not complete the study (data not shown). Of the 31 subjects completing the 12-month weight-loss intervention, 28 subjects returned their three-day food records for analysis at both baseline and the 12-month visit. Compared to baseline, after 12 months of dietary intervention, the daily energy intake decreased from 2239±703 to 1626±483 kcal (p<0.0001), while no significant changes were observed in macronutrient composition (carbohydrates 48.2±7.4% vs. 49.7±9.6%, p=0.432; protein 16.1±3.6% vs. 17.5±4.5%, p=0.112; fat 34.7±6.6% vs. 31.1±8.0%, p=0.062).
FIG. 1.
CONSORT chart. Forty-seven out of 71 overweight and obese subjects with a body mass index (BMI) at the point of enrollment of 25–45 kg/m2 completed at least three inpatient visits and were included in the analysis. Thirty subjects with a BMI of 19–24.9 kg/m2 underwent a single inpatient admission to allow for the cross-sectional analysis of anthropometric and thyroid hormone homeostasis parameters. The characteristics of the study participants are reported in Table 1.
Table 1.
Baseline Subject Population Characteristics
| |
|
Intervention groupa |
||
|---|---|---|---|---|
| Characteristic | Nonoverweight | Total | Completed the study | Did not complete the study |
|
N |
30 |
47 |
31 |
16 |
| Age (years) |
33.7±1.0 |
37.8±0.8 |
38.6±1.0 |
36.1±1.5 |
| Female, n (%) |
12 (40) |
27 (57.4) |
18 (58.1) |
9 (56.3) |
| Ethnicity, n (self-reported): | ||||
| African American |
8 |
15 |
11 |
4 |
| Caucasian |
18 |
21 |
14 |
7 |
| Hispanic |
3 |
8 |
4 |
4 |
| Asian |
1 |
2 |
1 |
1 |
| Other | 0 | 1 | 1 | 0 |
Data are expressed as mean±SEM.
Overweight and obese participants who completed at least three inpatient visits.
Anthropometrics
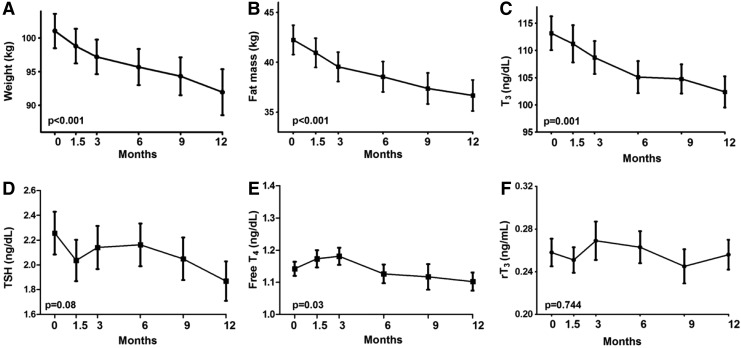
The intervention resulted in an average weight loss of 6.3±0.9 kg (6.5±1%) over a 12-month period (p<0.001; Fig. 2A). The weight loss was predominately due to a decrease in fat mass (5.1±0.8 kg; Fig. 2B). The summary of the data relative to the weight-loss intervention is reported in Table 2.
FIG. 2.
Longitudinal changes in anthropometric and thyroid hormone homeostasis parameters during the 12-month weight-loss intervention. During the weight-loss intervention (A and B), a significant decrease in serum T3 was observed (C). A trend toward a reduction in thyrotropin (TSH) and free T4 (fT4) was also observed (D and E). No significant difference was observed in the reverse T3 (rT3) serum concentration throughout the study. Repeated measures analysis of variance (ANOVA), data are reported as mean±SEM. Number of observations: time 0=47, 1.5=47, 3=47, 6=42, 9=34, 12=31.
Table 2.
Study Participants' Characteristics and Laboratory Data
| |
|
Intervention group |
|
|---|---|---|---|
| Parameter | Nonoverweight group | Baseline | 12 months |
| Weight (kg) |
65.7±2.1*** |
101.2±2.5 |
94.9±2.7### |
| BMI (kg/m2) |
22.2±0.4*** |
33.9±0.7 |
31.5±0.7### |
| Systolic blood pressure (mmHg) |
114.1±1.7 |
118.6±1.7 |
117.3±1.7 |
| Diastolic blood pressure (mmHg) |
68.7±1.2 |
69.0±1.1 |
68.5±1.2 |
| Fat mass (kg) |
15.1±1.07*** |
41.7±1.5 |
36.6±1.6### |
| TSH (ng/dL) |
1.8±0.16 |
2.2±0.2 |
1.9±0.2 |
| Free T4 (ng/dL) |
1.24±0.50** |
1.12±0.02 |
1.09±0.03 |
| Total T3 (ng/dL) |
100.6±3.1** |
112.7±3.1 |
101.8±2.6### |
| Reverse T3 (ng/mL) |
0.33±0.03* |
0.26±0.01 |
0.26±0.01 |
| Leptin (ng/dL) |
9.3±2.2*** |
39.1±3.6 |
34.5±3.4 |
| REE (kcal/24 h) | 1307.5±46.9*** | 1634.5±47.8 | 1612.9±43.1 |
Data are expressed as mean±SEM.
Comparison between control and intervention group at baseline: *p<0.05; **p<0.01; ***p<0.001.
Comparison between baseline and 12 months: #p≤0.05; ##p<0.01; ###p<0.001.
BMI, body mass index; TSH, thyrotropin, REE, resting energy expenditure.
Resting energy expenditure
At baseline, the intervention group had significantly higher REE compared with the nonoverweight group (1634.5±47.8 vs. 1307.5±46.9 kcal/24 h, p<0.001; Table 2), and not surprisingly, REE correlated significantly with fat-free mass at baseline and after intervention (p<0.001). REE also correlated significantly with fat mass at baseline (p=0.002, R=0.128) and after intervention (p=0.001, R=0.389) when the entire study population was analyzed as a whole, but the significance was lost when the correlation was corrected for fat-free mass (p=0.056). After weight loss, the decrease in measured REE was similar to the expected value after adjusting for changes in body composition (p=0.92).
Laboratory data
A summary of the laboratory data is presented in Table 2. At baseline, T3 was significantly higher in the intervention group compared to the nonoverweight group (112.7±3.1 vs. 100.6±3.1 ng/dL, p=0.003), while fT4 was lower (1.12±0.02 vs. 1.24±0.50 ng/dL, p=0.01). No significant differences in TSH were observed between the two groups. The intervention group had significantly lower rT3 compared to the nonoverweight group (0.26±0.01 vs. 0.33±0.03 ng/mL, p=0.02), and the T3:fT4 ratio was significantly higher in the intervention group compared to the nonoverweight group (101.6±2.7 vs. 83.8±3.6 ng/dL, p<0.001). At baseline, no significant difference was observed in the T3:rT3 ratio between the nonoverweight and intervention groups. As expected, the baseline leptin concentration was significantly higher in the intervention compared to the nonoverweight group (p<0.001).
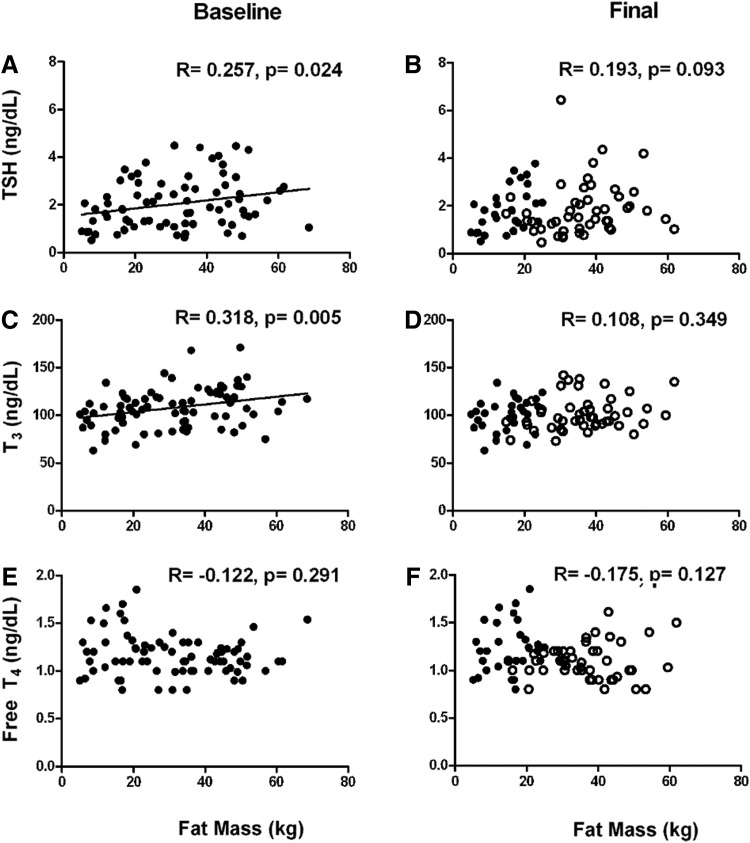
At baseline, TSH and T3 correlated with fat mass (R=0.257, p=0.024 and R=0.318, p=0.005, respectively) when the two groups were analyzed together (Fig. 3A and C); no correlation was observed between T3 or TSH and fat-free mass (data not shown). Furthermore, no significant correlation was observed between fT4 and fat mass (Fig. 3E). No significant correlation was observed between TSH, T3, fT4, and fat mass after the 12-month weight-loss intervention (Fig. 3B, D, and F).
FIG. 3.
Cross-sectional correlations between fat mass and thyroid hormone homeostasis parameters at baseline and after 12-month weight-loss intervention. The positive correlations between TSH (A) and T3 (C) were lost after a 12-month weight-loss intervention (B and D). No correlation was observed between fT4 and fat mass (E and F). Individuals who underwent the weight-loss intervention are represented by open circles. Data from the nonoverweight group (●) were included in both the baseline and final correlations (see text for details).
Compared to baseline, after the 12-month weight-loss intervention, T3 decreased significantly (from 112.7±3.1 to 101.8±2.6 ng/dL, p<0.001) in the absence of significant changes in TSH or fT4. After correcting T3 for changes in fat mass, the decrease from baseline remained statistically significant (p<0.001). As expected, when compared to noncompleters, study volunteers who completed the 12-month weight-loss intervention had lower indices of adiposity. The final T3 level was marginally lower in completers, while the rT3 level was minimally but significantly elevated. No significant differences were observed in the other laboratory or anthropometric parameters (Table 3).
Table 3.
Intervention Group Anthropometrics and Laboratory Data at Last Observation
| Parameter | Completers (n=31) | Noncompleters (n=16) |
|---|---|---|
| Weight (kg) |
90.5±3.0 |
103.4±5.1* |
| BMI (kg/m2) |
30.5±0.8 |
33.4±1.2* |
| Systolic blood pressure (mmHg) |
117.0±2.2 |
117.5±10.6 |
| Diastolic blood pressure (mmHg) |
69.7±1.8 |
66.2±4.6 |
| Fat mass (kg) |
33.7±1.8 |
42.1±2.4** |
| TSH (ng/dL) |
1.72±0.18 |
2.17±0.34 |
| Free T4 (ng/dL) |
1.09±0.03 |
1.09±0.05 |
| Total T3 (ng/dL) |
99.3±3.0 |
106.8±4.7 |
| Reverse T3 (ng/mL) |
0.28±0.02 |
0.21±0.02* |
| Leptin (ng/dL) |
33.7±4.1 |
35.7±6.7 |
| REE (kcal/24 h) | 1565.8±43.2 | 1701±94.9 |
Data are expressed as mean±SEM.
p<0.05; **p<0.01.
Longitudinally, TSH displayed a nonsignificant reduction (p=0.08), fT4 showed a small but significant reduction (p=0.03; Fig. 2D and E), and T3 had a more robust reduction (p=0.001; Fig. 2C). The decrease in serum T3 correlated with the decrease in weight (R=0.294, p<0.001). No significant correlation was observed between differences in T3 and REE (R=0.163, p=0.272), or between differences in leptin and T3 (R=0.134, p=0.369). In addition, the ratio of T3:fT4 decreased significantly between baseline and the final admission (100.4±4.1 vs. 90.6±4.0, p=0.02) in subjects that lost >5% of their body weight. No significant changes in rT3 were observed over the course of the study (p=0.744; Fig. 2F).
Discussion
In this study, we characterized the changes in thyroid homeostasis parameters during a controlled weight-loss intervention to explore whether a moderate decrease in fat mass is sufficient to induce a response in this hormonal axis and in the peripheral metabolism of TH.
TH action plays a pervasive role in the regulation of energy expenditure by its direct stimulation of REE and cold-induced thermogenesis (28), and the modulation of the metabolism of skeletal muscle, myocardium, and liver synthetic function (29–31). On the other hand, the HPT axis is directly affected by drastic changes in energy stores. Hence, TH concentration reflects, to a certain degree, the overall energy status of the organism. Our cross-sectional data support this hypothesis, since T3 and TSH correlate positively with adiposity (32–34), but it is worth noting that these findings have not been consistently replicated (35). Similar to others' observations, we found no correlation between fT4 and fat mass (36), although consistent with the observations of others (35,37), we noticed lower fT4 levels in the overweight group. On the other hand, our moderate weight-loss intervention resulted in a significant decrease in circulating T3, and only a marginal decrease in TSH and in fT4. Collectively, these observations indicate that even a moderate weight-loss intervention generates a perturbation in this axis. TH homeostasis did not reach a new equilibrium after a 12-month weight-loss intervention as indicated by the lack of correlation of TSH and T3 with fat mass at the end of the study period. This is not surprising since by the end of the study period many volunteers were still losing weight, and recent observations indicate the retention of hormonal changes for a prolonged period after weight stabilization (38).
In rodents, leptin plays a pivotal role in modulating energy expenditure, and stimulates the secretion of TRH in the paraventricular nucleus of the hypothalamus. This ultimately generates an increase in circulating concentrations of TH (39) by stimulating the HPT axis. Conversely, a reduction in fat mass results in a decrease in circulating leptin, ultimately inhibiting the HPT axis (39,40). Our data indicate that this pathway is not sufficient to explain the changes we observed, since the T3 concentration was primarily affected, and only a marginal decrease in fT4 and TSH was observed. Furthermore, when the final T3 concentration was corrected for changes in body composition, the values remained significantly lower than baseline, indicating that other mechanisms are at least in part responsible for the observed effects. Conversely, if the leptin-mediated inhibition of the HPT axis was the primary effecter, one would expect a net decrease in TSH and a concomitant reduction of both T3 and fT4. In keeping with our hypothesis, the correlation observed at baseline between T3 and leptin was lost at the end of the weight-loss intervention. The inclusion of leptin as a covariate did not affect the correlation between T3 and fat mass after weight loss. Moreover, the lack of a significant correlation between differences in leptin and T3 further supports this interpretation of the data.
A relative inhibition of peripheral 5′-deiodination of the prohormone T4 into T3 is likely responsible for some of the changes in the thyroid homeostasis, as indicated by the significant decrease in the T3:fT4 ratio, a sensitive index of conversion of TH (26) in the individuals who had a greater degree of weight loss, and the marginal increase in rT3 in the subgroup that completed the 12-month intervention. It is also important to note that, contrary to rodents, the pattern of secretion of the thyroid gland in humans is skewed toward the production of T4, and the peripheral conversion of this prohormone into its hormonally active metabolite plays a major role in determining the serum concentration of T3 (41,42). Drastic weight loss, and acute (23) and chronic (43) starvation, as seen with previously reported data, result in significant changes in TH homeostasis (17,44) consistent with a state of nonthyroidal illness commonly observed during cachectic states characterized by the central suppression of the HPT axis and an inhibition of the peripheral conversion of T4 into T3 (45). Our study does not fall into this category, since the weight-loss intervention was deliberately moderate and achieved during a relatively long period of time. The lack of significant changes throughout the study in rT3, the inactive metabolite resulting from the inner-ring deiodination of T4, is in line with this interpretation of the data. Previous drastic dietary manipulation experiments suggested that the carbohydrate content of the diet plays an important role in the activation of the outer ring deiodination, and that selective carbohydrate deficiency results in inhibition of the outer-ring deiodination of T4 (23). Our data do not support this hypothesis, since moderate weight loss was achieved without a significant change in the macronutrient composition of the diet.
Although TH plays a prominent role in regulating EE, we did not observe a correlation between T3 and REE. This is probably secondary to the small number of study participants and to the relative large variance in the measure. Furthermore, the ventilated hood technique cannot capture other important components of total EE such as thermic effect of food, spontaneous physical activity, and fidgeting, all affected by TH action.
It is important to note that the correlations between TH homeostasis and anthropometric and physiology parameters, although significant, were indeed small. Moreover, these findings were obtained in a relatively small group of individuals. Taken together, these findings indicate that TH homeostasis is at least modulated by the energy status of the individual. Conversely, the limited change in T3 levels does not warrant the supplementation with exogenous TH formulations during weight-loss interventions outside the confines of experimental settings.
The type of intervention was aimed by design to achieve a moderate weight loss, that is, the type of weight loss commonly achievable in the community setting. Hence, the results are applicable to the general population. The major strength of this study is represented by the strict a priori selection of volunteers devoid of comorbid conditions and of drug interference, which allowed a precise measurement of the physiologic and laboratory parameters, thus increasing the internal validity of the study. Furthermore, the multiple inpatient visits allowed assessment of trends over time across the studied variables in the intervention group. Finally, the inclusion of a nonoverweight group allowed the cross-sectional correlation of TH, metabolic parameters, and fat mass across a wide range of fat mass. Fat mass, rather than weight or BMI, was used as the dependent variable to increase the sensitivity of the analysis.
Major limitations of this study are represented by the relatively small number of volunteers and the attrition rate during the dietary intervention. These factors are balanced by the precision of the measures obtained in standardized conditions after an overnight stay in the Clinical Center. Furthermore, the study design allowed the use of data obtained from subjects who failed to complete the entire study. The relative imbalance in the sex distribution between the nonoverweight group and intervention group does not appear to have influenced the results, since no sex difference was observed in the TH homeostasis parameters. To avoid selection bias, the conservative intention to treat approach in the statistical analysis was used. The nonoverweight group was assessed only once and not followed longitudinally. Thus, the interpretation of the cross-sectional correlations observed at the end of the study are speculative. On the other hand, this factor is mitigated by the young age of our cohort, the lack of comorbid conditions, and the fact that the control group was weight stable at the point of enrollment. As a result, one can expect little variability in the parameters of TH homeostasis over a 12-month period (46).
In conclusion, the results of our study indicate that serum T3 concentration closely correlates with the individual nutritional status, and a moderate weight loss achieved by caloric restriction results in a decrease in T3 with minimal changes in other thyroid hormone homeostasis parameters. The data suggest that the effects of leptin on the HPT axis do not fully explain the changes in thyroid hormone homeostasis observed during weight loss and that a decrease in peripheral conversion of the prohormone T4 into its hormonally active metabolite T3 is at least in part responsible of the observed changes in TH.
Acknowledgments
The authors gratefully acknowledge the help and professionalism of the nursing, Nutrition Department, laboratory, and ancillary personnel of the NIH Metabolic Unit. The technical help of Mary Walter and Xiongce Zhao and the constructive criticism of Paul Lee are gratefully acknowledged. This research could not have been accomplished without the selfless participation of the study volunteers. This work was supported by the Intramural Research Program of the NIDDK program Z01-DK047057-02, and the Clinical Center, NIH.
Author Disclosure Statement
The authors have no actual or potential conflicts of interest to disclose in connection with this manuscript.
References
- 1.World Health Organization 2013 Obesity and overweight Available at: www.who.int/mediacentre/factsheets/fs311/en/index.html (accessed December2013)
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR.2010Prevalence and trends in obesity among U.S. adults, 1999–2008. JAMA 303:235–241 [DOI] [PubMed] [Google Scholar]
- 3.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M.2011Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378:815–825 [DOI] [PubMed] [Google Scholar]
- 4.Kraschnewski JL, Boan J, Esposito J, Sherwood NE, Lehman EB, Kephart DK, Sciamanna CN.2010Long-term weight loss maintenance in the United States. Int J Obes (Lond) 34:1644–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK.2007Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med 33:34–40 [DOI] [PubMed] [Google Scholar]
- 6.Wing RR, Phelan S.2005Long-term weight loss maintenance. Am J Clin Nutr 82:222S–225S [DOI] [PubMed] [Google Scholar]
- 7.Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE.2009Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs 24:58–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leibel RL.1990Is obesity due to a heritable difference in “set point” for adiposity? West J Med 153:429–431 [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon B, Nedergaard J.2009Thermogenesis challenges the adipostat hypothesis for body-weight control. Proc Nutr Soc 68:401–407 [DOI] [PubMed] [Google Scholar]
- 10.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA.2011Quantification of the effect of energy imbalance on bodyweight. Lancet 378:826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danforth E, Jr, Burger A.1984The role of thyroid hormones in the control of energy expenditure. Clin Endocrinol Metab 13:581–595 [DOI] [PubMed] [Google Scholar]
- 12.Onur S, Haas V, Bosy-Westphal A, Hauer M, Paul T, Nutzinger D, Klein H, Muller MJ.2005l-tri-iodothyronine is a major determinant of resting energy expenditure in underweight patients with anorexia nervosa and during weight gain. Eur J Endocrinol 152:179–184 [DOI] [PubMed] [Google Scholar]
- 13.Pucci E, Chiovato L, Pinchera A.2000Thyroid and lipid metabolism. Int J Obes Relat Metab Disord 24:S109–S112 [DOI] [PubMed] [Google Scholar]
- 14.Silva JE.2006Thermogenic mechanisms and their hormonal regulation. Physiol Rev 86:435–464 [DOI] [PubMed] [Google Scholar]
- 15.Braverman LUR 2004 Werner & Ingbar's The Thyroid—A Fundamental and Clinical Text Ninth edition. Lippincott Williams & Wilkins, New York [Google Scholar]
- 16.Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeold A, Bianco AC.2008Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blake NG, Eckland DJ, Foster OJ, Lightman SL.1991Inhibition of hypothalamic thyrotropin-releasing hormone messenger ribonucleic acid during food deprivation. Endocrinology 129:2714–2718 [DOI] [PubMed] [Google Scholar]
- 18.Vagenakis AG, Burger A, Portnary GI, Rudolph M, O'Brian JR, Azizi F, Arky RA, Nicod P, Ingbar SH, Braverman LE.1975Diversion of peripheral thyroxine metabolism from activating to inactivating pathways during complete fasting. J Clin Endocrinol Metab 41:191–194 [DOI] [PubMed] [Google Scholar]
- 19.Kiortsis DN, Durack I, Turpin G.1999Effects of a low-calorie diet on resting metabolic rate and serum tri-iodothyronine levels in obese children. Eur J Pediatr 158:446–450 [DOI] [PubMed] [Google Scholar]
- 20.Wimpfheimer C, Saville E, Voirol MJ, Danforth E, Jr, Burger AG.1979Starvation-induced decreased sensitivity of resting metabolic rate to triiodothyronine. Science 205:1272–1273 [DOI] [PubMed] [Google Scholar]
- 21.Chikunguwo S, Brethauer S, Nirujogi V, Pitt T, Udomsawaengsup S, Chand B, Schauer P.2007Influence of obesity and surgical weight loss on thyroid hormone levels. Surg Obes Relat Dis 3:631–635; discussion 635–636 [DOI] [PubMed] [Google Scholar]
- 22.Dall'Asta C, Paganelli M, Morabito A, Vedani P, Barbieri M, Paolisso G, Folli F, Pontiroli AE.2010Weight loss through gastric banding: effects on TSH and thyroid hormones in obese subjects with normal thyroid function. Obesity (Silver Spring) 18:854–857 [DOI] [PubMed] [Google Scholar]
- 23.Danforth E, Jr, Horton ES, O'Connell M, Sims EA, Burger AG, Ingbar SH, Braverman L, Vagenakis AG.1979Dietary-induced alterations in thyroid hormone metabolism during overnutrition. J Clin Invest 64:1336–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Heart, Lung, and Blood Institute 2005 Aim for a healthy weight (NIH Publication No. 05-5213) Department of Health and Human Services, Bethesda, MD [Google Scholar]
- 25.US Department of Health & Human Services 2008 Physical Activity Guidelines for Americans Available at: www.health.gov/paguidelines/default.aspx (accessed July, 2012)
- 26.Peeters RP, van der Deure WM, Visser TJ.2006Genetic variation in thyroid hormone pathway genes; polymorphisms in the TSH receptor and the iodothyronine deiodinases. Eur J Endocrinol 155:655–662 [DOI] [PubMed] [Google Scholar]
- 27.Muller MJ, Bosy-Westphal A, Later W, Haas V, Heller M.2009Functional body composition: insights into the regulation of energy metabolism and some clinical applications. Eur J Clin Nutr 63:1045–1056 [DOI] [PubMed] [Google Scholar]
- 28.Celi FS.2009Brown adipose tissue—when it pays to be inefficient. N Engl J Med 360:1553–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson WO, Thompson PK, Brailey AG, Cohen AC.1929The calorigenetic action of thyroxin at different levels of basal metabolism in myxedema. J Clin Invest 7:437–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianco AC, Sheng XY, Silva JE.1988Triiodothyronine amplifies norepinephrine stimulation of uncoupling protein gene transcription by a mechanism not requiring protein synthesis. J Biol Chem 263:18168–18175 [PubMed] [Google Scholar]
- 31.Freake HC, Oppenheimer JH.1995Thermogenesis and thyroid function. Annu Rev Nutr 15:263–291 [DOI] [PubMed] [Google Scholar]
- 32.Michalaki MA, Vagenakis AG, Leonardou AS, Argentou MN, Habeos IG, Makri MG, Psyrogiannis AI, Kalfarentzos FE, Kyriazopoulou VE.2006Thyroid function in humans with morbid obesity. Thyroid 16:73–78 [DOI] [PubMed] [Google Scholar]
- 33.Sari R, Balci MK, Altunbas H, Karayalcin U.2003The effect of body weight and weight loss on thyroid volume and function in obese women. Clin Endocrinol (Oxf) 59:258–262 [DOI] [PubMed] [Google Scholar]
- 34.Bray GA, Fisher DA, Chopra IJ.1976Relation of thyroid hormones to body-weight. Lancet 1:1206–1208 [DOI] [PubMed] [Google Scholar]
- 35.Knudsen N, Laurberg P, Rasmussen LB, Bulow I, Perrild H, Ovesen L, Jorgensen T.2005Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab 90:4019–4024 [DOI] [PubMed] [Google Scholar]
- 36.Manji N, Boelaert K, Sheppard MC, Holder RL, Gough SC, Franklyn JA.2006Lack of association between serum TSH or free T4 and body mass index in euthyroid subjects. Clin Endocrinol (Oxf) 64:125–128 [DOI] [PubMed] [Google Scholar]
- 37.Makepeace AE, Bremner AP, O'Leary P, Leedman PJ, Feddema P, Michelangeli V, Walsh JP.2008Significant inverse relationship between serum free T4 concentration and body mass index in euthyroid subjects: differences between smokers and nonsmokers. Clin Endocrinol (Oxf) 69:648–652 [DOI] [PubMed] [Google Scholar]
- 38.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J.Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 365:1597–1604 [DOI] [PubMed] [Google Scholar]
- 39.Friedman JM, Halaas JL.1998Leptin and the regulation of body weight in mammals. Nature 395:763–770 [DOI] [PubMed] [Google Scholar]
- 40.Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL.1997Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab 82:3647–3654 [DOI] [PubMed] [Google Scholar]
- 41.Chanoine JP, Braverman LE, Farwell AP, Safran M, Alex S, Dubord S, Leonard JL.1993The thyroid gland is a major source of circulating T3 in the rat. J Clin Invest 91:2709–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR.2005Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest 115:2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontana L, Klein S, Holloszy JO, Premachandra BN.2006Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab 91:3232–3235 [DOI] [PubMed] [Google Scholar]
- 44.Suda AK, Pittman CS, Shimizu T, Chambers JB., Jr1978The production and metabolism of 3,5,3′-triiodothyronine and 3,3′,5-triiodothyronine in normal and fasting subjects. J Clin Endocrinol Metab 47:1311–1319 [DOI] [PubMed] [Google Scholar]
- 45.Adler SM, Wartofsky L.2007The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am 36:657–672, vi [DOI] [PubMed] [Google Scholar]
- 46.Andersen S, Pedersen KM, Bruun NH, Laurberg P.2002Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab 87:1068–1072 [DOI] [PubMed] [Google Scholar]