Abstract
Background: According to Rundle's curve, Graves' ophthalmopathy (GO) worsens during an initial phase up to a peak of maximum severity, then improves and reaches a static plateau, with the activity curve preceding the severity curve by a few months. To our knowledge, no studies have tried to replicate Rundle's curve, and very few have investigated the natural history of GO. Here, we studied GO natural history retrospectively and tried to identify factors that may affect it.
Methods: A total of 65 patients with untreated GO underwent an eye assessment after a median of seven months after the appearance of GO and then after a median of 40 months. The primary endpoints were the variation of the single GO features and of the NOSPECS score, as well as the overall outcome of GO. The secondary endpoint was the influence of several variables (age, sex, smoking, GO and thyroid disease duration, thyroid treatment, thyroid status, thyroid volume, anti-TSH receptor autoantibodies) on the outcome of GO.
Results: The majority of patients had mild, minimally active GO, and only five had a Clinical Activity Score (CAS) >3. There was a significant reduction of CAS (p<0.0001) and NOSPECS (p=0.01) between the first and last observation, with a timing pattern resembling Rundle's curve. This difference was confirmed even when patients with a CAS >3 at first observation were excluded. At the last observation, 50.8% of patients had improved, 33.8% had remained stable, and 15.4% had worsened moderately or substantially. The overall outcome of GO was not affected by any of the variables under examination.
Conclusions: In confirmation of Rundle's observations, untreated GO improves spontaneously with time in the majority of patients, with an activity peak between 13 and 24 months, which may have implications in determining the proper timing of GO treatments.
Introduction
Therapies are aimed at modifying the spontaneous course of diseases, which implies the understanding and the knowledge of the natural history of a given pathological condition. In the case of Graves' ophthalmopathy (GO), this is particularly important, as timing of anti-inflammatory/immunosuppressive and then surgical treatments is considered fundamental to achieve the best possible outcome (1–6). Thus, it is generally believed that anti-inflammatory/immunosuppressive treatments should be given during the active, florid phase of the syndrome, but surgical procedures should be performed once GO is inactive (1–6). Nevertheless, most of the knowledge of GO natural history is anecdotal, and to our knowledge, very few studies on untreated patients are available.
The so-called Rundle's curve has been used for many years as a paradigm to describe the natural history of GO (7–9). The curve is based on two observational studies, one performed in 1945 and one in 1957 (10,11), both with a low number of patients. According to Rundle's curve, GO signs and symptoms worsen rapidly during an initial phase, up to a peak of maximum severity, and then improve and finally reach a static plateau without, however, resolving into a normal condition (7–11). The common interpretation is that the curve of GO activity, namely of GO inflammatory signs and symptoms, is slightly separated from the curve of GO severity, usually evaluated based on degree of proptosis, eyelid aperture, diplopia, and visual acuity (7–9). According to this model, the activity peak would precede the severity peak by a few months (7–9).
To our knowledge, no studies have tried to replicate Rundle's curve, and the very few available studies that have investigated the natural history of GO are mainly comparisons between GO signs and symptoms at the first and last patient observations (12–17). Here, we studied GO natural history retrospectively in a relatively large series of untreated patients followed up for various periods of time, from a few months up to several years, and tried to identify the factors that may affect GO natural history.
Patients and Methods
Study design and patients
A retrospective cohort study was conducted in order to investigate the natural history of GO in patients who met the following inclusion criteria: (i) GO signs or symptoms at our first observation for no longer than 24 months; (ii) no treatments for GO before first observation, as well as between first and last observation, with the exception of eye lubricants; (iii) no treatments for Graves' hyperthyroidism before first observation, with the exception of antithyroid drugs (methimazole [MMI] in all cases).
A total of 65 patients meeting the above-mentioned criteria were identified out of 740 consecutive patients who came to our GO clinic for a follow-up visit between September 2010 and December 2012. Demographical data on these patients are reported in Table 1. Signed informed consent was obtained from all patients prior to using their data.
Table 1.
Features of 65 Patients with Graves' Ophthalmopathy at First and Last Observations
| Feature | First observation | Last observation |
|---|---|---|
| Age |
42.4±12.6 years (range: 13–83) |
46.3±15.3 years (range: 16–83) |
| Sex |
Males: 19; Females: 46 |
n/a |
| Smoking Habits |
Non-smokers: 26 |
Non-smokers: 26 |
| |
Ex-smokers: 9 |
Ex-smokers: 16 |
| |
Current smokers: 30 |
Current smokers: 23 |
| |
Pack Years: 0.45 (IQR: 0–8.4; range: 0–70) |
Pack Years: 0.6 (IQR: 0–9.5; range: 0–80) |
| GO duration (time since GO appearance) |
7 months (IQR: 2–14; range: 1–24) |
40 months (IQR: 18.7–75.5; range: 3–258) |
| TD duration (time since diagnosis) |
11 months (IQR: 6–26.5; range: 1–24) |
50 months (IQR: 21.7–95.5; range: 3–279) |
| Follow-up |
31 months (IQR: 10.7–67.7; range: 1–203) |
n/a |
| Thyroid diagnosis |
Graves' disease: 56 |
|
| |
Autoimmune thyroiditis: 6 |
n/a |
| |
Euthyroid GO: 3 |
|
| Thyroid treatment |
None: 16 |
None: 9 |
| |
MMI: 44 |
MMI: 12 |
| |
LT4: 5 |
RI and then LT4: 11 |
| |
|
TX and then LT4: 28 |
| |
|
Only LT4: 5 |
| Thyroid status |
Hyperthyroid: 13 |
Hyperthyroid: 0 |
| |
Untreated: 12 |
Euthyroid: 63 |
| |
On MMI: 1 |
Untreated: 4 |
| |
Euthyroid: 50 |
On MMI: 11 |
| |
Untreated: 4 |
After MMI: 5 |
| |
On MMI: 41 |
On LT4: 43 |
| |
On LT4 5 |
Hypothyroid: 2 |
| |
Hypothyroid: 2 |
On MMI: 1 |
| |
On MMI: 2 |
On LT4: 1 |
| Thyroid volume |
25 mL (IQR: 12–33; range: 1–224) |
n/a |
| TRAb | 6.6 U/L (IQR: 1.8–19.5; range: 0–285) | 2.2 U/L (IQR: 0–7.6; range: 0–79) |
Numerical values are reported as median, with the exception of age (mean±SD).
GO, Graves' ophthalmopathy; n/a, not applicable; IQR, interquartile range; TD, thyroid disease; MMI, methimazole; RI, radioiodine; TX, thyroidectomy; TRAb, anti-TSH receptor autoantibodies.
Clinical and serological evaluation
All patients underwent an ophthalmological assessment at first and last observation, which included: (i) exophthalmometry; (ii) measurement of eyelid aperture; (iii) evaluation of the Clinical Activity Score (CAS) according to Mourits (18,19); (iv) assessment of diplopia; (v) assessment of corneal status; (vi) examination of fundi; and (vii) measurement of visual acuity. The overall degree of GO was evaluated using the NOSPECS score (18,20).* Serum FT3 and thyrotropin (TSH) were measured in all patients at first and last observation. Serum anti-TSH receptor antibodies (TRAb; Brahms, Berlin, Germany) were available in 62 patients at first observation and in 49 patients at last observation. Thyroid ultrasound was performed in all patients at first observation. Thyroid volume was calculated using the ellipsoid formula, as reported previously (21). Smoking habits were recorded in all patients. The general and thyroid features of patients are summarized in Table 1.
Endpoints
The primary endpoints of the study were: (i) the variation of the single GO features between first and last observation, (ii) the variation of the NOSPECS score, and (iii) the overall GO outcome at last observation. The latter was evaluated as reported previously (16). Briefly, GO was considered improved when at least one of the following criteria was fulfilled in one eye, without worsening in either eye (i) reduction in proptosis ≥2 mm; (ii) reduction of CAS ≥1/7 points; (iii) reduction in eyelid aperture ≥2 mm; and (iv) disappearance or improvement (change of degree from constant to inconstant or intermittent, or from inconstant to intermittent) of diplopia. GO was considered worsened when at least one of the following criteria was fulfilled: (i) increase in any of the NOSPECS classes; (ii) appearance of a new NOSPECS class; and (iii) decrease of visual acuity.
The secondary endpoint was the outcome of GO according to the following variables: (i) age; (ii) sex; (iii) smoking habits; (iv) GO duration (time since the appearance of GO symptoms) at last observation; (v) thyroid disease duration (time since diagnosis) at last observation; (vi) thyroid treatment; (vii) thyroid status; (viii) thyroid volume at first observation; and (ix) serum TRAb.
Data presentation and statistical analyses
Descriptive data are presented as median and interquartile range, unless otherwise specified. When appropriate and as indicated, the following tests were performed: (i) analysis of variance (ANOVA), (ii) paired sign, (iii) Kruskal–Wallis, and (iv) Fisher's exact test.
Results
Features of patients
The general and thyroid features of patients are reported in Table 1. The features of GO at first and last observation are reported in Table 2. Clearly, the majority of patients had mild, minimally active GO, which was basically why they did not undergo any major GO treatments. Thus, only five patients had a CAS >3. The median duration of GO at first observation was seven months, ranging up to 24 months. Nevertheless, the majority (45) of patients had GO lasting ≤12 months, whereas only 20 had GO for more than 12 months. The median duration of GO at last observation ranged from 3 to 258 months. Such a wide range was included in order to encompass the largest possible period of time. Only a few patients (10) had GO duration at last observation of ≤12 months. As reported in Table 2, some of the patients were treated with thyroidectomy and radioiodine between the first and last observation. In terms of GO features, including activity, there were no significant differences between patients treated with thyroidectomy and patients treated with radioiodine at the time these treatments were given (not shown), which occurred after a median time of three months after first observation. Likewise, at first observation, these two groups of patients did not differ from those treated with MMI (not shown).
Table 2.
Features and Outcome of Graves' Ophthalmopathy in 65 Untreated Patients at First and Last Observations
| Feature | First observation | Last observation | p |
|---|---|---|---|
| Exophthalmometry (most prominent eye) |
20 mm (IQR: 18–22; range: 17–24) |
20 mm (IQR: 19–22; range: 17–24) |
0.62 |
| Eyelid aperture (most affected eye) |
11 mm (IQR: 10.2–13; range: 7–15) |
11 mm (IQR: 10–13; range: 8–15) |
0.10 |
| CAS |
2 (IQR: 1–3; range: 0–6) |
1 (IQR: 0–2; range: 0–5) |
<0.0001 |
| Diplopia |
Absent: 49 |
Absent: 54 |
0.21 |
| |
Intermittent: 5 |
Intermittent: 3 |
|
| |
Inconstant: 11 |
Inconstant: 6 |
|
| |
Constant: 0 |
Constant: 0 |
|
| Visual acuity (most affected eye) |
9.9±0.1/10 (range: 9–10) |
9.9±0.2/10 (range: 9–10) |
0.08 |
| NOSPECS score |
2 (IQR: 1–3; range: 1–4) |
1 (IQR: 1–3; range: 1–4) |
0.01 |
| GO outcome |
Improved: 33 (50.8%) |
n/a |
|
| |
Stable: 22 (33.8%) |
||
| Worsened: 10 (15.4%) | |||
Numerical values are reported as median, with the exception of visual acuity (mean±SD). p Values were obtained by paired sign, with the exception of visual acuity (paired t-test) and diplopia (Fisher's exact test). Significant p values are presented in boldface.
CAS, Clinical Activity Score.
Behavior of GO over time
We examined the variation of the single GO features and of NOSPECS between first and last observation. As reported in Table 2, there was a significant reduction of CAS and NOSPECS, which was confirmed even when the five patients with a CAS >3 were excluded (CAS: p=0.0002; NOSPECS: p=0.04, both by paired sign). Likewise, the reduction of CAS and NOSPECS were confirmed when we considered separately the 45 patients with a GO duration at first observation ≤12 months (CAS: p=0.003; NOSPECS: p=0.03, both by paired sign) and, limited to CAS, also when we considered only the 20 patients with a GO duration at first observation >12 months (CAS: p=0.003; NOSPECS: p=0.45, both by paired sign). Exophthalmometry, eyelid aperture, diplopia, and visual acuity did not change significantly (Table 2), which was the case even when the five patients with a CAS >3 were excluded or when we considered separately patients with GO duration at first observation of ≤12 months or >12 months.
We then analyzed the overall GO outcomes at last observation, according to the criteria reported above. As reported in Table 2, compared with the first observation, at last observation 50.8% of patients had improved, 33.8% had remained stable, and 15.4% had worsened.
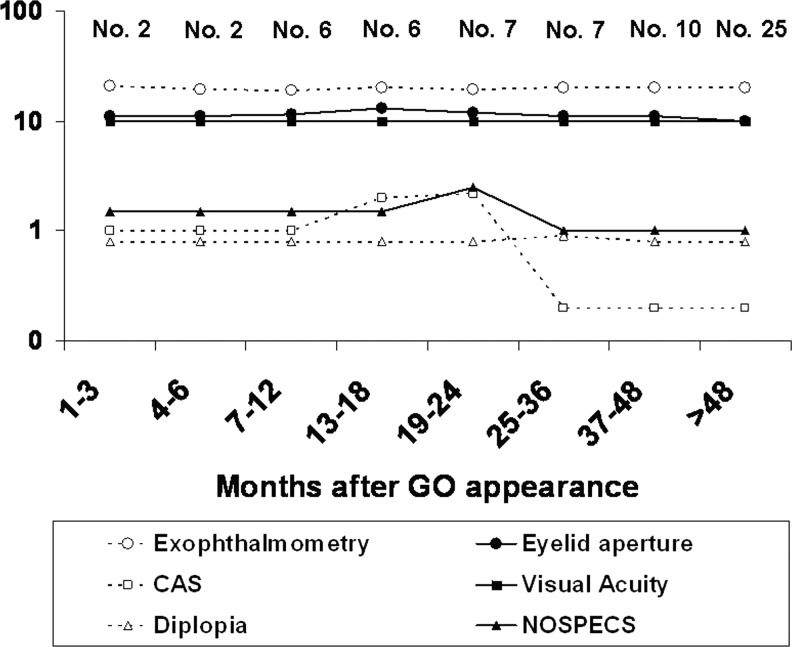
We then plotted findings for each patient over time, in order to determine to what extent GO features resembled Rundle's curve. As shown in Figure 1, CAS peaked between 13 and 24 months after the appearance of GO symptoms, and then decreased between 25 and 36 months and remained stable thereafter. The NOSPECS curve was slightly delayed, and thus peaked between 19 and 24 months, decreased between 25 and 36 months, and remained stable thereafter. Exophthalmometry, eyelid aperture, and visual acuity did not change much over time, in agreement with the findings reported above.
FIG. 1.
Behavior over time of exophthalmometry (mm), eyelid aperture (mm), Clinical Activity Score (CAS; points), visual acuity, diplopia (classes), and NOSPECS score in 65 patients with untreated Graves' ophthalmopathy (GO). The number of patients at each time period is indicated.
Analysis of the possible effects of several variables on GO behavior
We investigated whether there were any factors affecting GO natural history. However, none of the variables examined was found to be significantly correlated with GO change. Thus, as reported in Table 3, the overall outcome of GO was not significantly affected by age, sex, smoking habits, time since GO appearance, time since thyroid disease diagnosis, thyroid treatment, thyroid status (both at first and last observation), thyroid volume at first observation, and TRAb, the latter both at first and last observation.
Table 3.
Influence of Several Variables on the Outcome of Graves' Ophthalmopathy in 65 Untreated Patients
| Improved | Stable | Worsened | p | ||
|---|---|---|---|---|---|
| Age |
|
40.7±15.3 |
42.2±13 |
48.2±15.3 |
0.37a |
| Sex |
Males |
10/19 (52.6%) |
7/19 (36.8%) |
2/19 (10.5%) |
0.77b |
| |
Females |
23/46 (50%) |
15/47 (31.9%) |
8/47 (17%) |
|
| Smoking* |
Non-smokers |
16/26 (61.5%) |
8/26 (30.7%) |
2/26 (7.6%) |
0.32b |
| |
Ex-smokers |
4/9 (44.4%) |
2/9 (22.2%) |
3/9 (33.3%) |
|
| |
Smokers |
13/30 (43.3%) |
12/30 (40%) |
5/30 (16.6%) |
|
| |
Pack years |
0.4 (IQR: 0–7.2) |
0.2 (IQR: 0–8.4) |
4.8 (IQR: 1.1–9.8) |
0.46c |
| Smoking† |
Non-smokers |
16/26 (61.5%) |
8/26 (30.7%) |
2/26 (7.6%) |
0.50b |
| |
Ex-smokers |
6/16 (37.5%) |
6/16 (37.5%) |
4/16 (25%) |
|
| |
Smokers |
11/23 (47.8%) |
8/23 (34.7%) |
4/23 (17.3%) |
|
| |
Pack years |
0.9 (IQR: 0–9.7) |
1.1 (IQR: 0–9) |
3.8 (IQR: 0.2–11.2) |
0.58c |
| GO duration† |
|
42 months (IQR: 16.2–73.7) |
29 months (IQR: 21–72) |
45 months (IQR: 33–81) |
0.72c |
| TD duration† |
|
46 months (IQR: 19.7–100.2) |
49 months (IQR: 21–83) |
79.5 months (IQR: 43–140) |
0.36c |
| Thyroid treatment† |
None |
1/4 (25%) |
2/4 (50%) |
1/4 (25%) |
0.19b |
| |
MMI |
5/17 (29.4%) |
9/17 (52.9%) |
3/17 (17.6%) |
|
| |
RI |
6/11 (54.5%) |
5/11 (45.4%) |
0/11 (0%) |
|
| |
TX |
17/28 (60.7%) |
6/28 (21.4%) |
5/28 (17.8%) |
|
| |
LT4 |
4/5 (80%) |
0/5 (0%) |
1/5 (0%) |
|
| Thyroid status* |
Hyperthyroid |
7/13 (53.8%) |
4/13 (30.7%) |
2/13 (15.3%) |
0.78b |
| |
Euthyroid |
25/50 (50%) |
16/50 (32%) |
8/50 (16%) |
|
| |
Hypothyroid |
1/2 (50%) |
2/2 (100%) |
0/2 (0%) |
|
| Thyroid status† |
Hyperthyroid |
0/0 (0%) |
0/0 (0%) |
0/0 (0%) |
0.78b |
| |
Euthyroid |
32/63 (50.7%) |
21/63 (33.3%) |
10/63 (15.8%) |
|
| |
Hypothyroid |
1/2 (50%) |
1/2 (50%) |
0/2 (0%) |
|
| Thyroid volume* |
|
27 mL (IQR: 11.2–36.7) |
17.5 mL (IQR: 12–29) |
28 mL (IQR: 21–33.5) |
0.54c |
| TRAb* |
|
8.4 U/L (IQR: 3.3–26.3) |
3.5 U/L (IQR: 1.6–9.9) |
4.2 U/L (IQR: 1.3–15) |
0.08c |
| TRAb† |
|
3.9 U/L (IQR: 0–9.2) |
1.6 U/L (IQR: 0–5.3) |
4 U/L (IQR: 1–9.8) |
0.44c |
| TRAb reduction | 7 U/L (IQR: 2.2–14.3) | 1.8 U/L (IQR: −0.7–3.2) | 4.2 U/L (IQR: −5–22.9) | 0.07c |
LT4 refers only to patients given the medication for primary hypothyroidism. p-Values were obtained by: aANOVA, bFisher's exact test, and cKruskal–Wallis, as indicated.
First observation; †last observation.
Discussion
In 1945, Francis Felix Rundle reported two cases of untreated GO observed over a 30-month period (10). Based on those findings and observations in a subsequent study (11), the so-called Rundle's curve has been considered the model of GO natural history since that time (7–9). In the 68 years that have followed Rundle's publications, only a few studies have investigated the natural history of GO in untreated patients. In 1977, Teng and Yeo observed a spontaneous improvement of GO over a median period of about six years in ∼65% of 56 patients, ∼25% of whom remained stable and ∼10% of whom worsened, the two latter over shorter periods of time (5.6 and 2.1 years respectively) (12). Nearly 20 years later, Perros et al. studied 59 untreated GO patients followed up for a relatively short median period of time (12 months) and found that ∼65% of them improved spontaneously, ∼20% remained stable, and ∼15% worsened (13). In contrast, Streeten et al. previously observed an amelioration of proptosis only in ∼6% of 122 GO patients, the majority of whom had remained stable (14). More recently, in 2001, Noth et al. reported findings in 53 untreated GO patients with a median observational period of about three years (15). Approximately 45% of these patients improved spontaneously, and a similar proportion remained unchanged, whereas only a minority (∼4%) worsened. In the EUGOGO study on the effects of selenium on GO (16), ∼35% of patients in the untreated control group improved, ∼30% remained unchanged, and ∼25% worsened, as observed ∼18 months after the appearance of GO. Finally and very recently, Tanda et al. studied 43 untreated patients over an 18-month period and observed GO improvement in ∼60% of them (17). With the exception of the study from Streeten et al. (14), there were only slight differences between these studies, and the present study was mostly in accordance with them. These differences can be possibly explained by the different inclusion criteria and by the different criteria used to define the GO outcomes. Nevertheless, a spontaneous improvement of GO was almost invariably observed. All of these studies were performed in patients with mild GO, which is quite obvious considering that patients with moderate-to-severe GO, unlike those with a mild eye syndrome, are usually offered major treatments (1–6). Although these studies confirm that untreated, mild GO undergoes a spontaneous improvement in a substantial proportion of patients, none tried to determine the timing of this improvement (or worsening) and therefore to verify and possibly replicate Rundle's curve—information that is presumably of paramount importance for establishing the timing of GO treatment (1–6).
Here, we investigated 65 patients with untreated GO, of whom the vast majority had a mild eye syndrome, observed for a median period of 3.3 years from the beginning of GO symptoms, encompassing a time range from 3 to 258 months. Both GO activity (CAS) and severity (NOSPECS) improved spontaneously and significantly over the observational period, with a timing pattern resembling Rundle's curve (7–11), and with the activity curve preceding the severity curve by a few months, as postulated by Wiersinga (8). As in previous studies (12–17), the vast majority of our patients had mild GO, whereas Rundle's observations were based on patients with more severe forms of GO (7–11). Thus, although our curve is similar to the one of Rundle in terms of timing, we did not exactly replicate his observations. Nevertheless, although our findings cannot be applied with certainty to patients with moderate-to-severe GO, based on Rundle's studies, it is reasonable to postulate that the spontaneous behavior of GO could be similar regardless of its severity. In our series, a minority of patients had a more severe form of GO. However, this did not seem to affect the results, as the significant improvement of CAS and NOSPECS score was confirmed even when these patients were excluded.
While the majority of patients had a relatively brief history of GO at first observation (≤12 months), about one third of them had GO lasting for more than 12 months. We considered the possibility that this may have affected our results. However, the significant reductions of CAS and NOSPECS score were confirmed even when these two groups were considered separately, with the exception of the decrease of NOSPECS score, which was no longer significant in patients with GO duration at first observation of >12 months, possibly due to the limited number of subjects.
Exophthalmometry, eyelid aperture, visual acuity, and diplopia did not change significantly, suggesting that the amelioration of GO severity (the NOSPECS score) probably reflects the reduction of CAS, which is somehow part of the NOSPECS score (18,20). Nevertheless, the fact that the NOSPECS peak was slightly delayed compared with the CAS peak suggests that, at least to some extent, the other GO variables examined might have minimally changed over time.
As in the majority of previous studies (12–17), GO improved in about a half of the patients, remained stable in about 35%, and worsened in approximately 15%. However, none of the variables we examined (age, sex, smoking, time since GO appearance, time since thyroid disease diagnosis, thyroid treatment, thyroid status, thyroid volume, and TRAb) significantly affected the outcome of GO. This was relatively surprising concerning smoking and TRAb, two known risk factors for GO progression (1–6,22). Nevertheless, the relatively low number of patients has to be taken into account, which might not be sufficient for statistical significance to be reached, especially in view of the raw data (see Table 3) that seem to show a trend toward a relation of GO outcome with smoking and with TRAb reduction. In any case, because of a lack of statistical significance, no conclusions can be drawn at this time, and further studies are needed to investigate the effects of smoking and TRAb on the outcomes of untreated GO.
A possible limitation of the present study is that some of the patients underwent semi-ablative thyroid treatments, namely thyroidectomy or radioiodine, the latter being a known risk factor for GO progression (23–26). In addition, removal of thyroid antigens, either by thyroidectomy or radioiodine, may in theory have resulted in an attenuation of the autoimmune response also against orbital antigens, as suggested by previous studies on total thyroid ablation (27–29), a procedure that has been proposed to be beneficial for GO, although it cannot be regarded as a treatment of choice for Graves' hyperthyroidism in the absence of GO. However, thyroid treatment did not significantly affect the GO outcomes. In particular, in agreement with previous studies (1–6), among patients with Graves' disease, there was no significant difference in the GO outcome between those treated with MMI only and those treated with thyroidectomy or radioiodine. On the other hand, the number of patients treated with MMI, radioiodine, or thyroidectomy was quite different. Therefore, we cannot conclude with certainty that thyroid treatment does not affect the natural history of GO, and further studies in larger numbers of patients treated with either one of the three modalities are required.
As in previous studies and for the same reasons, our findings were obtained in patients with mild GO. Whether they can also be applied to patients with moderate-to-severe GO remains to be established. Nevertheless, if that were the case, one could speculate that, based on our curves, anti-inflammatory/immunosuppressive treatments, which are known to be effective especially during the active phase of the syndrome, should be given within 24 months after the appearance of GO symptoms (the activity peak), whereas surgical treatments should be recommended once the disease has become inactive, which we observed beginning at 36 months after the appearance of GO.
Footnotes
In 1969, the American Thyroid Association adopted the formal classification of Ocular Graves' disease, represented by the mnemonic NOSPECS. The disease process passes through 6 stages: (0) No signs or symptoms present; (I) Only symptoms of ocular irritation (dryness, tearing, foreign body sensation); (II) Soft tissue involvement (periorbital edema); (III) Proptosis; (IV) Extraocular muscle involvement (ophthalmoplegia); (V) Corneal involvement (dense punctate epitheliopathy, infiltration, and ulceration); (VI) Sight loss with or without visual field compromise secondary to compressive optic neuropathy. However, because the disease is recognized as variable, the formal classification was revised in 1974 to range from no manifestations to mild, moderate, or severe manifestations.
Acknowledgments
This study was supported by a grant from MIUR (Ministero dell'Istruzione, dell'Università e della Ricerca Scientifica; 2004068078 to M.M.).
Author Disclosure Statement
The authors declare that they do not have any commercial associations that might create conflicts of interest in connection with this manuscript.
References
- 1.Bahn RS.2010Graves' orbitopathy. N Engl J Med 362:726–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartalena L, Tanda ML.2009Clinical practice. Graves' orbitopathy. N Engl J Med 360:994–1001 [DOI] [PubMed] [Google Scholar]
- 3.Bartalena L, Pinchera A, Marcocci C.2000Management of Graves' ophthalmopathy: reality and perspectives. Endocr Rev 212:168–199 [DOI] [PubMed] [Google Scholar]
- 4.Bartalena L, Wiersinga WM, Pinchera A.2004Graves' orbitopathy: state of the art and perspectives. J Endocrinol Invest 27:295–301 [DOI] [PubMed] [Google Scholar]
- 5.Marcocci C, Marinò M.2012Treatment of mild, moderate-to-severe and very severe Graves' orbitopathy. Best Pract Res Clin Endocrinol Metab 26:325–337 [DOI] [PubMed] [Google Scholar]
- 6.Marcocci C, Pinchera A, Marinò M.2007A treatment strategy for Graves' orbitopathy. Nat Clin Pract Endocrinol Metab 3:430–436 [DOI] [PubMed] [Google Scholar]
- 7.Bartley GB.2011Rundle and his curve. Arch Ophthalmol 129:356–358 [DOI] [PubMed] [Google Scholar]
- 8.Wiersinga WM.1992Immunosuppressive treatment of Graves' ophthalmopathy. Thyroid 2:229–233 [DOI] [PubMed] [Google Scholar]
- 9.Perros P, Kendall-Taylor P.1998Natural history of thyroid eye disease. Thyroid 8:423–425 [DOI] [PubMed] [Google Scholar]
- 10.Rundle FF, Wilson CW.1945Development and course of exophthalmos and ophthalmoplegia in Graves' disease with special reference to the effect of thyroidectomy. Clin Sci 5:177–194 [PubMed] [Google Scholar]
- 11.Rundle FF.1957Management of exophthalmos and related ocular changes in Graves' disease. Metabolism 6:36–48 [PubMed] [Google Scholar]
- 12.Teng CS, Yeo PP.1977Ophthalmic Graves' disease: natural history and detailed thyroid function studies. Br Med J 29:273–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perros P, Crombie AL, Kendall-Taylor P.1995Natural history of thyroid associated ophthalmopathy. Clin Endocrinol (Oxf ) 42:45–50 [DOI] [PubMed] [Google Scholar]
- 14.Streeten DH, Anderson GH, Jr, Reed GF, Woo P.1987Prevalence, natural history and surgical treatment of exophthalmos. Clin Endocrinol (Oxf) 27:125–133 [DOI] [PubMed] [Google Scholar]
- 15.Noth D, Gebauer M, Müller B, Bürgi U, Diem P.2001Graves' ophthalmopathy: natural history and treatment outcomes. Swiss Med Wkly 131:603–609 [DOI] [PubMed] [Google Scholar]
- 16.Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, Altea MA, Nardi M, Pitz S, Boboridis K, Sivelli P, von Arx G, Mourits MP, Baldeschi L, Bencivelli W, Wiersinga W; European Group on Graves' Orbitopathy 2011Selenium and the course of mild Graves' orbitopathy. N Engl J Med 364:1920–1931 [DOI] [PubMed] [Google Scholar]
- 17.Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, Pariani N, Gallo D, Azzolini C, Ferrario M, Bartalena L.2013Prevalence and natural history of Graves' orbitopathy in a large series of patients with newly diagnosed Graves' hyperthyroidism seen at a single center. J Clin Endocrinol Metab 98:1443–1449 [DOI] [PubMed] [Google Scholar]
- 18.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L.1997Clinical Activity Score as a guide in the management of patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf ) 47:9–14 [DOI] [PubMed] [Google Scholar]
- 19.Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits MP, Perros P, Boboridis K, Boschi A, Currò N, Daumerie C, Kahaly GJ, Krassas G, Lane CM, Lazarus JH, Marinò M, Nardi M, Neoh C, Orgiazzi J, Pearce S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, von Arx G, Wiersinga WM.2008Consensus statement of the European group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Thyroid 18:333–346 [DOI] [PubMed] [Google Scholar]
- 20.Werner SC.1977Modification of the classification of the eye changes of Graves' disease: recommendations of the Ad Hoc Committee of the American Thyroid Association. J Clin Endocrinol Metab 44:203–204 [DOI] [PubMed] [Google Scholar]
- 21.Profilo MA, Sisti E, Marcocci C, Vitti P, Pinchera A, Nardi M, Rocchi R, Latrofa F, Menconi F, Altea MA, Leo M, Rago T, Marinò M.2013Thyroid volume and severity of Graves' orbitopathy. Thyroid 23:97–102 [DOI] [PubMed] [Google Scholar]
- 22.Eckstein A, Esser J, Mann K, Schott M.2010Clinical value of TSH receptor antibodies measurement in patients with Graves' orbitopathy. Pediatr Endocrinol Rev 7:198–203 [PubMed] [Google Scholar]
- 23.Bartalena L, Marcocci C, Bogazzi F, Panicucci M, Lepri A, Pinchera A.1989Use of corticosteroids to prevent progression of Graves' ophthalmopathy after radioiodine therapy for hyperthyroidism. N Engl J Med 321:1349–1352 [DOI] [PubMed] [Google Scholar]
- 24.Tallstedt L, Lundell G, Torring O, Wallin G, Ljunggren J-G, Blomgren H, Taube A, and the Thyroid Study Group 1992Occurrence of ophthalmopathy after treatment for Graves' hyperthyroidism. N Engl J Med 326:1733–1738 [DOI] [PubMed] [Google Scholar]
- 25.Tallstedt L, Lundell G, Blomgren H, Bring J.1994Does early administration of thyroxine reduce the development of Graves' ophthalmopathy after radioiodine treatment? Eur J Endocrinol 130:494–497 [DOI] [PubMed] [Google Scholar]
- 26.Bartalena L, Marcocci C, Bogazzi F, Manetti L, Tanda ML, Dell'Unto E, Bruno-Bossio G, Nardi M, Bartolomei MP, Lepri A, Rossi G, Martino E, Pinchera A.1998Relation between therapy for hyperthyroidism and the course of Graves' ophthalmopathy. N Engl J Med 338:73–78 [DOI] [PubMed] [Google Scholar]
- 27.Chiovato L, Latrofa F, Braverman LE, Pacini F, Capezzone M, Masserini L, Grasso L, Pinchera A.2003Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Int Med 139:346–351 [DOI] [PubMed] [Google Scholar]
- 28.Menconi F, Marinò M, Pinchera A, Rocchi R, Mazzi B, Nardi M, Bartalena L, Marcocci C.2007Effects of total thyroid ablation versus near-total thyroidectomy alone on mild to moderate Graves' orbitopathy treated with intravenous glucocorticoids. J Clin Endocrinol Metab 92:1653–1658 [DOI] [PubMed] [Google Scholar]
- 29.Leo M, Marcocci C, Pinchera A, Nardi M, Megna L, Rocchi R, Latrofa F, Altea MA, Mazzi B, Sisti E, Profilo MA, Marinò M.2012Outcome of Graves' Orbitopathy after total thyroid ablation and glucocorticoid treatment: follow-up of a randomized clinical trial. J Clin Endocrinol Metab 97:E44–E48 [DOI] [PubMed] [Google Scholar]