Abstract
Sepsis with subsequent multiple organ dysfunction is a pronounced systemic inflammatory response to concealed or known infection and is a leading cause of death in intensive care units. The survival rate of severe sepsis and septic shock has not markedly improved in recent decades despite a great number of receptors and molecules involved in its pathogenesis have been found and taken as therapeutic targets. It is essential to thoroughly understand the host cell-mediated immunity involved in the development of sepsis and sepsis-related organ injury. Recent studies indicate that innate immune cells (such as neutrophils, macrophages, dendritic cells, T lymphocytes, regulatory T cells, and natural killer T cells) play pivotal roles in the maintenance of peripheral homeostasis and regulation of immune responses during sepsis. Therefore, an understanding of the biological significance and pathophysiological roles of different cell populations might gain novel insights into the immunoregulatory mechanisms of sepsis. In this review, we focus on major immune cells that may play potential roles in the contribution of new therapeutic approaches for sepsis.
Introduction
Sepsis represents a complex clinical morbidity that results from a harmful or devastating host response to infection. Its treatment is yet unsatisfactory, and its mortality is still alarmingly high despite continuous progress in the development of novel treatment modalities and therapeutic strategies for severe sepsis. Many of the components of the innate immune response that is normally related to host defenses against infection might, under some circumstances, cause cell- and tissue- damage, and result in multiple organ dysfunction syndrome (MODS) or even multiple organ failure (MOF) (Yao and others 1998; Cohen 2002). For the purpose of this review, we will be focusing on the immunopathogenesis of sepsis, and in particular the regulatory mechanisms mediated by innate immune cells.
Sepsis develops when the initial, appropriate host response to an infection becomes amplified and is followed by dysregulation. The inflammatory response is partly mediated by innate immune cells [such as neutrophils, macrophages, dendritic cells (DC), T lymphocytes, regulatory T cells (Tregs), and natural killer T (NKT) cells], which can initiate or suppress host inflammation by producing pro-inflammatory cytokines [eg, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and high mobility group box-1 protein (HMGB1)] or inhibitory cytokines [eg, transforming growth factor (TGF)-β, interleukin (IL)-10, etc.] (Gautam and others 2012; Novotny and others 2012; Zhao and others 2012; Inoue and others 2013). This article is a brief overview of our understanding concerning the different types of innate immune cells, as to highlight recent investigations on potential roles and regulatory mechanisms of these immunological cells in the development of sepsis. In addition, a summary of the experimental results of our research laboratory in this field regarding these immune cells are presented in the following review, and finally we discuss the early diagnosis and rational treatment of severe sepsis in relation to the immune cells.
Sepsis and Host Immune Response
Traditionally, sepsis has been defined as a clinical syndrome, which develops fever, tachycardia, leukocytosis/leucopenia, and other manifestations of infection. It might represent an appropriate but inadequate response against an overwhelming infection or uncontrolled inflammation (Ghazal and others 2013; Silva and Dos 2013). As a result of a concerted effort to disclose the underlying pathogenetic mechanisms, there have been accumulating evidences to suggest that sepsis is described as the systemic inflammatory response syndrome (SIRS) resulting from infection (Drifte and others 2013). Insults, such as hemorrhagic shock, traumatic and severe tissue injury, thermal injury and ischemia-reperfusion injury, can lead to SIRS. Since that time, an alternative view is suggested by the clinical finding that activation of systemic inflammation from both infectious agents and insults can result in the metabolic and physiologic responses of sepsis, but the potential mechanisms of immunity and inflammation are not well defined.
To date, it is known that the complicated sepsis syndrome may lead to both widespread activation and dysfunction of the innate immune system (Souza and others 2010). The innate immune system is to coordinate a defensive response, including both humoral and cellular components. Through recognition of invading microbes or microbial products, the innate immune system responds with a generalized response pattern, which is mediated in large part by the release of secretory proteins or cytokines (Oberholzer and others 2001; Efron and Moldawer 2003). However, activation of host innate immunity may occur not only after a microbial invasion, but also subsequent to exposure to internal “danger” signals produced by cell injury, tissue ischemia, hypoxia, and necrosis (Oberholzer and others 2001). As the innate immune system activates severely enough, the host response itself can drive the patient to manifest SIRS, or even shock and MODS/MOF. Although some patients survive the initial SIRS insult, these patients remain at an increased risk of developing secondary or opportunistic infections because of the frequent onset of a compensatory anti-inflammatory response syndrome (CARS). At the initial stage of sepsis, there is a release of large quantities of pro-inflammatory mediators, including TNF-α, IFN-γ, IL-33, and IL-2 (Hirsiger and others 2012), whereas, with the progression of disease condition, immunosuppression would be elicited, including macrophage deactivation, reduced antigen presentation, suppression of reproductive activity of lymphocytes, and the release of a number of anti-inflammatory cytokines, such as IL-10, IL-13, and IL-27. Further, a shift in the T-helper cell (Th) pattern to a Th2 cells, accompanied by apoptosis of a large number of lymphocytes, might lead to an increase in susceptibility to infection (Luan and others 2012). A growing body of evidence shows that innate immune cells (including Tregs, regulatory DCs, and invariant NKT [iNKT]) from patients with severe sepsis are able to promote an upsurge in anti-inflammatory cytokines and reverse the Th2 type response, rendering the patient to enter a state of immune depression and CARS. Therefore, both innate immunity and inflammation must be considered in the development of severe sepsis.
Immune Regulation and Its Mechanism in Sepsis
The innate immune cells (such as neutrophils, macrophages, DCs, Tregs, and NK cells) are responsible not only for initiating an inflammatory event, but also for inducing widespread lymphocyte apoptosis (Wang and others 2008). Over a remarkably short period of time, studies of innate immunity revealed the discovery of the family of toll-like receptors (TLRs) on the surface of the innate immune cells (Yang and others 2013). These receptors could specifically recognize molecules shared by a variety of microbial components called pathogen-associated molecular patterns (PAMPs). Then, through the recognition of PAMPs, a wide array of cytokines and chemokines may be released from the immune cells, and the activation stimulus ignites the inflammatory process. In addition, apoptosis plays a significant role in maintenance of normal immune system, including the deletion of autoreactive lymphocytes and the termination of lymphocyte immune activity. However, it is also pathologic, as it is deprived of lymphoid or DC function (Chang and others 2007). Numerous studies demonstrated lymphocyte apoptosis increased during sepsis, contributing to great loss of lymphocytes with subsequent immunosuppression. Macrophages and immature DCs may accelerate the lymphocyte apoptosis in septic complications.
Neutrophil function in innate immune response in sepsis
Neutrophils are abundant in the blood but absent in normal tissues. They have the shortest life span among leukocytes, surviving only a few hours after leaving the bone marrow. In the early phase of sepsis, a considerable reserve pool of mature neutrophils within the bone marrow can be rapidly mobilized, resulting in a dramatic rise in circulating neutrophil numbers, and thereby the number of neutrophils available for recruitment into sites of sepsis increases (Alves-Filho and others 2010). Detectable in elevated concentrations of microparticles in inflammation, sepsis and metabolic disorders, these microparticles can be released from various cell types, such as neutrophils and erythrocytes. Microparticles derived from neutrophils may be beneficial in early sepsis, have a divergent effect on the immune response by activating phagocytic cells and deactivating bystander cells (Prakash and others 2012). Further, compared to mature neutrophils, immature neutrophils have a longer life span and resistance to spontaneous apoptosis, higher basal intracellular TNF-α/IL-10 ratio, and are also capable of mediating important innate immune functions but less efficiently (Drifte and others 2013).
Mechanisms governing neutrophil function in sepsis are complex. A failure of neutrophil migration in lethal sepsis and a reduced survival rate have been demonstrated. During sepsis neutrophil migratory responses can be regulated by bacterial products, cytokines/chemokines, leukotrienes, and immunomodulatory hormones via induction of cytoskeletal changes, disruption of polymorphonuclear leukocyte (PMN)-endothelial cell interactions, and alterations in G-protein-coupled receptor expression or signaling (Prakash and others 2012). It is known that the dysregulated PMN G-protein-coupled receptor and TLR expression and/or signaling can result in the alteration of leukotriene functions, further leading to pro-inflammatory and immunomodulatory genes suppression, and decreased production of reactive oxygen species in sepsis, In addition, recently an elevated levels of circulating form of C5aR (cC5aR) in serum, reduction of the C5a receptor on neutrophils were detected in septic shock (Unnewehr and others 2013). There are a series of effector functions performed by neutrophils, which represent an important mechanism of immunity against infections. The failure of neutrophil migration may lead to an increased number of bacteria in peritoneal exudates and blood, followed by tissue injury and systemic inflammation, which is characterized by increased levels of circulating cytokines and chemokines and neutrophil sequestration in the lung and other organs. Therefore, as an essential effector of the innate immune response, impaired recruitment and migration of neutrophils contribute to the pathogenesis of sepsis and are correlated with a poor outcome in severe sepsis.
The role of macrophage in immune response during sepsis
Macrophage can trap, engulf, destroy the invading pathogen, and stimulate the release of cytokines. In severe human sepsis and septic shock, secondary hemophagocytic lymphohistiocytic syndrome can be developed, followed by macrophage activation syndrome (MAS). MAS is characterized by excessive activation of macrophages, and the increased Th1 cytokine secretion such as IFN-γ, IL-12, and IL-18 during sepsis that promote the macrophage activation (Larroche and Mouthon 2004). Macrophages are important in the development of the innate immune response, the form of which is determined by their released cytokines. Type I IFN, released from splenic macrophages, can suppress the secretion of proinflammatory cytokines, and has been identified as the critical factor causing impairment of T-cell immunity after sepsis (Schwandt and others 2012).
In the setting of sepsis, inflammation and apoptosis can be considered as dual pathogenic factors. Apoptosis is a genetically programmed mechanism of cell death, the normal process of it includes regulation of immune homeostasis and elimination of autoreactive lymphocytes (Fadeel and Orrenius 2005), but the uncontrolled apoptosis in the immune cells may compromise the ability of the host to eliminate the invading pathogens. Neutrophils are the first line of defense against pathogens, and they also undergo constitutive programmed cell death. Constitutive apoptosis renders neutrophils unresponsive to extracellular stimuli and allows their recognition and removal by macrophages. In addition to apoptosis of neutrophils, recent studies of the accelerated inflammatory processes involved in sepsis have implicated apoptosis of macrophages in the pathogenesis of sepsis. Macrophages play key roles in initiating, maintaining, and resolving host inflammatory responses, and excessive apoptosis of them may lead to immune dysfunction and MODS subsequent to sepsis. Several mechanisms underlying apoptosis in macrophages have been reported. Sepsis is a highly complex disorder; multiple cytokine-associated signaling pathways may promote activation of apoptosis and accelerate inflammatory events during the disorder. Ample evidence is provided that some cytokines such as IL-1, IL-6, and granulocyte colony-stimulating factor can mediate inflammatory response but inhibit apoptosis, whereas other cytokines like TNF-α and HMGB1 can induce apoptosis in many cell populations (Kockara and Kayatas 2013). So macrophages apoptosis can occur in the absence of some cytokines or extensive expression of other cytokines. Thus, researchers have attempted to determine the cellular and humoral factors responsible for macrophage apoptosis in the development of sepsis. It has been demonstrated that HMGB1 play a vital role in macrophage apoptosis.
A prospective study found that treatment with cocultured medium of HMGB1-secreting WiDr human colon cancer cells led to growth inhibition and apoptosis of both PMA-U937 and U937 cells (human leukemic monocyte lymphoma cell line). In addition, a number of studies suggested that HMGB1 might correlate well with inducing apoptosis of mouse peritoneal macrophages, and its effect was dose- and time-dependent (Zhu and others 2009a). HMGB1 is a ubiquitously expressed and evolutionarily conserved chromosomal protein among species, regulating nucleosome function and transcription; thus it has more recently reported to be an extracellularly released mediator of inflammation, and it induces apoptosis of macrophages in sepsis (Lotze and Tracey 2005; Zhu and others 2009a). Also, HMGB1 is a specific and saturable ligand for the receptor for advanced glycation end products (RAGE). The relationship between macrophage apoptosis induced by HMGB1 and RAGE had been demonstrated when HMGB1 was prevented from interacting with RAGE by using rmRAGE/Fc and anti-RAGE antibody, indicating that RAGE was one of the major receptors involved in the process of macrophage apoptosis mediated by HMGB1 (Zhu and others 2009a). HMGB1-induced intracellular signaling through RAGE can activate 2 different cascades, one of which is the nuclear factor (NF) translocation of NF-κB, a transcription factor that upregulates expression of HMGB1 and other products of the activated macrophage (Wang and others 1999; Zheng and others 2013). Cysteinyl aspartate-specific protease (caspase) cascade is well known for its established function in triggering cell toxicity and programmed cell death, and macrophage treated with HMGB1 shows a significant increase in caspase-3 activity. In line with these reports, in vitro studies have shown that elevated caspase-3 activity might contribute to HMGB1-mediated apoptosis of macrophages (Zhu and others 2009a). In further, Jiang and others (2007) revealed that with lipopolysaccharide (LPS) stimulation, the extent of HMGB1 released from macrophage was correlated with the occurrence of apoptosis as measured by caspase-3 activation in vitro.
The T lymphocyte-mediated immunity and sepsis
The role of T lymphocytes is of great importance in all adaptive immune responses against microorganism invasion. T lymphocytes mature in the thymus, circulate in the blood, populate secondary lymphoid tissues, and then are recruited to peripheral sites of antigen exposure. They can recognize peptide fragments of foreign proteins bound to major histocompatibility complex (MHC) molecules by the antigen receptors expressed on the surface of them. According to distinct developmental pathways and unique biological functions, T lymphocyte subsets can be classified as CD4+ Th and CD8+ cytolytic T lymphocytes (Kumar and others 2005; Huang and others 2013). CD4+ Th cells recognize antigenic peptides in the context of class II MHC molecules expressed on professional antigen presenting cells (APCs), whereas cytotoxic CD8+ T cells recognize antigenic peptides in the context of class I MHC molecules (Miller and others 2007; Kasten and others 2010). CD4+ naive T cells are further subdivided into lineages of helper/effector T cells and Tregs (Fig. 1). Including Th17 subset, there are now more than 2 functionally unique populations of CD4+ T cells that are directly involved in the regulation of immune responses and maintain a balanced innate and cognate immune system. Th1 cells favor cell-mediated immunity and have evolved to respond to intracellular pathogens, Th2 cells contribute to humoral immunity and aid in the clearance of parasites (Shinkai and others 2002; Coffman 2006) and promote downregulation of host immunity. Th17 cells secrete the signature cytokine IL-17 to induce a series of tissue reactions and are critical in immune responses to infectious agents and mediate inflammation (Nakae and others 2003; Weaver and others 2007; Jager and Kuchroo 2010). CD4+CD25+Tregs secrete anti-inflammatory cytokines, such as IL-10 and TGF-β, and are responsible for immunosuppressive state described in the numerous forms of severe injuries and sepsis (MacConmara and others 2006). Besides, γδ-T cells may play a potential role in the postburn survival and sepsis, cytokine formation by Th1 and Th2 cells, initiation of neutrophil-mediated tissue damage, and wound healing. In the setting of major burns, CD8+ CD11b+γδ-T cells are present in more numbers in splenic tissue, and also inhibit the lymphocytic proliferation; in contrast to γδ-T cells, CD8+ CD11b+γδ-T cells mainly secrete Th2 cytokine, and the retransfusion of these cells to normal mouse can increase the susceptibility to septic challenge (Tschop and others 2008). More recently, it has been found that hypoxia-inducible factor 1α (HIF-1α) protein would support LPS-dependent expression of IL-6 by preventing depletion of ATP and be selectively required for downregulation of apoptosis signal-regulating kinase 1 activated during LPS-induced TLR4 downstream signaling, deletion of HIF-1α gene in T cells may prevent their inhibition in hypoxic inflamed tissues and improve survival of septic patients (Lall and others 2008). In the development of sepsis, several lines of evidence indicated that T lymphocytes played central roles in cell-mediated immune response, including remarkably attenuated reproductive activity and the predominant Th2 immune reaction. The initiation of immunological reaction mediated by Th2 cells and accompanied by apoptosis of a large number of lymphocytes might lead to an increase in susceptibility to infection (Hirsiger and others 2012).
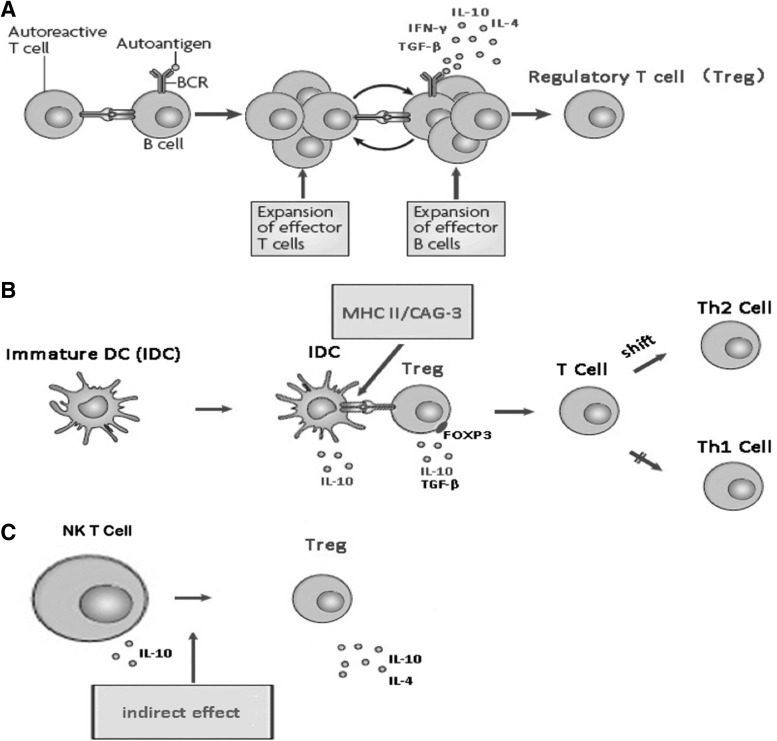
FIG. 1.
The intercommunication of the regulatory T cells, DCs, and NKT cells. (A) The initiation of cognate interactions between autoreactive T and B cells and the establishment of a B and T cell-dependent reaction, the production of cytokines (eg, IL-4, IL-6, IL-10, IFN-γ, TGF-β, etc.) were involved, in turn promoting the activation of Treg. (B) The production of Treg or effector T lymphocytes induced by DCs depended on the immature status, referring to the ratio of immature DCs and mature DCs. Cytokines released by regulatory DCs (immature DCs) might affect the immune activity, and exert immunoregulatory effects. (C) NKT cells possess more similarities to Tregs than other cells. The activation of NKT cells was directly enhanced by certain ligands, but also indirectly through activation of Tregs by cytokines secretion, and NKT cells influenced other T cell functions via regulation of their cytokine responses. DC, dendritic cell; NKT cell, natural killer T cell; IFN-γ, interferon-γ; TGF-β, transforming growth factor-β; Tregs, regulatory T cells; IL, interleukin.
Apoptosis appears to be critically involved in the pathophysiology of sepsis. In light of recent data, T lymphocyte apoptosis has been suggested as the potential factor in the immunosuppression and determination of sepsis severity (Lang and Matute-Bello 2009; Alves-Filho and others 2010). Clinical findings also correlated well with the results from septic mouse after cecal ligation and puncture (CLP), as assessed by transferase-mediated dUTP nick-end labeling (TUNEL) staining (Matsuda and others 2010). Previous studies effectively illustrated that multiple apoptotic pathways might be involved in sepsis, including the intrinsic and extrinsic-mediated pathways (Ma and others 2008). The intrinsic pathway is highly dependent on the expression of proapoptotic and of antiapoptotic proteins. During sepsis, there is an increase in proapoptotic protein Bim and a decrease in level of antiapoptotic molecules (Bcl-2 and Bcl-xL) (Table 1) in T cells (Weber and others 2008). Transgenic mice that selectively overexpress Bcl-2 and Bcl-xL in T lymphocytes displayed appreciable protection against lymphocyte apoptosis and significantly improved survival in CLP-induced sepsis (Hotchkiss and others 1999; Schwulst and others 2008). The extrinsic pathway of apoptosis can be initiated by different Fas ligand (FasL) and Fas-associated receptors. It has been shown in CLP-induced septic mice that downregulation of caspase-8 decreased T lymphocyte apoptosis and improved survival of mice, as caspase-8 was related with other executioner caspases activity and NF-κB activity. In addition to the intrinsic and extrinsic pathways, endoplasmic reticulum (ER) stress-mediated pathway is also involved in the apoptotic process of T lymphocytes via activation of numerous overlapping cascades (caspase-12) (Jimbo and others 2003), however, the precise regulatory mechanism has yet to be fully elucidated. More recently, the crosstalk of apoptotic pathways during sepsis has been implicated in both intrinsic pathways and the ER-mediated or extrinsic pathway (Oakes and others 2006). T cell apoptosis can affect innate immune response, such as decreased IFN-γ and IL-17 within the first 24 h, reduced ability to limit macrophage phagocytosis of dead T cells, and the subsequent production of IL-10 and TGF-β. Septic thymocyte apoptosis can be promoted by glucocorticoid, and it has been regarded as the most clinically relevant treatment for sepsis. The cell death in the adaptive immune system is beneficial to the host, by downregulating the inflammatory response to sepsis, but the extensive loss of immune cells may compromise the ability of the host to eliminate the invading pathogens, and finally lead to septic death, demonstrating that increased apoptosis in T lymphocytes plays a critical role in the adverse outcome to sepsis.
Table 1.
The Immunoregulatory Mechanisms Associated with Immune Cells During Sepsis
|
Effector molecules/ |
Different immune cell subsets |
||||
|---|---|---|---|---|---|
| pathways | Macrophage | T cell | DC | Treg | NKT cell |
| Cytokines |
TNF-α, IL-6, IL-18 |
IL-2, IFN-γ, TNF-α |
IL-12/IL-10 |
IL-2, IL-4, TGF-β, IL-10 |
IL-2, IFN-γ/IL-4 |
| Signature receptor/septic molecular |
RAGE |
TCR/BcL-2, Bcl-xL, TIPE2 |
TLR2,4/CD80, CD86, MHC II |
TLR4, α7nAChR/Foxp3, CTLA-4, TIPE2 |
TCR, α-C52R/CD1d |
| Primary signaling regulator | JAK-STAT, caspase-3 signaling | NF-κB, NF-AT, p38-MAPK | NF-κB, UPR pathway | NF-AT, JAK-STAT, TLR pathway | NF-κB |
DC, dendritic cell; Treg, regulatory T cell; NKT cell, natural killer T cell; IL, interleukin; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; NF-κB, nuclear factor-κB; JAK-STAT, janus kinase-signal transducer and activator of transcription; NF-AT, nuclear factor of activated T cell; RAGE, receptor for advance glycation end products; TIPE2, tumor necrosis factor α-induced protein 8 like-2; Foxp3, Forkhead/wing helix transcription factor p3; CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; TLR, toll-like receptor; α7nAChR, α7 nicotinic acetylcholine receptor; α-C52R, α-C52 receptor; UPR, unfolded protein response; MAPK, mitogen-activated protein kinases; TGF-β, transforming growth factor-β; MHC, major histocompatibility complex; TCR, T cell receptor.
Involvement of Tregs in regulating immune system during sepsis
Several subpopulations of Tregs have been described based on their development, specificity, and role of action; thus, they can be classified into naturally (native) occurring Tregs and acquired (inducible) Tregs. Generally, naturally occurring Tregs become mature in the thymus and then enter the peripheral lymphoid tissue. A small population of CD4+CD25+ Treg has been assigned to play an essential role in the control of adaptive and innate immune responses (Hanschen and others 2012). Inducible Treg is a group of mature T cells (CD4+CD25−T cells), developing in peripheral lymphoid tissues with an opposite function from naturally occurring Tregs. They appear in response to a particular condition of antigen-experienced T lymphocytes after being exposed to a specific antigen or immunosuppressive cytokines, and they are referred as TGF-β-producing Th3 cells and IL-10-producing T regulatory type 1 cells (Bach 2003). Tregs can proliferate in vivo and inhibit immunoreactivity mediated by effector T cells and other cells of innate immune system. There have been 4 different immune regulatory mechanisms described so far, however, the most important mechanisms are immunosuppressive properties and immune nonreactivity (Sakaguchi and others 2006).
During experimental and clinical sepsis, the percentage of Tregs has been found to be increased, and the expansion/conversion of Tregs were investigated in the postseptic immune system. In these context, investigations of the mechanisms of Treg function have identified growing expressions of constitutive and high surface molecules, such as cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and the glucocorticoid-induced TNFR (Jonuleit and others 2001; Peng and others 2004), and all of them correlated with the immunosuppressive properties of Tregs. There are also other factors, including the Forkhead/wing helix transcription factor p3 (Foxp3) and TLRs, which contribute to the suppressor function of Tregs (Fontenot and others 2005; Banham 2006). Recently, α7 nicotinic acetylcholine receptor (α7 nAChR) has been found in CD4+CD25+Tregs, the receptor regulates the expression of CTLA-4 and Foxp3, and has been described as an important regulator of immunodepressive function of CD4+CD25+Tregs (Wang and others 2010). Treg-mediated immunosuppressive state is mainly dependent on the drift of Th1/Th2 caused by the activation of T cell receptor (TCR) signal. Tregs can release a variety of anti-inflammatory cytokines (IL-4, IL-10), which might markedly promote the Th2 immune reaction. It is clear that Th1 clones synthesize IL-2, IFN-γ, and lymphotoxin, whereas these lymphokines are not detectably expressed in Th2 clones; conversely, only Th2 clones synthesize detectable amounts of IL-4 and IL-10 (Cherwinski and others 1987). MacConmara and others (2006) demonstrated that compared with normal control group, suppressive potency of Tregs was reinforced in patients 7 days after injury, and this might be the result of differentiation of CD4+ T cells to Th2 cells, while production of IL-10 induced the differentiation. As observed in humans, an injury also primed circulating human Tregs for enhanced immunosuppressive activity. In the mouse model of septic shock induced by CLP, the adoptive transfer of Tregs before or 6 h after CLP had a protective effect in preventing the development of severe sepsis (Heuer and others 2005). These in vivo and in vitro results indicate that Tregs appear to be essential for the active suppression of autoimmunity both in sepsis and injury.
With regard to the function of immune nonreactivity in Treg during the course of sepsis, several reports showed that CD4+CD25+Tregs might contribute to maintenance of immune tolerance, manifesting nonreactivity to the antigenic stimulation and a cease of IL-2 production. They are activated and proliferated with the presence of a large amount of IL-2, but the extent of proliferation is much lower than that of CD4+CD25−T cells (Sakaguchi and others 1995; Hesse and others 2004; Tatura and others 2012). In addition to immune nonreactivity, apoptotic cell death may trigger sepsis-induced anergy, and recent work has shown that cells may undergo to die by apoptosis. The increased percentage of Treg after shock has been described, it is not due to their proliferation, but a decrease in effector T cell number, and it might be related to the resistance of Treg to apoptotic processes occurring during septic shock. Moreover, Treg can induce and increase apoptosis of autoreactive thymic T cell and monocyte by a mechanism involving the Fas/FasL pathway (Venet and others 2006), and the contributory mechanism of which is the binding of FasL and Fas, resulting in activation of caspase-8. These results suggest that Treg may play a critical role during the process of immunologic derangement in sepsis, although the precise mechanisms involved in the process remain to be elucidated.
TNF-α-induced protein 8-like 2 (TIPE2) shares considerable sequence homology with members of the TNF-α-induced protein 8 (TNFAIP8) family, and it is preferentially expressed in lymphoid-derived and marrow-derived cells, but it may also be induced in other cell types by TNF-α (Li and others 2009; Zhang and others 2009) (Fig. 2). Experimental evidence indicated that septic shock was dramatically accelerated and exacerbated in TIPE2−/− mice as compared to wild-type controls, suggesting TIPE2 was directly responsible in preventing septic shock (Sun and others 2008). As it possesses a negative immune function similar to Treg in sepsis, we examined the expression of TIPE2 by western blot and reverse transcription–polymerase chain reaction, and found that Tregs from naive BALB/c mice positively expressed TIPE2, which was a cytoplasmic protein. It was unclear whether TIPE2 deficiency could affect the immune regulatory function of Tregs. To address this question, we investigated the potential effects of TIPE2 in CD4+CD25+ Tregs that were treated with TIPE2 gene silenced by siRNA. Accordingly, we found that expressions of cell surface molecules (CTLA-4 and Foxp3) and cytokines (IL-10 and TGF-β) were markedly downregulated in TIPE2 deficiency Tregs in vitro (Luan and others 2011). Similarly, TIPE2 knockdown increased the ratio of IFN-γ/IL-4 and enhanced NF-AT activation in T lymphocytes. Consistent with these results, T-cell proliferation and differentiation were also significantly promoted, and the levels of IL-2 expression were upregulated in TIPE2 knockdown Tregs as compared with normal controls. Above results imply that TIPE2 seems to be a novel negative regulator, which may affect the immune suppressive function mediated by Tregs during sepsis.
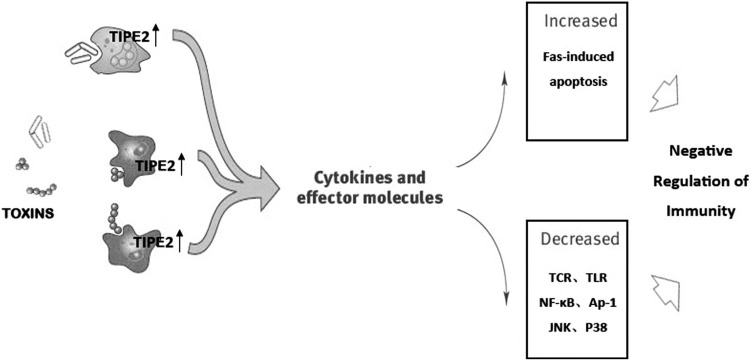
FIG. 2.
Regulation of innate and adaptive immunity by TIPE2 in T cells. The c-Jun N-terminal kinase (JNK), p38 and NF-κB pathways were found to be regulated by the TIPE2 action in T cells. TIPE2 can inhibit activation of AP-1 and NF-κB, and it is also an essential negative regulator of TLR and T cell receptor signal activation. In addition, TIPE2 may significantly enhance the immune suppressive activity in Tregs via influencing the levels of cytokines and receptors. NF-κB, nuclear factor-κB; TIPE2, TNF-α induced protein 8 like-2; TLR, Toll-like receptors.
NKT cell and its role in maintaining immune homeostasis in sepsis
It is well known that septic shock undermines immune homeostasis by inducing an initial exacerbated inflammation that is rapidly followed by anti-inflammatory process. This latter immunoparalysis phase is characterized by lowering the function of T cells, NK cells, and B cells, and increasing anti-inflammatory cytokine release. NKT cells are a subset of αβ T cells, referred to as a part of the innate and adaptive immunity, and capable of potently regulating immunity through production of Th1 and Th2 cytokines and chemokines (Pastille and others 2011), activation of other components of the immune system, and cytolytic effector activity. NKT cells can be broadly divided into 2 different types: type I NKT cells, also called iNKT and type II NKT cells (Godfrey and others 2004). Both of their activation is restricted by the MHC I-related glycoprotein CD1d, which presents glycolipid and lipid antigens for recognition by NKT cells. In contrast to type II NKT cells, iNKT cells have not extensively studied up to now. As a subset of T lymphocytes, iNKT cells, which express semi-invariant α/β TCRs consisting of an invariant Vα24/Jα18 chain in human (Vα14/Jα18 in mouse) and a restricted β chain(Godfrey and others 2004), have the ability to promptly secrete large amounts of cytokines after TCR engagement (Bendelac and others 2007), such as IL-2, IL-4, and IFN-γ. Several research results have demonstrated that NKT cells might be involved in the development of sepsis.
NKT cells play major roles in maintaining immune homeostasis in face of a variety of disease situations. More recently, some data demonstrated a detrimental role for NKT cells during severe sepsis. At the early phase of sepsis, a number of pro-inflammatory cytokines become amplified, but as sepsis protracted, inhibitory cytokines ensue, including IL-10 and IL-4, which could limit the strength of immune cell activation and expansion, and negatively modulate inflammation (Fig. 3). NKT cells are capable of secreting copious amounts of cytokines including pro-inflammatory and inhibitory cytokines (IFN-γ and IL-4), respectively, upon stimulation. It has been documented that activated iNKT cells produced IL-4 more rapidly than most other cytokines (Wu and others 2009). Cytokines secreted by iNKT cells can also activate a variety of other types of cells, including DCs, macrophages, NK cells, B cells, and conventional T cells (Van Kaer 2004; Prasad 2007). NKT cells have been shown to have a number of cell surface markers and functions, which are similar to both T and NK cells. Like T cells, NKT cells express TCR and respond to diverse immune challenge by recognizing multiple antigens. Almost all NK cells express NK1.1, but not all NKT cells, both of them are engaged in crosstalks with other immune cells and bridge the innate and adaptive immune systems (Sun and Lanier 2009).
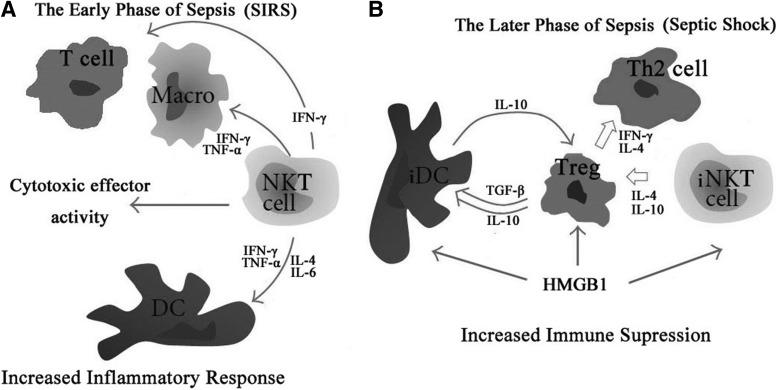
FIG. 3.
The immunoregulatory effect of immune cells in different phases of sepsis. (A) In the early phase of sepsis, macrophages, DCs, T lymphocytes, Tregs, and NKT cells play pivotal roles in the maintenance of peripheral homeostasis and regulation of inflammatory response by releasing various pro-inflammatory cytokines, including TNF-α and INF-γ. NKT cells also possess cytotoxic effector activity via Fas/Fas ligand and perforin pathway. (B) In the later phase of sepsis, inhibitory cytokines ensued, including IL-10 and IL-4, which limit the strength of immune cell activation and expansion and negatively modulate inflammation. In addition, extracellular HMGB1 has been shown to be able to provoke inflammation, to regulate functions of macrophage and T lymphocytes, and to mediate immune function of DCs and Tregs. HMGB1, high mobility group box-1 protein; TNF-α, tumor necrosis factor-α.
Notably, NKT cells are more similar to Tregs than other cells. Through release of cytokines such as TGF-β and IL-10, Tregs can induce anti-inflammatory or immunosuppressive response during the stages of severe sepsis and septic shock. Similar to Tregs, several reports showed that NKT cells could produce or induce these cytokines, but the immune response was shifted to Th1 or Th2 direction depending on the activating conditions (Berzofsky and Terabe 2008). In addition to cytokine production, NKT cells may induce cytotoxic effector activity via Fas/FasL and perforin pathway, which is also active in Tregs (Liu and others 2011c).
Recent experimental studies support the view that NKT cells are critically involved in the initiation and regulation of inflammatory and immune cascades in the setting of sepsis. In an endotoxemia model induced by a single intravenous injection of LPS, the number of iNKT cells were larger, and also produced mostly IFN-γ but undetectable levels of IL-4 after LPS exposure (Nagarajan and Kronenberg 2007). Fusakio and others (2011) demonstrated that naive NKT and NK cells expressed mRNA levels of complement receptor C5a (C5aR) and C5a receptor-like 2 (C5L2). NKT cells appeared to have a strong correlation between septic mortality and NKT cell activity, because NKT cells in the absence of C5aR showed greater survival together with reduced serum IFN-γ and TNF-α levels. Further, when mices were primed with anti-CD1d-blocking antibody in vivo, not only a strong reduction in IL-6 and IL-10 levels was observed in septic mice but also higher survival, suggesting that the activation of NKT cells played a primary role in regulating the innate immune/systemic inflammatory response and survival during early sepsis. These findings support the concept that NKT cells participate in the control of immunoreaction and are affected by acute insults and septic challenge.
The effect of DCs on immune function in sepsis
DCs are crucial in pathogen recognition and induction of specific immune responses to eliminate pathogens from the infected host. They are professional APCs specialized in the capturing, processing, and transporting of the antigen to lymphoid organs from the secondary lymphoid tissue (Banchereau and Steinman 1998; Hirsiger and others 2012). On the condition of different factors, DCs present diverse functions in implementing the biological effect. When nonspecific immune system is activated by pathogenic microorganisms and organ lesions, DCs not only present foreign antigen and homoantigen, but also activate effector T lymphocytes and induce Treg proliferation, thus determining the type of immune responses toward inflammation or anti-inflammation. Nevertheless, the production of Treg or effector T lymphocytes induced by DCs depends on their status of maturity, referring to the ratio of immature DCs and mature DCs. From a functional standpoint, DCs can be separated into immature antigen-capturing cells and mature immune-stimulating cells. Compared with mature DCs, the population of immature DCs can present with a low expression CD11c and a high expression of CD45RB, additionally, and they can express low amounts of costimulatory molecules (CD80, CD86, and MHC II), characterized by the appearance of plasmacytoid and immature phenotype, and secrete high levels of IL-10 after activation (Moore and others 2001; Wakkach and others 2003). These CD11clowCD45RBhigh DCs can significantly suppress pro-inflammatory cytokine production and protect mice from severe sepsis after trauma/burns (Liu and others 2011b).
DCs play important roles in host resistance and immunogenicity, and exhibit different expression of TLRs and cytokines by stimulation of various pathogens. Stimulation of TLRs may activate myeloid differentiation primary response protein 88 (MyD88)-dependent signaling pathways, which enhance the release of a range of pro-inflammatory cytokines. DCs, which are differentiated from peripheral monocytes, express TLR1, 2, 4, 5, and 8, and upon LPS stimulus, they strongly produce TNF-α and IL-6 (Kadowaki and others 2001). TLR2 and TLR4 are involved in the mechanisms leading to depletion of splenic DC following polymicrobial sepsis (Pene and others 2009). Immature CD11c+ DCs predominantly express TLR1, 2, and 3, they secrete high levels of IL-10 following activation and induce T-regulator type 1 cells both in vivo and in vitro (Akdis and Akdis 2007). Following severe trauma, burns, and sepsis, in addition to TLRs, DCs have the remarkable capacity to present processed antigens via major costimulatory molecules (CD80 and CD86) and MHC and initiate the development of innate and adaptive immune responses. At different time points after CLP, it was revealed that the expression of CD86 but not CD80 was strongly enhanced on DC isolated from the spleen as soon as 8 h after CLP, in contrast, lymph node DC equally upregulated the CD86 and CD80 expression (Nolan and others 2009). CD80-deficient mice had improved survival after CLP when compared with wild-type or CD86-deficient mice. In humans, upregulation of CD80 and loss of constitutive CD86 expression on monocytes displayed higher severity of illness, indicating the differential role for CD80 and CD86 in regulation of inflammation in the innate immune response to sepsis (Nolan and others 2009). Recently, several reports have indicated that endoplasmic reticulum stress (ERS) response might be involved not only in differentiation and plasticity of T cells but also development and maturation of DC (Bravo and others 2012). ER, one of the most important organelles in eukaryotic cells, is extremely sensitive to alterations in homeostasis. In response to ERS, mammalian cells trigger unfolded protein response (UPR) signaling pathways to cope with stressful conditions and to monitor the protein folding capacity of the ER and transmit that information to mechanisms that can modulate the ER environment, regulate various aspects of cellular metabolism, and even influence the fate of the cell (Amodio and others 2009; Bravo and others 2012; Zhu and others 2012). Accordingly, Zhu and others (2012) demonstrated that HMGB1 might induce the maturation and activation of DCs, and regulate its function by modulating the ERS response and UPR pathway, thus providing intensive insights into the critical mechanism for endogenous sources of cellular stress in the central role of DCs in immunity during sepsis.
More recently, the potential regulatory function of a DC subset, characterized by their particular surface marker expression of CD11clowCD45RBhigh, has also been investigated. Regulatory CD11clowCD45RBhigh DCs may affect the immunological activity through releasing high levels of suppressor cytokines (IL-10) but low levels of inflammatory cytokines (IL-12), thereby exerting immunoregulatory effects. Fujita and others found that these regulatory DCs generated in vitro by culturing bone marrow cells obtained from mice protected against septic response to microbial pathogens in innate immunity (Fujita and others 2006). Likewise, we reported that as a largely neglected subset of DCs, CD11clowCD45RBhigh DC could induce antigen-specific tolerance and protect mice from LPS-induced host inflammatory responses after thermal injury (Pene and others 2009). When a high dose of LPS was injected into mice, the regulatory DCs and its counterpart of the conventional DCs was rapidly expanded, indicating these cells could be triggered by a high inflammatory stimulus (Gehrie and others 2011). It is well known that sepsis was associated with a loss of resident DC in the bone marrow; however, in response to polymicrobial sepsis, DC precursor cells in the bone marrow develop into regulatory DC that impaired Th1 priming and NK cell activity, and thus mediating immunosuppression. Therefore, the regulatory function of these DCs has been shown that increased regulatory capacity of CD11clowCD45RBhigh DC can be associated with uncontrolled inflammatory responses followed by tissue and organ destruction. The potential roles of different sets of DCs and their exact molecular mechanisms in pathologic conditions such as sepsis have not yet fully been clarified. Concurrently, it has been reported the relationship between DCs and HMGB1 during the process of cellular activation, and the effect of HMGB1 on the naturally existing CD11clowCD45RBhigh DCs was investigated. The results showed that compared to CD11chighCD45RBlow DCs, the ratio of CD11clowCD45RBhigh DCs was significantly elevated after being treated with HMGB1, indicating that HMGB1 can promote the differentiation of DCs to CD11clowCD45RBhigh DCs, which induce suppression of T lymphocyte immune function and shifting of Th1 to Th2 in vitro (Zhu and others 2009b; Zhang and others 2010).
Therapeutic Modulation of Immune Function in Severe Sepsis
It is extremely important that approaches to immunoregulation could be applied in the treatment of severe human sepsis. Currently, many molecular techniques have been developed and successfully used to detect or identify microorganisms in infectious diseases, including early diagnosis of sepsis in critical illness. Meanwhile, there are also advances in the treatment of severe sepsis during recent years. It has been noticed that there were increased levels of leptin initially while subsequently declined levels during sepsis, leptin has the ability to enhance inflammation and the host response and is considered to be a potential therapeutic agent (Tschop and others 2010). Cannabinoid receptor 2, the expression of which is upregulated in sepsis, may reduce leukocyte activation and inflammatory mediator formation in acute experimental sepsis (Lehmann and others 2012). Treatment with cannabinoid receptor 2-agonist also showed decreased serum IL-6 levels and enhanced neutrophil activation and p38 activity during sepsis (Tschop and others 2009). Although there are conflicting results regarding the effects of cannabinoid receptor 2 during the immune response to an overwhelming infection, the therapeutic potential in sepsis is confirmed. Wang and others (2011) found Liver X receptor, a transcription factor of the nuclear receptor family, played an important role in protection against liver injury in experimental sepsis induced by CLP. Drugs targeting these pathways and genes may have therapeutic potential in the setting of sepsis. In addition, subsets of innate immune cells, which are involved in both protective immunity and immunopathology, have been showed to play critical roles during the different stages of severe sepsis and septic shock. It is believed that the pathophysiology of sepsis involves the hyperactivation of complex inflammatory cascades that are related to the immune cell activation and the exuberant inflammatory cytokines secreted by these cells. Therefore, it is especially important to introduce effective immune regulatory therapies, avoiding inappropriate use of antibiotics that might be associated with increased risk for poor outcome among septic patients.
In severe sepsis, there exists apoptosis of a variety of immune cells, such as macrophages, T cells, DCs, and vascular endothelial cells in the development of MODS. Multiple studies have demonstrated that cell apoptosis is presented in the pathophysiology of sepsis, therefore, prevention of cell death seems to be an attractive therapeutic target for severe sepsis. Glucocorticoids have been commonly used in the treatment of severe sepsis and septic shock. However, recent studies showed that high-dose glucocorticoid therapy might accelerate sepsis-induced cell apoptosis, which increased the risk of immune depression and the incidence of secondary infections, although Annane and others (2002) showed low-dose treatment with hydrocortisone could reduce the morbidity and improve survival of septic shock. Apoptosis is regulated by a number of intrinsic and extrinsic signaling pathways, and also ERS-mediated pathway. Ample evidence had shown that both Bid- and Bim-deficient mice survived better with CLP-induced sepsis, and overexpression of Bcl-2 in T cells was responsible for the decreased mortality (Prakash and others 2012), indicating that administration of siRNA might be a promising therapy for sepsis. Moreover, the function of extrinsic pathway-associated proteins has been examined in the treatment of severe sepsis, such as inhibition of multiple death receptors or their ligands, downregulation of the expression of FasL (Chung and others 1998) and Fas with siRNA, resulting in lowering the mortality following septic challenge. Synthetic siRNA, as a potential inhibitor of active caspases, such as caspase-8 and caspase-3 siRNAs, can improve survival of mice after CLP (Wesche-Soldato and others 2005). In addition to targeting therapy in the intrinsic and extrinsic apoptotic pathways, cytokines may also represent novel therapeutic approaches to improve outcome in sepsis. Currently, IL-7 was showed to have the ability to promote T cell survival by upregulating the expression of of Bcl-2, and IL-7 treatment in septic mice could improve their survival rate by protecting CD4+ and CD8+ T cells from apoptosis, and promoting function of immune effector T cells (Unsinger and others 2010). Altogether, many cytokines and intrinsic and extrinsic pathways may be closely associated with sepsis-induced apoptosis of T cells, so that siRNA therapy may offer a unique alternative treatment by reducing cellular apoptosis, and thus a number of targets in apoptotic pathway are revealed, showing that anti-apoptotic therapies could be considered for clinical trials in the treatment of severe sepsis.
The DC is now also examined as a target to manipulate the immune system. Some in vitro studies showed that DCs had the ability to induce T cell-mediated immune response, and they had been used in vaccine therapies in a variety of diseases. DC immunization may protect the mouse against tumors (Tuting and others 1997), viruses, and bacteria. Modifying the phenotypic response by DCs during the septic response represents a novel therapeutic approach that we have been pursuing for the past several years. In the septic condition, increased T cell apoptosis in vivo appears to be associated with DC phenotype, modifying which would improve the outcome of sepsis. In contrast to T cell death, DC depletion in the CLP models was delayed relatively, and this process appeared to be important in the development of sepsis (Sagar and others 2012). At present, it is practical to interrupt the pathological process of sepsis through genetic and viral manipulations of the DC phenotype. Additionally, DC phenotype can be modified by transfection with adenovirus. Though this altered phenotype has been successfully used in the treatment of a number of autoimmune diseases, the treatment of acute inflammatory diseases including severe sepsis is presently underway. In a further study, by considering the central role of these cells in regulating innate and acquired immunity, Laudanski (2012) demonstrated that transplantation of DCs could reverse several aspects of the long-term postsepsis immunodepression. In response to polymicrobial sepsis, DC precursor cells in the bone marrow may develop into regulatory DCs that impair the function of Th1 cells and NK cells (Pastille and others 2011), leading to immune dissonance. Therefore, normalization of DC differentiation from precursor cells in the bone marrow may reduce the susceptibility of the host to secondary infections, and it is a promising candidate for therapy against sepsis.
It is also clear that in patients and in animals the suppressive capacity of Tregs is amplified after severe injury or sepsis. Treg depletion, as induced by administration of anti-CD25 antibody, tends to show its protective effect in sepsis-induced damage. Nevertheless, CD25 is not the only marker for Tregs; Foxp3 and CTLA-4 are also involved in regulation of Tregs. For the development of more specific targeting methods, it was observed that pretreatment of septic mice with Astragalus polysaccharides (APS), Foxp3 expression, and IL-10 production were significantly decreased, and it was followed by restoration of proliferative activity of IL-2 and IL-2Rα expression on effector T cells (Liu and others 2011a), suggesting that APS might impair CD4+CD25+Treg activity and immunosuppressive function. This phenomenon might be taken as a potential strategy for the treatment of the immunosuppressive conditions in sepsis. Moreover, Hiraki and others (2012) found a marked increase in the percentage of Tregs in peripheral blood lymphocytes isolated from septic patients, and they observed that regulation of Tregs by neutralizing IL-10 or TGF-β provided a potential valuable for the treatment of sepsis. At present, the effect of hemoperfusion with polymyxin B-immobilized fibers (PMX-F) on the recovery from immunosuppression associated with sepsis has been evaluated. Ono and his group showed that PMX-F therapy significantly decreased the number of Tregs and serum IL-6 and IL-10 levels in patients with septic shock (Ono and others 2013), indicating that it might be an effective therapy for severe sepsis by removing Tregs by hemoperfusion with PMX-F. As mentioned above, these findings seem to confirm the pivotal role of Tregs in modulating the anti-inflammatory immune response after trauma/sepsis. Based on the known properties of Tregs, Zhao and others (2012) investigated the effect of curcumin (a phytochemical obtained from the rhizome of the plant Curcuma longa) on Tregs and its mechanism; they found that the expression of Foxp3 and CTLA-4, inhibitory cytokine secretion, IL-2 and IL-2Rα production, and nuclear translocation of p65 and c-Rel were all significantly downregulated in Tregs with curcumin stimulation, suggesting that it might be feasible to use curcumin as an immunotherapy for Treg-related disorders during sepsis. In addition, it is interesting to note that TIPE2 plays a critical role in the Treg-mediated immunity, as an increase in Treg activity in vitro was not present in TIPE2-deficient mice (Luan and others 2011). If additional animal studies or clinical trials continue to support the efficacy of TIPE2 in the treatment of sepsis, TIPE2 would be also considered for a clinical trial in the treatment of sepsis.
In addition to above immune cells, NKT cells have also been considered to be involved in the regulation of septic response. It has been documented that type II NKT cell activation can be achieved by treatment with sulfatide, and they can mediate anergy in iNKT cells. The potential therapeutic applications for inflammatory diseases have been strongly implicated in iNKT cells, such as antitumor immunity, autoimmune disease, and asthma (Halder and others 2007). Presently, targeted NKT cell therapies are developed in severe sepsis by various strategies to enhance immune regulation of NKT cells. During the development of septic shock, there appears to be disproportionate Th1 cytokine secretion. In a study, with the administration of alpha-galactosylceramide (α-GalCer) (Parekh and others 2005), the survived animals exhibited iNKT cell proliferation, lower levels of Th1 cytokines, but an increase in Th2 cytokines. These results suggested a potential therapeutic application for α-GalCer in modulating and influencing iNKT cell response to immunosuppressive state. Further, experimental studies revealed that apolipoprotein E (apoE) could modulate the septic immune response, influencing septic morbidity by NKT cells via the low-density lipoprotein receptor (LDLR) (Wang and others 2009). Recent evidence has implicated that apoE antagonism (inhibition of apoE's LDLR-binding site) protected mice against septic mortality, following diminished NKT proliferation and cytokine release (Chuang and others 2011). For the potential therapeutic value of NKT cells, new approaches should also allow modulation of the immune function of NKT cells during the different phases of severe sepsis, providing more targeted therapies with fewer side effects.
Conclusions
Severe sepsis and septic shock occur with a high incidence and prevalence in emergency departments and intensive care units, and they may result in both the widespread activation and dysfunction of the innate and adaptive responses in immune system. A large amount of information concerning subsets of innate immune cells in sepsis has implicated that these cells (including neutrophils, macrophages, DCs, T lymphocytes, Tregs, and NKT cells) have profound effects on immunoreactivity during acute insults/sepsis through modulating multiple receptor expression or cytokine secretion, in turn affecting the development and outcome of severe sepsis. Despite the evolution of aggressive surgical techniques, extensive methods of supportive care, and a vast array of anti-microbial choices, unfortunately, these therapeutic strategies have failed to sufficiently reduce mortality in severely septic patients. Contributions from a number of laboratories have demonstrated critical roles of immune cells in the innate and acquired immunity of sepsis, such as accelerating lymphocyte apoptosis during sepsis induced by macrophages and immature DCs, and Treg-mediating immunosuppression through direct cell–cell contact and/or soluble mediators. From a greater understanding of the pathogenesis of critical illness, we have now concluded that sepsis syndromes are truly a disease associated with the immune system. Similar to other autoimmune diseases, the innate immune response to sepsis is modulated by different immune cells and environmental factors that contribute to a dysfunctional phenotype. Therefore, the findings on the basic mechanisms involved in the innate immune cells in severe sepsis will provide an opportunity to accurately evaluate the patient's immune status, and develop novel immunomodulatory strategy at the early stage of septic complications.
Acknowledgments
This study was supported, in part, by grants from the National Natural Science Foundation (Nos. 81130035, 81071545, 81272090, and 81121004), the National Basic Research Program of China (No. 2012CB518102), and the Medical Research Foundation of Chinese PLA (Nos. AWS11J008 and BWS12J050).
Author Disclosure Statement
No competing financial interests exist.
References
- Akdis M, Akdis CA. 2007. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol 119(4):780–791 [DOI] [PubMed] [Google Scholar]
- Alves-Filho JC, Spiller F, Cunha FQ. 2010. Neutrophil paralysis in sepsis. Shock 34Suppl 1:15–21 [DOI] [PubMed] [Google Scholar]
- Amodio G, Renna M, Paladino S, Venturi C, Tacchetti C, Moltedo O, Franceschelli S, Mallardo M, Bonatti S, Remondelli P. 2009. Endoplasmic reticulum stress reduces the export from the ER and alters thearchitecture of post-ER compartments. Int J Biochem Cell Biol 41(12):2511–2521 [DOI] [PubMed] [Google Scholar]
- Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troché G. 2002. Effect of treatment with low doses of hydrocortisone and fludrocortisone onmortality in patients with septic shock. JAMA 288(7):862–871 [DOI] [PubMed] [Google Scholar]
- Bach JF. 2003. Regulatory T cells under scrutiny. Nat Rev Immunol 3(3):189–198 [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392(6673):245–252 [DOI] [PubMed] [Google Scholar]
- Banham AH. 2006. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3+ regulatory T cells. Trends Immunol 27(12):541–544 [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. 2007. The biology of NKT cells. Annu Rev Immunol 25:297–336 [DOI] [PubMed] [Google Scholar]
- Berzofsky JA, Terabe M. 2008. A novel immunoregulatory axis of NKT cell subsets regulating tumor immunity. Cancer Immunol Immunother 57(11):1679–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Gutierrez T, Paredes F, Gatica D, Rodriguez AE, Pedrozo Z, Chiong M, Parra V, Quest AF, Rothermel BA. 2012. Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int J Biochem Cell Biol 44(1):16–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, Hotchkiss RS. 2007. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J 21(3):708–719 [DOI] [PubMed] [Google Scholar]
- Cherwinski HM, Schumacher JH, Brown KD, Mosmann TR. 1987. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med 166(5):1229–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KI, Leung B, Hsu N, Harris HW. 2011. Heparin protects against septic mortality via apoE-antagonism. Am J Surg 202(3):325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CS, Xu YX, Wang W, Chaudry IH, Ayala A. 1998. Is Fas ligand or endotoxin responsible for mucosal lymphocyte apoptosis in sepsis. Arch Surg 133(11):1213–1220 [DOI] [PubMed] [Google Scholar]
- Coffman RL. 2006. Origins of the T(H)1-T(H)2 model: a personal perspective. Nat Immunol 7(6):539–541 [DOI] [PubMed] [Google Scholar]
- Cohen J. 2002. The immunopathogenesis of sepsis. Nature 420(6917):885–891 [DOI] [PubMed] [Google Scholar]
- Drifte G, Dunn-Siegrist I, Tissieres P, Pugin J. 2013. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit Care Med 41(3):820–832 [DOI] [PubMed] [Google Scholar]
- Efron P, Moldawer LL. 2003. Sepsis and the dendritic cell. Shock 20(5):386–401 [DOI] [PubMed] [Google Scholar]
- Fadeel B, Orrenius S. 2005. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med 258(6):479–517 [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. 2005. Regulatory T cell lineage specification by the forkhead transcription factorfoxp3. Immunity 22(3):329–341 [DOI] [PubMed] [Google Scholar]
- Fujita S, Seino K, Sato K, Sato Y, Eizumi K, Yamashita N, Taniguchi M, Sato K. 2006. Regulatory dendritic cells act as regulators of acute lethal systemic inflammatory response. Blood 107(9):3656–3664 [DOI] [PubMed] [Google Scholar]
- Fusakio ME, Mohammed JP, Laumonnier Y, Hoebe K, Kohl J, Mattner J. 2011. C5a regulates NKT and NK cell functions in sepsis. J Immunol 187(11):5805–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A, Dixit S, Embers M, Gautam R, Philipp MT, Singh SR, Morici L, Dennis VA. 2012. Different patterns of expression and of IL-10 modulation of inflammatory mediators from macrophages of Lyme disease-resistant and -susceptible mice. PLoS One 7(9):e43860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrie E, Van der Touw W, Bromberg JS, Ochando JC. 2011. Plasmacytoid dendritic cells in tolerance. Methods Mol Biol 677:127–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P, Dickinson P, Smith CL. 2013. Early life response to infection. Curr Opin Infect Dis 26:213–218 [DOI] [PubMed] [Google Scholar]
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. 2004. NKT cells: what's in a name. Nat Rev Immunol 4(3):231–237 [DOI] [PubMed] [Google Scholar]
- Halder RC, Aguilera C, Maricic I, Kumar V. 2007. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest 117(8):2302–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanschen M, Tajima G, O'Leary F, Hoang K, Ikeda K, Lederer JA. 2012. Phospho-flow cytometry based analysis of differences in T cell receptor signaling between regulatory T cells and CD4+ T cells. J Immunol Methods 376(1–2):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever AW, Shevach EM, Wynn TA. 2004. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol 172(5):3157–3166 [DOI] [PubMed] [Google Scholar]
- Heuer JG, Zhang T, Zhao J, Ding C, Cramer M, Justen KL, Vonderfecht SL, Na S. 2005. Adoptive transfer of in vitro-stimulated CD4+CD25+regulatory T cells increases bacterial clearance and improves survival in polymicrobial sepsis. J Immunol 174(11):7141–7146 [DOI] [PubMed] [Google Scholar]
- Hiraki S, Ono S, Tsujimoto H, Kinoshita M, Takahata R, Miyazaki H, Saitoh D, Hase K. 2012. Neutralization of interleukin-10 or transforming growth factor-beta decreases the percentages of CD4+ CD25+ Foxp3+ regulatory T cells in septic mice, thereby leading to an improved survival. Surgery 151(2):313–322 [DOI] [PubMed] [Google Scholar]
- Hirsiger S, Simmen HP, Werner CM, Wanner GA, Rittirsch D. 2012. Danger signals activating the immune response after trauma. Mediators Inflamm 2012:315941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, et al. . 1999. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol 162(7):4148–4156 [PubMed] [Google Scholar]
- Huang HW, Tang JL, Han XH, Peng YP, Qiu YH. 2013. Lymphocyte-derived catecholamines induce a shift of Th1/Th2 balance toward Th2 polarization. Neuroimmunomodulation 20(1):1–8 [DOI] [PubMed] [Google Scholar]
- Inoue S, Sato T, Suzuki-Utsunomiya K, Komori Y, Hozumi K, Chiba T, Yahata T, Nakai K, Inokuchi S. 2013. Sepsis-induced hypercytokinemia and lymphocyte apoptosis in aging-accelerated Klotho knockout mice. Shock 39(3):311–316 [DOI] [PubMed] [Google Scholar]
- Jager A, Kuchroo VK. 2010. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol 72(3):173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Bell CW, Pisetsky DS. 2007. The relationship between apoptosis and high-mobility group protein 1 release frommurine macrophages stimulated with lipopolysaccharide orpolyinosinic-polycytidylic acid. J Immunol 178(10):6495–6503 [DOI] [PubMed] [Google Scholar]
- Jimbo A, Fujita E, Kouroku Y, Ohnishi J, Inohara N, Kuida K, Sakamaki K, Yonehara S, Momoi T. 2003. ER stress induces caspase-8 activation, stimulating cytochrome release andcaspase-9 activation. Exp Cell Res 283(2):156–166 [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. 2001. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med 193(11):1285–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J Exp Med 194(6):863–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten KR, Tschop J, Adediran SG, Hildeman DA, Caldwell CC. 2010. T cells are potent early mediators of the host response to sepsis. Shock 34(4):327–336 [DOI] [PubMed] [Google Scholar]
- Kockara A, Kayatas M. 2013. Renal cell apoptosis and new treatment options in sepsis-induced acute kidney injury. Ren Fail 35(2):291–294 [DOI] [PubMed] [Google Scholar]
- Kumar A, Kumar A, Michael P, Brabant D, Parissenti AM, Ramana CV, Xu X, Parrillo JE. 2005. Human serum from patients with septic shock activates transcription factorsSTAT1, IRF1, and NF-kappaB and induces apoptosis in human cardiac myocytes. J Biol Chem 280(52):42619–42626 [DOI] [PubMed] [Google Scholar]
- Lall H, Coughlan K, Sumbayev VV. 2008. HIF-1alpha protein is an essential factor for protection of myeloid cells against LPS-induced depletion of ATP and apoptosis that supports Toll-like receptor4-mediated production of IL-6. Mol Immunol 45(11):3045–3049 [DOI] [PubMed] [Google Scholar]
- Lang JD, Matute-Bello G. 2009. Lymphocytes, apoptosis and sepsis: making the jump from mice to humans. Crit Care 13(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larroche C, Mouthon L. 2004. Pathogenesis of hemophagocytic syndrome (HPS). Autoimmun Rev 3(2):69–75 [DOI] [PubMed] [Google Scholar]
- Laudanski K. 2012. Adoptive transfer of naive dendritic cells in resolving post-sepsis long-term immunosuppression. Med Hypotheses 79(4):478–480 [DOI] [PubMed] [Google Scholar]
- Lehmann C, Kianian M, Zhou J, Kuster I, Kuschnereit R, Whynot S, Hung O, Shukla R, Johnston B, Cerny V, Pavlovic D, Spassov A, Kelly ME. 2012. Cannabinoid receptor 2 activation reduces intestinal leukocyte recruitment and systemic inflammatory mediator release in acute experimental sepsis. Crit Care 16(2):R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Song L, Fan Y, Li X, Li Y, Chen J, Zhu F, Guo C, Shi Y, Zhang L. 2009. Down-regulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Immunol 133(3):422–427 [DOI] [PubMed] [Google Scholar]
- Liu QY, Yao YM, Yu Y, Dong N, Sheng ZY. 2011a. Astragalus polysaccharides attenuate postburn sepsis via inhibiting negative immunoregulation of CD4+ CD25high T cells. PLoS One 6(6):e19811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QY, Yao YM, Zhang SW, Yan YH, Wu X. 2011b. Naturally existing CD11clowCD45RBhigh dendritic cells protect mice from acute severe inflammatory response induced by thermal injury. Immunobiology 216(1–2):47–53 [DOI] [PubMed] [Google Scholar]
- Liu Y, Teige A, Mondoc E, Ibrahim S, Holmdahl R, Issazadeh-Navikas S. 2011c. Endogenous collagen peptide activation of CD1d-restricted NKT cells ameliorates tissue-specific inflammation in mice. J Clin Invest 121(1):249–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. 2005. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5(4):331–342 [DOI] [PubMed] [Google Scholar]
- Luan YY, Yao YM, Sheng ZY. 2012. Update on the immunological pathway of negative regulation in acute insults and sepsis. J Interferon Cytokine Res 32(7):288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan YY, Yao YM, Zhang L, Dong N, Zhang QH, Yu Y, Sheng ZY. 2011. Expression of tumor necrosis factor-alpha induced protein 8 like-2 contributes to the immunosuppressive property of CD4+CD25+ regulatory T cells in mice. Mol Immunol 49(1–2):219–226 [DOI] [PubMed] [Google Scholar]
- Ma T, Han L, Gao Y, Li L, Shang X, Hu W, Xue C. 2008. The endoplasmic reticulum stress-mediated apoptosis signal pathway is involved in sepsis-induced abnormal lymphocyte apoptosis. Eur Surg Res 41(2):219–225 [DOI] [PubMed] [Google Scholar]
- MacConmara MP, Maung AA, Fujimi S, McKenna AM, Delisle A, Lapchak PH, Rogers S, Lederer JA, Mannick JA. 2006. Increased CD4+ CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann Surg 244(4):514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Teramae H, Futatsugi M, Takano K, Yamamoto S, Tomita K, Suzuki T, Yokoo H, Koike K, Hattori Y. 2010. Up-regulation of histamine H4 receptors contributes to splenic apoptosis inseptic mice: counteraction of the antiapoptotic action of nuclear factor-kappaB. J Pharmacol Exp Ther 332(3):730–737 [DOI] [PubMed] [Google Scholar]
- Miller AC, Rashid RM, Elamin EM. 2007. The “T” in trauma: the helper T-cell response and the role of immunomodulation in trauma and burn patients. J Trauma 63(6):1407–1417 [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’ Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683–765 [DOI] [PubMed] [Google Scholar]
- Nagarajan NA, Kronenberg M. 2007. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol 178(5):2706–2713 [DOI] [PubMed] [Google Scholar]
- Nakae S, Nambu A, Sudo K, Iwakura Y. 2003. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol 171(11):6173–6177 [DOI] [PubMed] [Google Scholar]
- Nolan A, Kobayashi H, Naveed B, Kelly A, Hoshino Y, Hoshino S, Karulf MR, Rom WN, Weiden MD, Gold JA. 2009. Differential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsis. PLoS One 4(8):e6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny AR, Reim D, Assfalg V, Altmayr F, Friess HM, Emmanuel K, Holzmann B. 2012. Mixed antagonist response and sepsis severity-dependent dysbalance of pro- and anti-inflammatory responses at the onset of postoperative sepsis. Immunobiology 217(6):616–621 [DOI] [PubMed] [Google Scholar]
- Oakes SA, Lin SS, Bassik MC. 2006. The control of endoplasmic reticulum-initiated apoptosis by the BCL-2 family of proteins. Curr Mol Med 6(1):99–109 [DOI] [PubMed] [Google Scholar]
- Oberholzer A, Oberholzer C, Moldawer LL. 2001. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 16(2):83–96 [DOI] [PubMed] [Google Scholar]
- Ono S, Kimura A, Hiraki S, Takahata R, Tsujimoto H, Kinoshita M, Miyazaki H, Yamamoto J, Hase K, Saitoh D. 2013. Removal of increased circulating CD4+CD25+Foxp3+ regulatory T cells in patients with septic shock using hemoperfusion with polymyxin B-immobilized fibers. Surgery 153(2):262–271 [DOI] [PubMed] [Google Scholar]
- Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. 2005. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest 115(9):2572–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastille E, Didovic S, Brauckmann D, Rani M, Agrawal H, Schade FU, Zhang Y, Flohe SB. 2011b. Modulation of dendritic cell differentiation in the bone marrow mediates sustained immunosuppression after polymicrobial sepsis. J Immunol 186(2):977–986 [DOI] [PubMed] [Google Scholar]
- Pene F, Courtine E, Ouaaz F, Zuber B, Sauneuf B, Sirgo G, Rousseau C, Toubiana J, Balloy V, et al. . 2009. Toll-like receptors 2 and 4 contribute to sepsis-induced depletion of spleen dendritic cells. Infect Immun 77(12):5651–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. 2004. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci U S A 101(13):4572–4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash PS, Caldwell CC, Lentsch AB, Pritts TA, Robinson BR. 2012. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J Trauma Acute Care Surg 73(2):401–406; discussion 406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AS. 2007. Zinc: mechanisms of host defense. J Nutr 137(5):1345–1349 [DOI] [PubMed] [Google Scholar]
- Sagar D, Lamontagne A, Foss CA, Khan ZK, Pomper MG, Jain P. 2012. Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2chemotaxis at the blood-brain barrier through paracellular transmigration and ERK activation. J Neuroinflammation 9:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155(3):1151–1164 [PubMed] [Google Scholar]
- Sakaguchi S, Setoguchi R, Yagi H, Nomura T. 2006. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in self-tolerance and autoimmune disease. Curr Top Microbiol Immunol 305:51–66 [DOI] [PubMed] [Google Scholar]
- Schwandt T, Schumak B, Gielen GH, Jüngerkes F, Schmidbauer P, Klocke K, Staratschek-Jox A, van Rooijen N, Kraal G, Ludwig-Portugall I, Franken L, Wehner S, Kalff JC, Weber O, Kirschning C, Coch C, Kalinke U, Wenzel J, Kurts C, Zawatzky R, Holzmann B, Layland L, Schultze JL, Burgdorf S, den Haan JM, Knolle PA, Limmer A. 2012. Expression of type I interferon by splenic macrophages suppresses adaptive immunity during sepsis. EMBO J 31(1):201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwulst SJ, Muenzer JT, Peck-Palmer OM, Chang KC, Davis CG, McDonough JS, Osborne DF, Walton AH, Unsinger J, McDunn JE, Hotchkiss RS. 2008. Bim siRNA decreases lymphocyte apoptosis and improves survival in sepsis. Shock 30(2):127–134 [DOI] [PubMed] [Google Scholar]
- Shinkai K, Mohrs M, Locksley RM. 2002. Helper T cells regulate type-2 innate immunity in vivo. Nature 420(6917):825–829 [DOI] [PubMed] [Google Scholar]
- Silva JM, Dos SSS. 2013. Sepsis in AIDS patients: clinical, etiological and inflammatory characteristics. J Int AIDS Soc 16:17344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza HP, Lima-Salgado T, da CNLM. 2010. Toll-like receptors in sepsis: a tale still being told. Endocr Metab Immune Disord Drug Targets 10(3):285–291 [DOI] [PubMed] [Google Scholar]
- Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, Kong L, Xu L, Hilliard B, Hu S, Shen H, Yang X, Chen YH. 2008. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 133(3):415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Lanier LL. 2009. Natural killer cells remember: an evolutionary bridge between innate and adaptive immunity. Eur J Immunol 39(8):2059–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatura R, Zeschnigk M, Adamzik M, Probst-Kepper M, Buer J, Kehrmann J. 2012. Quantification of regulatory T cells in septic patients by real-time PCR-based methylation assay and flow cytometry. PLoS One 7(11):e49962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop J, Dattilo JR, Prakash PS, Kasten KR, Tschop MH, Caldwell CC. 2010. The leptin system: a potential target for sepsis induced immune suppression. Endocr Metab Immune Disord Drug Targets 10(4):336–347 [DOI] [PubMed] [Google Scholar]
- Tschop J, Kasten KR, Nogueiras R, Goetzman HS, Cave CM, England LG, Dattilo J, Lentsch AB, Tschöp MH, Caldwell CC. 2009. The cannabinoid receptor 2 is critical for the host response to sepsis. J Immunol 183(1):499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop J, Martignoni A, Goetzman HS, Choi LG, Wang Q, Noel JG, Ogle CK, Pritts TA, Johannigman JA, Lentsch AB, Caldwell CC. 2008. Gammadelta T cells mitigate the organ injury and mortality of sepsis. J Leukoc Biol 83(3):581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuting T, DeLeo AB, Lotze MT, Storkus WJ. 1997. Genetically modified bone marrow-derived dendritic cells expressing tumor-associated viral or “self” antigens induce antitumor immunity in vivo. Eur J Immunol 27(10):2702–2707 [DOI] [PubMed] [Google Scholar]
- Unnewehr H, Rittirsch D, Sarma JV, Zetoune F, Flierl MA, Perl M, Denk S, Weiss M, Schneider ME, Monk PN, Neff T, Mihlan M, Barth H, Gebhard F, Ward PA, Huber-Lang M. 2013. Changes and regulation of the C5a receptor on neutrophils during septic shock in humans. J Immunol 190:4215–4225 [DOI] [PubMed] [Google Scholar]
- Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, McDonough JS, Tschoep J, Ferguson TA, McDunn JE, Morre M, Hildeman DA, Caldwell CC, Hotchkiss RS. 2010. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol 184(7):3768–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kaer L. 2004. Regulation of immune responses by CD1d-restricted natural killer T cells. Immunol Res 30(2):139–153 [DOI] [PubMed] [Google Scholar]
- Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, Powell WS, Monneret G. 2006. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand- dependent mechanism. J Immunol 177(9):6540–6547 [DOI] [PubMed] [Google Scholar]
- Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. 2003. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 18(5):605–617 [DOI] [PubMed] [Google Scholar]
- Wang DW, Zhou RB, Yao YM, Zhu XM, Yin YM, Zhao GJ, Dong N, Sheng ZY. 2010. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J Pharmacol Exp Ther 335(3):553–561 [DOI] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285(5425):248–251 [DOI] [PubMed] [Google Scholar]
- Wang H, Christensen DJ, Vitek MP, Sullivan PM, Laskowitz DT. 2009. APOE genotype affects outcome in a murine model of sepsis: implications for a new treatment strategy. Anaesth Intensive Care 37(1):38–45 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhu S, Zhou R, Li W, Sama AE. 2008. Therapeutic potential of HMGB1-targeting agents in sepsis. Expert Rev Mol Med 10:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Ryg U, Dahle MK, Steffensen KR, Thiemermann C, Chaudry IH, Reinholt FP, Collins JL, Nebb HI, Aasen AO, Gustafsson JA, Wang JE. 2011. Liver X receptor protects against liver injury in sepsis caused by rodent cecal ligation and puncture. Surg Infect (Larchmt) 12(4):283–289 [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25:821–852 [DOI] [PubMed] [Google Scholar]
- Weber SU, Schewe JC, Lehmann LE, Muller S, Book M, Klaschik S, Hoeft A, Stuber F. 2008. Induction of Bim and Bid gene expression during accelerated apoptosis in severe sepsis. Crit Care 12(5):R128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche-Soldato DE, Chung CS, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A. 2005. In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood 106(7):2295–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Gabriel CL, Parekh VV, Van Kaer L. 2009. Invariant natural killer T cells: innate-like T cells with potent immunomodulatory activities. Tissue Antigens 73(6):535–545 [DOI] [PubMed] [Google Scholar]
- Yang X, Jiang X, Chen G, Xiao Y, Geng S, Kang C, Zhou T, Li Y, Guo X, Xiao H, Hou C, Wang R, Lin Z, Li X, Feng J, Ma Y, Shen B, Li Y, Han G. 2013. T cell Ig mucin-3 promotes homeostasis of sepsis by negatively regulating the TLR response. J Immunol 190(5):2068–2079 [DOI] [PubMed] [Google Scholar]
- Yao YM, Redl H, Bahrami S, Schlag G. 1998. The inflammatory basis of trauma/shock-associated multiple organ failure. Inflamm Res 47(5):201–210 [DOI] [PubMed] [Google Scholar]
- Zhang LT, Yao YM, Yao FH, Huang LF, Dong N, Yu Y, Sheng ZY. 2010. Association between high-mobility group box-1 protein release and immune function of dendritic cells in thermal injury. J Interferon Cytokine Res 30(7):487–495 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang J, Fan C, Li H, Sun H, Gong S, Chen YH, Shi Y. 2009. Crystal structure of TIPE2 provides insights into immune homeostasis. Nat Struct Mol Biol 16(1):89–90 [DOI] [PubMed] [Google Scholar]
- Zhao GJ, Lu ZQ, Tang LM, Wu ZS, Wang DW, Zheng JY, Qiu QM. 2012. Curcumin inhibits suppressive capacity of naturally occurring CD4+CD25+regulatory T cells in mice in vitro. Int Immunopharmacol 14(1):99–106 [DOI] [PubMed] [Google Scholar]
- Zheng D, Sun Q, Su Z, Kong F, Shi X, Tong J, Shen P, Peng T, Wang S, Xu H. 2013. Enhancing specific-antibody production to the rag B vaccine with GITRL that expand Tfh, IFN-gamma(+) T cells and attenuates porphyromonas gingivalis infection in mice. PLoS One 8(4):e59604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XM, Yao FH, Yao YM, Dong N, Yu Y, Sheng ZY. 2012. Endoplasmic reticulum stress and its regulator XBP-1 contributes to dendriticcell maturation and activation induced by high mobility group box-1 protein. Int J Biochem Cell Biol 44(7):1097–1105 [DOI] [PubMed] [Google Scholar]
- Zhu XM, Yao YM, Liang HP, Liu F, Dong N, Yu Y, Sheng ZY. 2009a. Effect of high mobility group box-1 protein on apoptosis of peritoneal macrophages. Arch Biochem Biophys 492(1–2):54–61 [DOI] [PubMed] [Google Scholar]
- Zhu XM, Yao YM, Liang HP, Xu S, Dong N, Yu Y, Sheng ZY. 2009b. The effect of high mobility group box-1 protein on splenic dendritic cell maturation in rats. J Interferon Cytokine Res 29(10):677–686 [DOI] [PubMed] [Google Scholar]