Abstract
A rapid method combining microwave-assisted extraction and high-speed counter-current chromatography was applied for preparative separation of six bioactive compounds including loganic acid (I), isoorientin-4'-O-glucoside (II), 6'-O-β-d-glucopyranosyl gentiopicroside (III), swertiamarin (IV), gentiopicroside (V), sweroside (VI) from traditional Tibetan medicine Gentiana crassicaulis Duthie ex Burk. Microwave-assisted extraction parameters were predicted by central composite design-response surface methodology. That is, 5.0 g dried roots of G. Crassicaulis was extracted with 50 mL 57.5% aqueous ethanol under 630 W for 3.39 min. The extract (gentian total glycosides) was separated by high-speed counter-current chromatography with n-butanol/ethyl acetate/methanol/1% acetic acid water (7.5:0.5:0.5:3.5, v/v/v/v) using upper phase mobile in tail-to-head elution mode. 16.3 mg, 8.8 mg, 12.8 mg, 25.1 mg, 40.7 mg and 21.8 mg of compounds I–VI were obtained with high purities in one run from 500 mg of original sample. The purities and identities of separated components were confirmed using HPCL with photo diode array detection and quadrupole time-of-flight MS and NMR spectroscopy. The study reveals that response surface methodology is convenient and highly predictive for optimizing extraction process, microwave-assisted extraction coupled with high-speed counter-current chromatography could be an expeditious method for extraction and separation of phytochemicals from ethnomedicine.
Keywords: Gentiana crassicaulis Duthie ex Burk., High-speed counter-current chromatography, Microwave-assisted extraction, Quadrupole time-of-flight mass spectrometry, Response surface methodology
1. Introduction
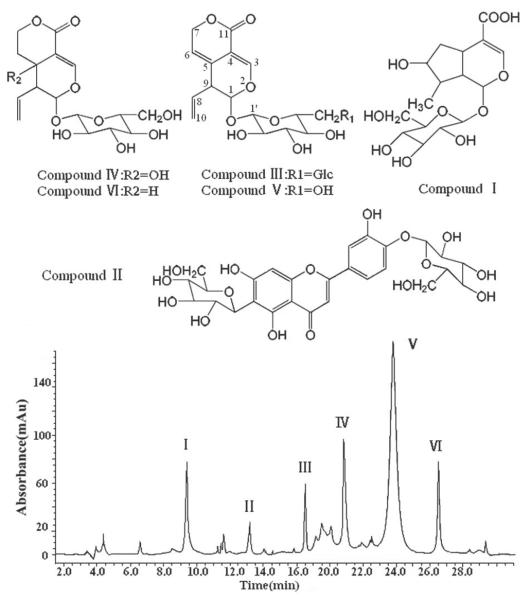
Gentiana crassicaulis Duthie ex Burk., a Tibetan medicinal herb which belongs to Gentianaceae family [1], is used for clinical treatment of pyrexia, rheumatoid arthritis, icteric hepatitis, etc. [2]. Developed in anoxic high altitude area, Tibetan medicine revealed many unique biological activities [2, 3]. Phytochemical study revealed that gentian total glycosides (GTG) is the main bioactive fraction in G. Crassicaulis [4], in which loganic acid, isoorientin-4'-O-glucoside, 6'-O-β-d-glucopyranosyl gentiopicroside, swertiamarin, gentiopicroside and sweroside (chemical structures shown in Fig. 1) are representative. These chemical constituents, frequently used as markers for quality evaluation of G. crassicaulis and related complex prescriptions [5, 6], showed interesting pharmacological effects [7–9]. GTG, trade name JIAOLONG capsule (Xi'an C. P. Pharmaceutical, China; approval number: Z20030101), is on sale as a gastroprokinetic drug in China for years, moreover, gentiopicroside, tradename QINGLONG KUSU powder-injection (Harbin Gloria Pharmaceutical, China; approval number: 2003L03260), is a novel hepatoprotective agent and is undergoing phase III clinical trials now, both drugs above were discovered and developed by our research group. For clinical use, plenty of pure substance of gentiopicroside are required, meanwhile, other constituents contained in GTG also attract considerable attention, so preparative separation method of gentiopicroside and other compounds contained in GTG is urgently needed. Some techniques such as hot reflux, ultrasonic-assisted extraction [10, 11], followed by macroporous resin and silica gel column chromatography [12, 13] were reported for separating only one or two compounds from G. Crassicaulis. Worse yet, these methods are inefficient, small scale and time consuming. Hence, this paper mainly focuses on developing an efficient preparation method of gentiopicroside and other compounds contained in GTG, which can be applied for clinical trials, pharmaceutical analysis and further bioactivity research.
Fig. 1.
Chemical structures of target compounds from G. crassicaulis and HPLC–PDA chromatogram of GTG.
Microwave-assisted extraction (MAE) is a process that enables solvent homogeneous heating and plant internal superheating which facilitates the extraction [14], its key advantages are shortened extraction time and high yield [15, 16]. Central composite design response surface methodology (CCD-RSM) is a collection of mathematical and statistical techniques that can be used for studying the effect of several factors at different level and their influence on each other [17, 18]. It is more informative than the classical one-variable-at-a-time approach and it needs reduced experimental trials [19, 20]. Therefore, CCD-RSM is an efficient method to model and optimize biochemical processes such as extraction of natural compounds.
On the other hand, high-speed counter-current chromatography (HSCCC) is a support-free liquid-liquid partition chromatography [21] with key advantages of rapid separation speed and none irreversible adsorption [22–24]. Thus far, no study concerning the application of MAE, predicted by CCD-RSM, together with HSCCC for preparation of phytochemicals was published. In addition, neither HSCCC nor other techniques were ever used for simultaneous preparative separation of gentiopicroside and other compounds contained in GTG (loganic acid, isoorientin-4'-O-glucoside, 6'-O-β-d-glucopyranosyl gentiopicroside, swertiamarin, gentiopicroside and sweroside) from any medicinal plant according to previous reports.
In the present study, an expeditious method combing MAE and HSCCC has been developed, gentiopicroside and five analogs were extracted and separated from G. Crassicaulis, even from natural herbs by this method for the first time. CCD-RSM comprising a three-factor-five-level experimental design was used for predicting and optimizing of MAE, the selected extraction process takes 3.39 min; the HSCCC separation was operated in one run using upper phase mobile in tail-to-head elution mode; furthermore, the combination of HPLC with photo diode array detection and quadrupole time-of-flight MS (HPLC–PDA–Q-TOF-MS) and NMR spectroscopy made the purity and structural elucidation of the obtained compounds more accurate and much faster. The results demonstrate the preparative capability of MAE coupled with HSCCC for separating phytochemicals from ethnomedicine.
2. Materials and methods
2.1. Apparatus
MAE experiments were carried out on MSP-100E microwave extraction apparatus (Leiming Science and Technology, Beijing, China). HSCCC equipment was a TBE-300A HSCCC instrument (Tauto Biotech, Shanghai, China) with three multilayer coil separation columns connected in series (I.D. of the tubing = 1.5 mm, volume = 280 mL) and a 20 mL sample loop. The β values of the multilayer coil varied from 0.5 at the internal terminal to 0.8 at the external terminal. The solvent was monitored with a Model 500A-UV Detector. The HPLC analysis were performed with a Varian 212-LC equipped with a PDA detector and a Q-TOF Premier (Waters, USA). The NMR spectra were recorded with an INOVA spectrometer (Varian, USA).
2.2. Reagents and materials
All organic solvents used for extraction and separation were of analytical grades and purchased from Tianjin Chemical Factory (Tianjin, China). Acetonitrile and acetic acid used for HPLC analysis were of chromatographic grade and purchased from Supervision of Kermel Chemical Reagents Development Center (Tianjin, China). Water was purified in a Milli-Q plus system (Millipore, Madrid, Spain). The roots of G. crassicaulis were provided by Xi'an C. P. Pharmaceutical (Xi'an, China).
2.3. Optimization of extraction of GTG by CCD-RSM
A three-factor-five-level (±β, ±1, 0; where β=23/4=1.682) CCD was employed to determine the best combination of MAE parameters. Loganic acid, swertiamarin and gentiopicroside were selected as the model compounds owing to relatively high content. The variables, which is most influential parameters for MAE, and their level selected were: ethanol concentration (X1; 24.77, 35, 50, 65, 75.23 %), microwave power (X2; 432, 500, 600, 700, 768 W), irradiation time (X3; 1.32, 2, 3, 4, 4.68 min). Six replicates (standard run number 15–20) at the center of the design were used for estimation of error sum of squares. Experiments were randomized to maximize the effects of unexplained variability in the observed responses. For each run, 5.0 g dried roots of G. Crassicaulis was used and the solid/liquid ratio was 1:10 g/mL which was optimized by mono-factor test. The experimental data shown in Table 1 were used for determining the regression coefficients and fitted to a quadratic polynomial equation by Design-Expert (version 7.1.3 Stat-Ease, Minneapolis, USA):
| (1) |
where Y is the total yield of model compounds analyzed by HPLC–PDA which was expressed with the weight proportion of extracted model compounds to the crude drug; β0 is a constant, βi, βii and βij are the linear, quadratic and interactive coefficients of the model, respectively; Xi and Xj are independent variables. The response surface and contour plots were generated using the same software in order to visualize the reactions of any two independent variables, while holding the value of the third one at central (0) level.
Table 1.
CCD layout and ANOVA results.
| CCD experiments | ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Run | X1 (%) | X2 (W) | X3(min) | Y (%) | Source | Sum of Squares | df | Mean Square | F value | P value |
| 1 | 35 | 500 | 2 | 7.86 | Model | 1.84 | 9 | 0.20 | 19.71 | 0.0001a) |
| 2 | 35 | 700 | 2 | 7.98 | X 1 | 0.45 | 1 | 0.45 | 43.77 | 0.0001a) |
| 3 | 65 | 500 | 2 | 8.29 | X 2 | 0.042 | 1 | 0.042 | 4.07 | 0.0713 |
| 4 | 65 | 700 | 2 | 8.37 | X 3 | 0.30 | 1 | 0.30 | 28.86 | 0.0003a) |
| 5 | 35 | 500 | 4 | 8.08 | X 1 X 2 | 0.00031 | 1 | 0.00031 | 0.030 | 0.8656 |
| 6 | 35 | 700 | 4 | 8.17 | X 1 X 3 | 0.00101 | 1 | 0.00101 | 0.098 | 0.7610 |
| 7 | 65 | 500 | 4 | 8.45 | X 2 X 3 | 0.00011 | 1 | 0.00011 | 0.011 | 0.9191 |
| 8 | 65 | 700 | 4 | 8.53 | X 1 2 | 0.76 | 1 | 0.76 | 72.92 | 0.0001a) |
| 9 | 50 | 432 | 3 | 8.33 | X 2 2 | 0.22 | 1 | 0.22 | 20.94 | 0.0010a) |
| 10 | 50 | 768 | 3 | 8.56 | X 3 2 | 0.29 | 1 | 0.29 | 27.99 | 0.0004a) |
| 11 | 24.77 | 600 | 3 | 7.86 | Residual | 0.10 | 10 | 0.10 | ||
| 12 | 75.23 | 600 | 3 | 8.42 | Lack of fit | 0.1 | 5 | 0.020 | 3.74 | 0.1173 |
| 13 | 50 | 600 | 1.32 | 8.01 | Pure error | 0.00175 | 5 | 0.00035 | ||
| 14 | 50 | 600 | 4.68 | 8.77 | Cor Total | 1.88 | 19 | |||
| 15–20 | 50 | 600 | 3 | 8.73 | ||||||
Significant at P < 0.05.
2.4. Selection of the two-phase solvent system and mobile phase
The two-phase solvent system was selected depending on the partition coefficient (K) of each target component as follows: a suitable amount of original sample was delivered into a 10 mL test tube to which about 2 mL of each phase of the pre-equilibrated solvent system were added. The capped tube was shaken vigorously for 1 min to thoroughly equilibrate the sample between two phases. Then, an equal volume of each phase was transferred and evaporated separately. The residue of each phase was dissolved in an equal volume of methanol and analyzed by HPLC–PDA to determine K value of each component. The peak area of the stationary phase was recorded as As and that of the mobile phase was recorded as Am. The K value was calculated based on the equation: K = As /Am.
Either upper or lower phase could be chosen as the mobile phase which would require the different elution mode. In order to retain a sufficient amount of the stationary phase in the column, the heavier mobile phase should be introduced through the head toward the tail of the column, and the lighter mobile phase in the opposite direction by changing the direction of column rotation or the in/outlet. In addition the settling time of the two-phase solvent system should be less than 20 seconds [21].
2.5. HSCCC Separation Procedure
Selected solvent system was prepared with appropriate volume, the column was first filled with the stationary phase followed by rotating the HSCCC apparatus and pumping the mobile phase. After a clear mobile phase eluting out indicating that hydrodynamic equilibrium was reached, an original sample was loaded into the injection valve. The effluent was monitored and each separated fraction was collected according to the chromatogram peak and evaporated to dryness under reduced pressure.
2.7. HPLC analysis and NMR spectroscopy
HPLC analysis was accomplished with a Welchrom C18 column (250 mm × 4.6 mm I.D., 5 μm) at 30°C. The mobile phase was acetonitrile/water (containing 0.1% acetic acid) (9: 91, v/v). The flow rate was 1.0 mL/min and PDA detection was set at 256 nm. The Q-TOF-MS was operated in positive-ionization mode and the conditions were as follows: drying gas (N2) flow rate, 9.0 L/min; drying gas temperature, 330 °C; nebulizer, 40 psi; capillary voltage, 1.5 kV; scan spectra, from m/z 200–800. The linearities, LODs and recoveries for loganic acid, swertiamarin and gentiopicroside were 12.96–1296.00 μg/mL, 0.29 μg/mL and 102.6%, 7.36–736.00 μg/mL, 0.21 μg/mL and 97.7%, 35.60–3560.00 μg/mL, 0.35 μg/mL and 97.3%, respectively. Analysis of the extract and determination of K values were carried out by HPLC–PDA. Purity and structural elucidation of the separated compounds were performed by HPLC–PDA–Q-TOF-MS and NMR spectroscopy.
3. Results and discussion
3.1. Optimization of extraction of GTG by CCD-RSM
The independent and dependent variables were analyzed to get regression equation, which was an empirical relationship between the yield of model compounds and MAE parameters, to predict the response under the given range. The quadratic polynomial equation obtained was as follows:
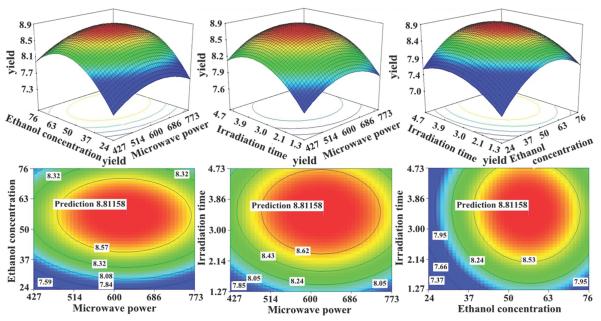
In order to ensure a good model, significance tests of regression model, lack of fit and individual coefficients were carried out by analysis of variance (ANOVA), As seen in Table 1, with very small model P value (less than 0.0001), insignificant lack of fit (P value>0.05), low pure error and suitable determination coefficient (r=0.9787), the quadratic polynomial model was valid and highly significant to represent the actual relationship between variables and response. The effect of ethanol concentration (X1), irradiation time (X3), the quadratic effect of ethanol concentration (X12), microwave power (X22) and irradiation time (X32) on the yield of model compounds were significant and the ranking was: X1=X12>X3>X32>X2.
To investigate the interactive effects of two factors on the response values, three dimensional surface and contour plot were drawn (Fig. 2). Comprehensively considering three variables, with the help of regression equation, the maximum yield of 8.812% could be obtained and the corresponding MAE procedure is: 5.0 g dried roots of G. Crassicaulis was extracted with 50 mL 57.5% aqueous ethanol under 630 W for 3.39 min. The extract was concentrated to give 1.47 g of GTG which was submitted to HSCCC separation.
Fig. 2.
Response surface and contour plots showing interactive effects of any two factors on total yield of three model compounds.
3.2. Selection of solvent system, mobile phase and other conditions of HSCCC
GTG was analyzed by HPLC-PDA and the chromatogram is given in Fig. 1. In order to achieve an ideal separation of target compounds, a series of solvent systems were screened (Table 2). According to the golden rules of HSCCC [21], before optimizing the mobile phase, lower phase may be temporarily used as the mobile phase. The solvent system composed of n-butanol/methanol/1% aqueous acetic acid system (from 4:0:5 to 9:0.5:4, v/v/v) gave too small K values. Meanwhile, the volume of upper phase was much more than that of lower phase, which resulted in solvent wastage. When the volume ratio was modified to 10:0.5:4 (v/v/v) for the sake of larger K values, the solvent system formed a single phase. So the upper phase was tried as the mobile phase, the solvent system n-butanol/methanol/1% aqueous acetic acid (9:0.5:4, v/v/v) contributed to suitable K values, but somewhat yielded low stationary phase retention (35%). Thus, this system was considered as an alternative proposal. Solvent system composed of n-butanol/ethyl acetate/methanol/1% aqueous acetic acid was tested with lower phase used as the mobile phase at first, the volume ratios (from 4:0.5:0.5:5 to 7.5:0.5:0.5:3.5, v/v/v/v) also produced too small K values for the lower phase mobile with unacceptable volume ratios of each phase, and the solvent system formed a single phase when the volume ratio was adjusted to increase K values. Then upper phase were used as the mobile phase, the system (7.5:0.5:0.5:3.5, v/v/v/v) led to suitable K values with relatively satisfactory retention of the stationary phase (52%) and shorter settling time than n-butanol/methanol/1% aqueous acetic acid system (9:0.5:4, v/v/v, upper phase mobile). Consequently, HSCCC separation was performed with solvent system composed of n-butanol/ethyl acetate/methanol/1% aqueous acetic acid (7.5:0.5:0.5:3.5, v/v/v/v) using the upper phase mobile in tail-to-head elution mode, where six compounds were isolated successfully with satisfactory separation factors (α = K1 /K2, K1>K2).
Table 2.
The K values of compounds I–VI in various solvent systems.
| Solvent System | Mobile phase | Ratio |
K
|
|||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | |||
| n-butanol/methanol/1% acetic acid in water | Lower Aqueous phase | 4:0:5 | 0.01 | - | - | 0.01 | 0.02 | - |
| 5:1:5 | 0.05 | 0.05 | 0.22 | 0.30 | 0.41 | 0.44 | ||
| 7:0.5:4 | 0.22 | 0.31 | 0.44 | 0.58 | 0.70 | 0.89 | ||
| 9:0.5:4 | 0.29 | 0.35 | 0.54 | 0.72 | 0.90 | 1.19 | ||
| 10:0.5:4 | solvent system formed a single phase | |||||||
|
| ||||||||
| Upper organic phase | 9:0.5:4 | 3.44 | 2.85 | 1.85 | 1.38 | 1.11 | 0.84 | |
|
| ||||||||
| n-butanol/ethyl acetate/methanol/1% acetic acid in water | Lower Aqueous phase | 4:0.5:0.5:5 | - | - | - | - | 0.21 | - |
| 6:0.5:0.5:3.5 | 0.23 | 0.31 | 0.50 | 0.66 | 0.84 | 1.11 | ||
| 7.5:0.5:0.5:3.5 | 0.35 | 0.50 | 0.57 | 0.67 | 0.94 | 1.30 | ||
| 8.5:0.5:0.5:3.5 | solvent system formed a single phase | |||||||
|
| ||||||||
| Upper organic phase | 7.5:0.5:0.5:3.5 | 2.82 | 1.99 | 1.75 | 1.48 | 1.06 | 0.77 | |
| α | 1.42 | 1.14 | 1.18 | 1.40 | 1.38 | |||
Other conditions such as flow rate of the mobile phase, separation temperature and rotational speed were also investigated. To shorten the separation time while still maintaining an adequate resolution, the flow rate was set at 1.7 mL/min (0–340 min) and 2.5 mL/min (after 340 min). Raising temperature improves stationary phase retention and separation factor, but it tends to form air bubbles in the column, therefore, the separation temperature was set at 30°C. High rotational speed increased the retention of the stationary phase but resulted in loss of peak resolution, so the rotational speed was selected as 850 rpm.
3.3. Purity and structural elucidation of the obtained fractions
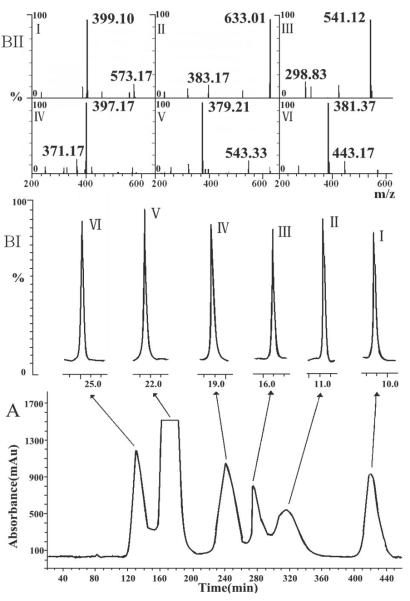
16.3, 8.8, 12.8, 25.1, 40.7 and 21.8 mg of peaks I–VI were yielded from 500 mg of original sample with purities of 98.6, 96.7, 98.1, 99.1, 99.4 and 98.7%, respectively. Fig. 3 shows the HSCCC chromatogram of GTG under the optimized conditions with HPLC–PDA–Q-TOF-MS analysis of each peak for confirming the chemical structures. The UV, MS and 1H NMR data are as follows:
Fig. 3.
(A) HSCCC chromatogram of GTG. Solvent system: n-butanol/ethyl acetate/methanol/1% acetic acid in water (7.5:0.5:0.5:3.5, v/v/v/v); mobile phase: upper organic phase; elution mode: tail to head; flow rate: 1.7 mL/min (0–340 min), 2.5 mL/min (after 340 min); rotational speed: 850 rpm; column temperature: 30 °C; detection wavelength: 256 nm; settling time: 18 s; retention of stationary phase: 52%; original sample: 500 mg dissolved in 10 mL mixture solution of the two phase. (B) HPLC–Q-TOF-MS analysis of HSCCC peak fractions: TIC chromatogram (BI) and MS spectra (BII) of peaks I–VI.
Peak I: λmax = 238 nm; MS (m/z): 399 [M+Na]+; 1H NMR (400 MHz, CD3OD): δ: 0.96 (3H, d, J = 6.6 Hz, CH3-10), 1.51 (1H, m, H-8), 1.53 (1H, ddd, J = 12.4, 8.7, 3.4 Hz, H-6β), 1.78 (1H, ddd, J = 12.4, 9.0, 4.8 Hz, H-6α), 1.94 (1H, ddd, J = 12.3, 9.2, 8.1 Hz, H-9), 2.09 (1H, dtd, J = 12.3, 8.7, 1.0 Hz, H-5), 3.20–3.69 (6H, m, H-2'-6'), 3.70 (1H, dt, J = 8.6, 3.4 Hz, H-7), 5.38 (1H, d, J = 7.7 Hz, H-1'), 5.70 (1H, d, J = 9.2 Hz, H-1), 7.38 ppm (1H, d, J = 1.0 Hz, H-3).
Peak II: λmax = 270, 326 nm, MS (m/z): 633 [M+Na]+; 1H NMR (400 MHz, DMSO-d6): δ: 3.28–4.24 (12H, m, H-2”-6”, 2”'-6”'), 4.88 (1H, d, J = 9.2 Hz, H-1”'), 5.77 (1H, d, J = 7.6 Hz, H-1”), 5.82 (1H, br s, OH-7), 6.53 (1H, br s, H-8), 6.61 (1H, br s, H-3), 6.99 (1H, d, J = 7.5 Hz, H-5'), 7.09 (1H, d, J = 1.5 Hz, H-2'), 7.11 (1H, br s, OH-3'), 7.48 (1H, dd, J = 7.5, 1.5 Hz, H-6'), 9.04 ppm (1H, br s, OH-5).
Peak III: λmax = 252, 270 nm, MS (m/z): 541 [M+Na]+; 1H NMR (400 MHz, CD3OD): δ: 3.09–3.87 (12H, m, H-2'-6', 2”-6”), 3.38 (1H, m, H-9), 4.82 (2H, d, J = 6.2 Hz, CH2-7), 4.99 (1H, m, H-10β), 5.03 (1H, m, H-10α), 5.37 (1H, d, J = 8.9 Hz, H-1), 5.38 (2H, d, J = 8.0 Hz, H-1', 1”), 5.64 (1H, td, J = 6.2, 1.0 Hz, H-6), 5.71 (1H, m, H-8), 7.43 ppm (1H, br s, H-3).
Peak IV: λmax = 238 nm, MS (m/z): 397 [M+Na]+; 1H NMR (400 MHz, CD3OD): δ: 1.75 (1H, dt, J = 12.4, 5.2 Hz, H-6β), 2.00 (1H, dt, J = 12.4, 5.2 Hz, H-6α), 2.67 (1H, m, H-9), 3.15–3.60 (6H, m, H-2'-6'), 4.08 (1H, dt, J = 12.4, 5.2 Hz, H-7β), 4.20 (1H, dt, J = 12.4, 5.2 Hz, H-7α), 4.97 (1H, d, J = 2.2 Hz, H-1), 5.03 (1H, m, H-10β), 5.07 (1H, m, H-10α), 5.38 (1H, d, J = 7.7 Hz, H-1'), 5.70 (1H, ddd, J = 16.8, 10.0, 6.2 Hz, H-8), 7.41 ppm (1H, br s, H-3).
Peak V: λmax = 244, 272 nm, MS (m/z): 379 [M+Na]+; 1H NMR(400 MHz, CD3OD): δ: 3.17–3.90 (6H, m, H-2'-6'), 3.39 (1H, m, H-9), 4.82 (2H, d, J = 6.2 Hz, CH2-7), 4.98 (1H, m, H-10β), 5.02 (1H, m, H-10α), 5.28 (1H, d, J = 8.8 Hz, H-1), 5.38 (1H, d, J = 7.6 Hz, H-1'), 5.64 (1H, td, J = 6.2, 1.0 Hz, H-6), 5.71 (1H, m, H-8), 7.43 ppm (1H, br s, H-3).
Peak VI: λmax = 244 nm, MS (m/z): 381 [M+Na]+; 1H NMR (400 MHz, CD3OD): δ: 1.77 (1H, m, Hβ-6), 1.90 (1H, m, Hα-6), 2.49 (1H, dtd, J = 11.9, 8.7, 1.0 Hz, H-5), 2.79 (1H, m, H-9), 3.20–3.70 (6H, m, H-2'-6'), 4.08 (1H, dt, J = 12.4, 6.4 Hz, H-7β), 4.18 (1H, dt, J = 12.4, 6.4 Hz, H-7α), 4.83 (1H, d, J = 9.1 Hz, H-1), 5.03 (1H, m, Hβ-10), 5.07 (1H, m, Hα-10), 5.38 (1H, d, J = 7.4 Hz, H-1'), 5.70 (1H, ddd, J = 16.8, 10.0, 6.2 Hz, H-8), 7.41 ppm (1H, d, J = 1.0 Hz, H-3).
Compared with the data from Refs. [25–28], peaks I–VI were identified as loganic acid, isoorientin-4'-O-glucoside, 6'-O-β-d-glucopyranosyl gentiopicroside, swertiamarin, gentiopicroside, and sweroside, respectively.
4. Concluding remarks
This paper describes a method for the preparation of bioactive components from G. Crassicaulis, which is more efficient than conventional ones due to rapid extraction, separation and identification procedure. MAE, predicted by CCD-RSM, was applied for fast extraction; by optimization of solvent system, mobile phase and elution mode, six compounds were successfully separated by HSCCC; combined use of HPLC–PDA–Q-TOF-MS and NMR spectroscopy made the purity and structural elucidation more accurate and faster. Our study clearly demonstrates the preparative capability of MAE coupled with HSCCC for separating phytochemicals from ethnomedicine.
Acknowledgements
Financial support from Funds of scientific research launched project of Northwest University (PR12079) is gratefully acknowledged.
Abbreviations
- CCD-RSM
central composite design-response surface methodology HSCCC, high-speed counter-current chromatography
- HPLC–PDA–Q-TOF-MS
high-performance liquid chromatography with photo diode array detection and quadrupole time-of-flight mass spectrometry
- MAE
microwave-assisted extraction
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi:10.1002/jssc.201300897.
The authors have declared no conflict of interest.
References
- [1].Liu Y, Zhang H, Liu C, Peng LX, Shang YH, Meng QY. LISHIZHEN. Med. Mater. Med. Res. 2006;17:1631–1633. [Google Scholar]
- [2].Liang JR, Zhu S, Zhang XX, Su Q, He J, Sun WJ. J. Chin. Med. Mater. 2012;35:495–499. [Google Scholar]
- [3].Cui JR, Zhao XY, Zhang JS. J Peking. Univ. 1992;24:225–227. [Google Scholar]
- [4].Li JM, Li FA, Li XY. Nat. Prod. Res. Dev. 2004;16:225–227. [Google Scholar]
- [5].Cao XY, Wang ZZ. Chin. J. Pharm. Anal. 2010;30:623–625. [Google Scholar]
- [6].Su Q, Shang PP, Zhang YM, Jia N, He J, Zhao WN, Sun WJ. Chin. Herb. Med. 2012;4:245–251. [Google Scholar]
- [7].Recio MC, Giner RM, Manez S, Rios JL. Planta. Med. 1994;60:232–234. doi: 10.1055/s-2006-959465. [DOI] [PubMed] [Google Scholar]
- [8].Chen L, Liu JC, Zhang XN, Guo YY, Xu ZH, Cao W, Sun XL, Sun WJ, Zhao MG. Neuropharmacol. 2008;54:1175–1181. doi: 10.1016/j.neuropharm.2008.03.007. [DOI] [PubMed] [Google Scholar]
- [9].Hou JW, Yao S, Huang LM, Sun WJ, Xie RM. Pharmacol. Clin. Chin. Mater. Med. 2007;23:105–107. [Google Scholar]
- [10].Zhao Y, Zhang R, Sun WJ. J. Chin. Med. Mater. 2007;30:1583–1586. [PubMed] [Google Scholar]
- [11].Zhao Y, Shang PP, Sun WJ. J. Chin. Med. Mater. 2003;26:37–38. [Google Scholar]
- [12].Li MX, Jia ZP, Zhang RX, Wang JH, Ge X. Chin. Trad. Pat. Med. 2005;27:1384–1386. [Google Scholar]
- [13].Chen QL, Shi ZY, Tu GZ, Sun WJ. Chin. J. Chin. Mater. Med. 2005;30:1519–1522. [PubMed] [Google Scholar]
- [14].Qiu HY, Xiao XH, Li GK. J. Sep. Sci. 2012;35:901–906. doi: 10.1002/jssc.201100995. [DOI] [PubMed] [Google Scholar]
- [15].Xiao XH, Si XX, Yuan ZQ, Xu XF, Li GK. J. Sep. Sci. 2012;35:2313–2317. doi: 10.1002/jssc.201200231. [DOI] [PubMed] [Google Scholar]
- [16].Naeeni MH, Yamini Y, Rezaee M, Seidi S. J. Sep. Sci. 2012;35:2469–2475. doi: 10.1002/jssc.201100978. [DOI] [PubMed] [Google Scholar]
- [17].Liu L, Liu RL, Zhang J, Zhang ZQ. J. Sep. Sci. 2012;35:3412–3420. doi: 10.1002/jssc.201200495. [DOI] [PubMed] [Google Scholar]
- [18].Yang L, Cao YL, Jiang JG, Lin QS, Chen J, Zhu L. J. Sep. Sci. 2010;33:1349–1355. doi: 10.1002/jssc.200900776. [DOI] [PubMed] [Google Scholar]
- [19].Zhou J, Ma XM, Qiu BH, Chen JX, Bian L, Pan LM. J. Sep. Sci. 2013;36:383–390. doi: 10.1002/jssc.201200647. [DOI] [PubMed] [Google Scholar]
- [20].Zhong M, Huang KL, Zeng JG, Li S, She JM, Li GY, Zhang L. J. Sep. Sci. 2010;33:2160–2167. doi: 10.1002/jssc.201000085. [DOI] [PubMed] [Google Scholar]
- [21].Ito Y. J. Chromatogr. A. 2005;1065:145–168. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- [22].Liang JR, He J, Zhu S, Zhao WN, Zhang YM, Ito Y, Sun WJ. J. Liq. Chromatogr. Related Technol. 2013;36:983–999. [PMC free article] [PubMed] [Google Scholar]
- [23].Wang Y, Liu MZ, Zheng LL, Yin LH, Xu LN, Qi Y, Ma XC. J. Sep. Sci. 2012;35:1977–1984. doi: 10.1002/jssc.201200011. [DOI] [PubMed] [Google Scholar]
- [24].Ha IJ, Kang M, Na YC, Park Y, Kim YS. J. Sep. Sci. 2011;34:2559–2565. doi: 10.1002/jssc.201100326. [DOI] [PubMed] [Google Scholar]
- [25].Zhang XZ, Xu Q, Xiao HB, Liang XM. Phytochemistry. 2003;64:1341–1344. doi: 10.1016/s0031-9422(03)00501-6. [DOI] [PubMed] [Google Scholar]
- [26].Sherwood RT, Shamma M, Moniot JL, Kroschewsky JR. Phytochemistry. 1973;12:2275–2278. [Google Scholar]
- [27].Kakuda R, Iijima T, Yaoita Y, Machida K, Kikuchi M. J. Nat. Prod. 2001;64:1574–1575. doi: 10.1021/np010358o. [DOI] [PubMed] [Google Scholar]
- [28].Zhou Y, Di YT, Gesang SL, Peng SL, Ding LS. Helv. chim. acta. 2006;89:94–102. [Google Scholar]