Abstract
Background
We have isolated mesenchymal stromal cells (MSCs) from tracheal aspirates of premature infants with respiratory distress. Under the influence of transforming growth factor-β, MSCs differentiate into α-smooth muscle actin-expressing myofibroblasts. Myofibroblasts are increased in the lungs of patients with bronchopulmonary dysplasia (BPD), a chronic lung disease of prematurely-born infants.
Objective
We tested whether isolation of MSCs from tracheal aspirates of premature infants with respiratory distress during the first week of life correlates with BPD.
Patients and Methods
Eighty-four infants born at gestational age <33 weeks and requiring mechanical ventilation were studied. Aspirates were collected during suctioning and centrifuged. Cell pellets were resuspended in culture medium and plated. Adherent cells were grown to confluence.
Results
MSCs were isolated from the tracheal aspirates of 56 infants; 28 infants showed no MSCs. There was no statistical difference in gestational age or birth weight between the ‘MSC’ and ‘no MSC’ groups. In the MSC group, 12 died and 25 developed BPD, as defined by a requirement for supplemental O2 at 36 wks post-menstrual age. In the ‘no MSC’ group, 6 infants died and 1 developed BPD. Accounting for the potential influences of gender, birth weight, gestational age, number of tracheal aspirate samples taken and the duration of endotracheal intubation (up to 7 days), isolation of MSCs increased the adjusted odds ratio of BPD over 21-fold (95% confidence intervals: 1.82, 265.85).
Conclusions
Isolation of tracheal aspirate MSCs predicts the development of BPD, which suggests that MSCs play an important role in the pathogenesis of this disease.
Keywords: chronic lung disease, lipofibroblast, myofibroblast
INTRODUCTION
With improvements in neonatal care, the survival of very premature infants has increased. However, improvements in survival have been accompanied by a corresponding increase in the incidence of bronchopulmonary dysplasia (BPD), a fibrotic lung disease requiring supplemental oxygen for months or years 1. Survivors of BPD have abnormal lung function even as young adults 2, 3, making BPD one of the leading causes of pediatric lung disease.
Before the surfactant era, airway remodeling and parenchymal fibrosis were prominent histologic findings in BPD. More recently, the lungs of infants dying from BPD have shown less fibrosis and more uniform inflation. However, there are larger and fewer alveoli, as well as poorly formed secondary crests, indicating an interference with septation 4, 5. Further, alveolar septa are thickened with collagen and elongated cells resembling fibroblasts 6. A study of infants with respiratory distress syndrome (RDS) showed the appearance of α-smooth muscle actin-positive myofibroblasts in the alveolar septa four days after birth 7. Myofibroblasts featured intense immunoreactivity for transforming growth factor (TGF)-β, a stimulus for myofibroblastic differentiation. TGF-β overexpression in neonatal mouse lungs results in structural changes of BPD including abnormal alveolar structure and vascular development 8. TGF-β also induces proliferation of α-smooth muscle actin-positive cells within the septal walls. Together, these results indicate that BPD may result in part from the abnormal differentiation or proliferation of alveolar mesenchymal progenitor cells to myofibroblasts.
We have shown that tracheal aspirates from week-old premature infants undergoing mechanical ventilation for RDS contain fibroblast-like cells with colony-forming potential, surface markers and differentiation potential typically found in mesenchymal stem cells 9. Recent work suggests that there is a hierarchy of multipotent mesenchymal stromal cells ranging from true self-renewing stem cells with multilineage differentiation capacity to those with more restricted differentiation potential, until a state of complete restriction to the fibroblast is reached 10. Since we have not thoroughly tested the clonogenicity or self-renewal of neonatal lung mesenchymal cells, we now refer to them as mesenchymal stromal cells (MSCs), which have a more restricted differentiation potential. Based on their potential to differentiate into myofibroblasts 11, we tested whether isolation of MSCs from tracheal aspirates of premature infants with respiratory distress during the first week of life would correlate with development of BPD.
PATIENTS AND METHODS
Patients
We examined tracheal aspirates from 77 infants admitted to the University of Michigan C.S. Mott Children’s Hospital Holden Newborn Intensive Care Unit between July 2005 and August 2009. We also examined tracheal aspirates from 7 infants admitted to the St. Joseph Mercy Hospital between January 2007 and June 2008. Entry criteria included gestational age at birth ≤33 wk, mechanical ventilation for respiratory distress, and age ≤7 d. Infants with acute sepsis during the first week of life were excluded. Chorioamnionitis and necrotizing enterocolitis were diagnosed on clinical grounds. Informed consent was obtained. This study was approved by both institutions’ Investigational Review Boards.
Tracheal aspirate collection and cell culture
Endotracheal tube suctioning was performed as needed to maintain tube patency. The need for suctioning was determined by the nurse and respiratory therapist. Specimens were centrifuged (1200 × g for 5 min at 15°C) and supernatants stored at −80°C. Cell pellets were resuspended in 2 ml Dulbecco’s Modified Essential Medium with 10% fetal bovine serum, 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine and 500 μg/ml Fungizone. Cell culture medium was refreshed daily for three days and three times a week thereafter. Adherent cells were incubated at 37°C in 5% CO2. MSC colony forming units were identified by eye under light microscopy.
We examined a total of 142 tracheal aspirates from 84 infants. 37 babies had one sample taken, 37 babies had two samples taken, 9 babies had three samples taken, and 1 baby had four samples taken. Some subjects had at least one sample from which MSCs were isolated and at least one sample from which they were not. These babies were classified as positive for MSCs.
Measurement of latent TGF-β1 in the tracheal aspirates
TGF-β1 levels were measured by ELISA (R&D Systems, Minneapolis, MN). Latent TGF-β1 was activated to immunoreactive TGF-β1 by acidification with 1 N HCl (10 min in room temperature). Samples were neutralized with 1.2 N NaOH/0.5 M HEPES. To control for sample dilution, TGF-β levels were normalized for the secretory component of IgA (scIgA) 12.
Statistical analysis
Group mean data are expressed as mean±SD, except for days on mechanical ventilation and supplemental O2, which were described as median (interquartile range). Demographic and clinical data were compared by unpaired t-test, Mann-Whitney test or Fisher’s exact test, as required. The diagnosis of BPD was the primary outcome variable. BPD was defined as the need for supplemental O2 to maintain an acceptable O2 saturation by pulse oximeter at 36 wks post-menstrual age. Logistic regression was used to estimate the relative odds of BPD as a function of MSCs, gender, gestational age, birth weight, number of tracheal aspirate samples taken, and days of endotracheal intubation (up to seven), with simultaneous mutual adjustment of these odds for each other, specified a priori. The last two variables were included in the analysis to test whether multiple sampling and a longer duration of endotracheal intubation (thereby allowing more opportunities for suctioning) could explain a higher yield of MSCs in infants developing BPD, if present. Days of mechanical ventilation and supplemental O2 were considered secondary outcome variables. The fraction of patients yielding MSCs was correlated with gestational age at birth using weighted linear regression, with each point weighted by the number of infants at each age. Similarly, the fraction of samples yielding MSCs was correlated with postnatal age, with each point weighted by the number of samples at each age. A significance level of 0.05 was used for all hypothesis tests.
Based on our initial outcomes data 9, we estimated the number of patients needed to find a significant difference in the proportion of infants developing BPD between the MSC and non-MSC groups. Assuming a first group proportion of 0.75, second group proportion of 0.14 and a ratio of sample sizes of 0.81, the sample size calculation to achieve a 90% power for detecting a difference in the proportion of infants developing BPD was 17 in the MSC group and 13 in the no MSC group 13. Therefore, the current study was sufficiently powered to accept or reject the null hypothesis that the proportion of infants developing BPD would be no different in subjects who did or did not have MSCs isolated from the tracheal aspirate fluid, provided that a difference of the magnitude specified above actually exists.
RESULTS
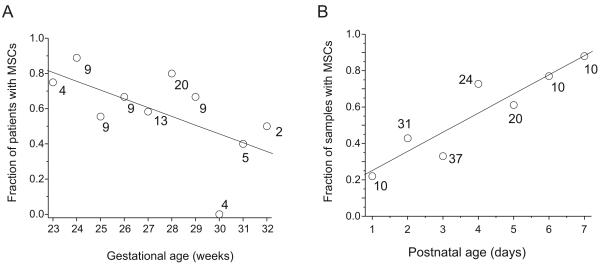
We examined tracheal aspirates from 84 infants. MSCs were isolated from 56 infants (Figure 1). Colony forming units developed 7-14 days after tracheal aspirate plating. MSC isolation negatively correlated with gestational age at birth (p<0.001, Figure 2a). There was also a significant positive correlation between postnatal age and MSC yield (p<0.001, Figure 2b).
Figure 1. MSC colony forming units-fibroblast (CFU-F) develop between 7-14 days after plating of tracheal aspirates.
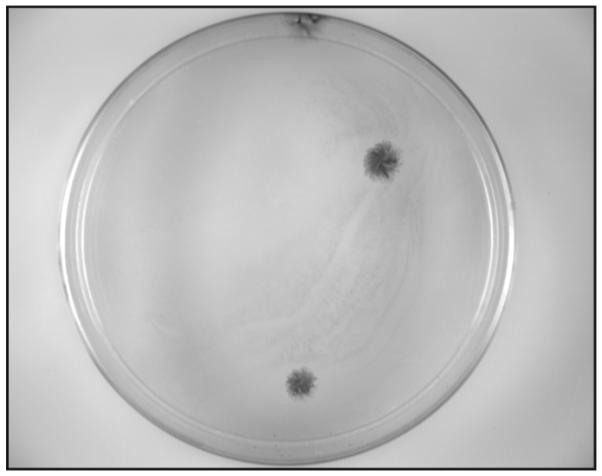
Colonies are stained with crystal violet. Three CFU-F are shown.
Figure 2. Relationships between the isolation of MSCs from tracheal aspirate fluid and gestational age (A) and postnatal age (B).
In A, number of infants is plotted against gestational age at birth; numbers next to each data point represent the total number of infants for that point. In B, number of samples is plotting against postnatal age; numbers next to each data point represent the total number of samples for that point.
We compared the demographics and outcomes of infants from whom tracheal aspirate MSCs were isolated (n=56) to those from whom MSCs were not isolated (n=28)(Table 1). There were no differences in the groups with regard to gender, incidence of necrotizing enterocolitis or incidence of late-onset sepsis. Birth weight and gestational age at birth tended to be lower, the number of surfactant doses tended to be higher, and the history of maternal chorioamnionitis higher in the MSC group. In the MSC group, 12 patients died (10 before 36 wks post-menstrual age) and 5 were lost to follow up (for example, they were transferred to another hospital prior to 36 wks post-menstrual age). Of the 42 patients evaluated at 36 wks post-menstrual age, 25 developed BPD. In the non-MSC group, 6 patients died before 36 wks post-menstrual age and 2 were lost to follow up. Of the 20 patients evaluated at 36 wks post-conceptual age, only 1 developed BPD (p < 0.001). Considering the two secondary outcome variables, the MSC group showed greater days of mechanical ventilation and supplemental O2 administration (both p<0.001). Sepsis and necrotizing enterocolitis were the most common causes of death (Table 2).
Table 1.
Comparison of demographics and clinical outcomes in babies with and without MSCs in their tracheal aspirate (mean±SD where appropriate).
| MSCs | no MSCs | test statistic | |
|---|---|---|---|
| N | 56 | 28 | |
| Male gender (% of total) | 29 (52%) | 17 (61%) | Fisher exact test, p = 0.49 |
| Died (% of total) | 12 (21%) | 6 (21%) | Fisher exact test, p = 1 |
| Lost to follow up (% of total) | 5 (9%) | 2 (7%) | Fisher exact test, p = 1 |
| GA (wks, mean±SD) | 27.2±0.3 | 28.1±0.5 | t-test, p = 0.09 |
| BW (gms, mean±SD) | 1013±43 | 1150±89 | t-test, p = 0.12 |
| Surfactant doses | 2.1±0.6 | 1.7±0.7 | Mann-Whitney test, p =0.072 |
| Suspected clinical chorioamniotitis (% of total) |
10 (18%) | 1 (4%) | Fisher exact test, p = 0.09 |
| Necrotizing enterocolitis (% of total) |
7 (13%) | 1 (4%) | Fisher exact test, p = 0.26 |
| Culture-proven late-onset sepsis (% of total) |
8 (14%) | 4 (14%) | Fisher exact test, p = 1 |
| BPD (% of patients evaluated at 36 wks post-menstrual age) |
25 (60%) | 1 (5%) | Fisher exact test, p < 0.001 |
| BPD or death (% of total) | 43 (77%) | 7 (25%) | Fisher exact test, p < 0.001 |
| Vent days, median (IQR) | 22 (25) | 4 (9) | Mann-Whitney test, p <0.001 |
| O2 days, median (IQR) | 57 (110) | 19 (32) | Mann-Whitney test, p <0.001 |
| O2 28 days or greater (% of patients evaluated at 28 days postnatal age) |
35 (81%) | 10 (50%) | Fisher exact test, p = 0.016 |
Abbreviations: MSC, mesenchymal stromal cell; SD, standard deviation; N, number; GA, gestational age; BW, birthweight; BPD, bronchopulmonary dysplasia; vent days, days requiring mechanical ventilation; O2 days, days requiring supplemental O2; IQR, interquartile range.
Table 2.
Mortality.
| Cause of death | Age of death, day of life (weeks post-conception) |
|---|---|
| MSCs isolated (n=12) | |
| Sepsis (E. coli)/necrotizing enterocolitis | 19 (29) |
| Sepsis (C. albicans)/necrotizing enterocolitis | 17 (26) |
| Presumed sepsis/necrotizing enterocolitis | 11 (29) |
| Presumed sepsis | 46 (32) |
| Necrotizing enterocolitis | 4 (24) |
| Sepsis (S. aureus) | 13 (27) |
| Necrotizing enterocolitis | 13 (30) |
| Necrotizing enterocolitis | 9 (27) |
| Respiratory failure, renal failure | 110 (40) |
| Sepsis (P. aeruginosa) | 6 (29) |
| Respiratory failure, presumed sepsis | 69 (37) |
| Necrotizing enterocolitis | 12 (27) |
| MSCs not isolated (n=6) | |
| Necrotizing enterocolitis | 21 (30) |
| Presumed sepsis | 7 (24) |
| Sepsis (S. aureus) | 7 (25) |
| Presumed sepsis | 21 (28) |
| Pulmonary hemorrhage | 3 (25) |
| Refractory hypotension, pulmonary hypertension, pulmonary interstitial emphysema, intraventricular hemorrhage |
9 (27) |
We reasoned that the observed difference in BPD prevalence between the “no MSC” and “MSC” groups could be due disparities in sample collection between infants who ultimately develop BPD and those who do not. For example, patients developing BPD were intubated for a longer period of time than patients without BPD, allowing a longer period for sample collection within the first week of life (Table 3). Accordingly, patients with BPD had more samples collected than patients who did not develop BPD. Also, average postnatal age at the time of the first tracheal aspirate collection was higher in the BPD group. Since MSC yield increased with postnatal age, these factors would tend to increase the likelihood of isolating MSCs from the tracheal aspirates of patients developing BPD.
Table 3.
Comparison of sample collection variables for babies who did not and did develop BPD (mean±SD).
| No BPD | BPD | test statistic | |
|---|---|---|---|
| N | 36 | 26 | |
| Last day of endotracheal intubation during the 7-day collection period (postnatal age in days) |
4.3±2.6 | 6.7±1.2 | Mann-Whitney test, p<0.001 |
| Number of samples | 1.5±0.7 | 1.9±0.7 | Mann-Whitney test, p=0.036 |
| Day of first specimen collection (postnatal age in days) |
2.5±1.4 | 3.6±1.2 | Mann-Whitney test, p<0.001 |
Abbreviations: SD, standard deviation; N, number.
To determine the independent predictive value of MSC isolation for the development of BPD, we performed multivariate logistic regression analysis. This analysis took into account the factors of gender, gestational age, birthweight, number of samples collected and days of endotracheal intubation (up to 7). Patients dying or lost to follow up prior to 36 wks post-menstrual age were eliminated from this analysis, leaving 42 in the MSC group and 20 in the non-MSC group. While univariate analysis showed that MSC isolation, gestational age, birthweight, and days of endotracheal intubation each affected the development of BPD (each p<0.001), only birth weight and MSC isolation were independent predictors of BPD (Table 4), with MSC isolation being by far the strongest predictor. If MSCs were present, the odds of BPD as defined here increased nearly 22 times, adjusting for the other risk factors (adjusted odds ratio = 21.98, 95% confidence interval = 1.818, 265.854). Birth weight was a negative predictor of BPD (adjusted odds ratio = 0.561, 95% confidence interval = 0.331, 0.950). A one hundred-gram increase in birth weight decreased the odds of BPD by 43.9%. The number of samples collected or days of endotracheal intubation did not increase the adjusted odds of BPD development.
Table 4.
Estimated logistic regression model comparing the effects of MSC isolation, gender, gestational age, birthweight (in units of 100 g), sample number, and days of endotracheal intubation (up to 7) on the development of BPD.
| Variables | Odds ratio | 95% C.I. for Odds Ratio | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| MSC | 21.983 | 1.818 | 265.854 | 0.015 |
| Gender (male) | 0.232 | 0.039 | 1.362 | 0.106 |
| Gestational age | 1.400 | 0.635 | 3.086 | 0.404 |
| Birth weight | 0.561 | 0.331 | 0.950 | 0.032 |
| Number of samples: 2 | 1.294 | 0.238 | 7.041 | 0.766 |
| Number of samples: 3 | 2.152 | 0.226 | 20.544 | 0.505 |
| Last day of intubation | 1.300 | 0.740 | 2.284 | 0.361 |
Given the role of TGF-β in the fibroproliferative response, we measured latent TGF-β in tracheal aspirates from 28 infants, 13 of whom went on to develop BPD. There was no difference in latent TGF-β between groups (no BPD, 223±133 pg/ml, 0.24±0.13 pg/ng scIgA; BPD, 255±183 pg/ml, 0.30±0.26 pg/ng scIgA; mean±SD). On the other hand, normalized TGF-β levels were higher in patients with MSCs (cell-negative, 162± 113 pg/ml, 0.129±0.096 pg/ng scIgA; cell-positive, 263±163 pg/ml, 0.317±0.209 pg/ng scIgA, p=0.038), consistent with our finding that MSCs produce TGF-β 11.
DISCUSSION
The precise mechanisms responsible for BPD remain unclear. Hyperoxia, mechanical ventilation and maternal chorioamnionitis have been implicated as inciting factors. Interleukin (IL)-8, IL-1β and tumor necrosis factor-α are elevated in tracheal aspirates and bronchoalveolar lavage fluid of preterm infants who develop BPD 14-16 and IL-1β overexpression disrupts postnatal alveolar septation in the mouse 17, suggesting that inflammation plays a role. Expression of vascular endothelial growth factor (VEGF) is decreased in infants dying with BPD 6 and VEGF promotes lung angiogenesis and prevents alveolar damage in hyperoxia-exposed rats 18, 19, evidence that angiogenesis participates in the alveolarization process. Infants with RDS and BPD show the appearance of α-smooth muscle actin- and TGF-β-positive myofibroblasts in the alveolar septa 7, and tracheal aspirate TGF-β1 levels are increased in preterm infants who develop BPD 20. TGF-β overexpression in neonatal mouse lungs results in structural changes of BPD including proliferation of α-actin-positive myofibroblasts within the alveolar septal walls, abnormal alveolar structure and vascular development 8. Together, these results indicate that BPD may result in part from the abnormal differentiation of alveolar mesenchymal progenitor cells to myofibroblasts, under the influence of TGF-β.
We have shown that tracheal aspirates from premature infants with RDS contain fibroblast-like cells with colony-forming potential, surface markers and differentiation potential found in MSCs 9. We also observed that MSCs express mRNAs encoding contractile and extracellular matrix proteins, consistent with a myofibroblast progenitor phenotype. Treatment of MSCs with TGF-β induced myofibroblastic differentiation 11. We therefore reasoned that MSCs are more likely to be isolated from the tracheal aspirates of premature infants who develop BPD than those who do not. We found that, if MSCs were present, the adjusted odds of BPD increased nearly 22 times. Further, in the “MSC group,” 25 of 42 patients evaluated at 36 wks post-menstrual age developed BPD. In contrast, in the “no MSC group,” only 1 of 20 patients developed BPD.
The high predictive value of MSC isolation for the development of BPD suggests that these cells play a significant physiologic role in the development of chronic lung disease. As noted above, MSCs could serve as precursors for myofibroblasts, the number of which are increased in BPD 7. The myofibroblast is the key effector cell in fibrogenesis 21, 22. Myofibroblasts are therefore likely to contribute to alveolar fibrosis and abnormal alveolar development. On the other hand, it is also conceivable that MSCs play a positive role. During development, alveolar myofibroblasts are required for the formation of secondary septi 23, 24. Cultured MSCs may also be differentiated to lipofibroblasts 9, which provide type II cells with neutral lipid for surfactant synthesis 25.
On the basis of the high odds ratio and the binary nature of this measurement, isolation of MSCs from the tracheal aspirate may also represent a promising biomarker for the development of chronic lung disease. However, it may take up to two weeks for colonies of MSCs to appear on plastic culture dishes, which may diminish the utility of this marker.
We defined BPD as the need for supplemental O2 to maintain an acceptable O2 saturation by pulse oximeter at 36 wks post-menstrual age. This definition includes infants with both “moderate” and “severe” BPD 1. Premature infants requiring supplemental O2 at 36 wks post-menstrual age have a significantly greater need for pulmonary medications and incidence of rehospitalization at 18-22 months’ corrected age than infants not requiring supplemental O2 26. However, we acknowledge that, with increasing survival of very low birth weight, the duration of O2 therapy, including treatment with supplemental O2 at 36 wks post-menstrual age, may no longer identify all infants with chronic lung disease. For example, one study found that, while the 36 wk cutoff was more accurate in predicting poor pulmonary outcomes compared to earlier post-menstrual ages, the overall accuracy was only 63% 27.
It is possible that other variables account for the higher prevalence of BPD in the MSC group. Birth weight and gestational age tended to be lower, and the incidence of a history of maternal chorioamnionitis higher, in the MSC group. Low birth weight and chorioamnionitis have each been previously been identified as risk factors for the development of BPD 28. There was also a negative correlation between gestational age and MSC isolation. It is therefore possible that the observed relationship between MSC isolation and BPD might simply reflect the known association between low birth weight and chronic lung disease. However, multivariate logistic regression analysis showed that, while there was an association between birth weight and development of BPD, isolation of MSCs was a much stronger predictor of poor pulmonary outcome. In addition, patients developing BPD were intubated for a longer period of time than patients not developing BPD, allowing a longer period of time for sample collection. Accordingly, patients with BPD had more samples collected than patients without BPD. In addition, the average postnatal age of the first tracheal aspirate collection was significantly higher in the BPD group, and MSC yield increased with postnatal age. Together, these data suggest the possibility that infants who did not develop BPD, who were more likely to be extubated earlier in the course of their disease, had a lower yield of MSCs simply because they were sampled at earlier time points and sampled fewer times than those who developed BPD. Consistent with this, the only patient in the “non-MSC” group who developed BPD was sampled on day 1 of life, when the yield of MSCs was low. To test this, we included the number of samples per subject and the number of days which we had access to tracheal aspirate samples (days the subject was endotracheally intubated, up to seven) in our multivariate logistic regression analysis. While inclusion of these variables decreased the predictive value of MSC isolation for the development of BPD (odds ratio decreased from 31.50 to 21.98, data not shown), they were not independent predictors of BPD. Thus, the independent association of MSC isolation with the development of BPD most likely reflects the degree of injury in the lungs of these patients, rather than simply a sampling bias.
Organs carry their own population of pluripotent MSCs in a perivascular compartment 29. MSCs have been noted to arise from both the large and small arteries 30, 31 as well as capillaries 29. Together with the finding that tracheal aspirates sample more proximally in the tracheobronchial tree than bronchoalveolar lavage 12, we speculate that MSCs are derived from the perivascular tissue of pulmonary and bronchial arteries.
One potential limitation of our study concerns the wide confidence interval for the MSC odds ratio. This reflects the fact that there was very little variance in infants from whom no MSCs were isolated; only one infant in this group developed BPD. Another limitation concerns our choice of BPD as the primary outcome variable. Because death is a competing outcome for BPD, some investigators have selected BPD or death, rather than BPD alone, as a primary outcome variable. Had we employed BPD or death rather than BPD alone as a primary outcome variable, the difference between “MSC” and “no MSC” groups would still have been highly significant.
CONCLUSIONS
Isolation of tracheal aspirate MSCs predicts the development of BPD. MSCs may therefore play an important role in the pathogenesis of BPD. MSC may also represent a biomarker for adverse pulmonary outcomes in prematurely-born infants.
ACKNOWLEDGEMENTS
The authors thank Brady T. West, M.A. and the University of Michigan Center for Statistical Consultation and Research for their assistance with statistical analysis.
Supported by NIH grant HL90134 (M.B.H.)
Abbreviations
- MSC
mesenchymal stem cell
- BPD
bronchopulmonary dysplasia
- RDS
respiratory distress syndrome
- TGF
transforming growth factor
REFERENCES
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy JD. Lung function outcome in children of premature birth. J Paediatr Child Health. 1999;35:516–521. doi: 10.1046/j.1440-1754.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 3.Eber E, Zach MS. Paediatric origins of adult lung disease · 8: Long term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy) Thorax. 2001;56:317–323. doi: 10.1136/thorax.56.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussain NA, Siddiqui NH, Stocker JR. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 5.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 7.Toti P, Buonocore G, Tanganelli P, Catella AM, Palmeri ML, Vatti R, Seemayer TA. Bronchopulmonary dysplasia of the premature baby: an immunohistochemical study. Pediatr Pulmonol. 1997;24:22–28. doi: 10.1002/(sici)1099-0496(199707)24:1<22::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol. 2004;31:650–656. doi: 10.1165/rcmb.2004-0092OC. [DOI] [PubMed] [Google Scholar]
- 9.Hennrick KT, Keeton AG, Nanua S, Kijek TG, Goldsmith AM, Sajjan US, Bentley JK, Lama VN, Moore BB, Schumacher RE, Thannickal VJ, Hershenson MB. Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med. 2007;175:1158–1164. doi: 10.1164/rccm.200607-941OC. [DOI] [PubMed] [Google Scholar]
- 10.Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS One. 2009;4:e6498. doi: 10.1371/journal.pone.0006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popova AP, Bozyk PD, Goldsmith AM, Linn MJ, Lei J, Bentley JK, Hershenson MB. Autocrine production of TGF-β1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am J Physiol Lung Cell Mol Physiol. 2010;298:L735–43. doi: 10.1152/ajplung.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dargaville PA, South M, McDougall PN. Comparison of two methods of diagnostic lung lavage in ventilated infants with lung disease. Am J Respir Crit Care Med. 1999;160:771–777. doi: 10.1164/ajrccm.160.3.9811048. [DOI] [PubMed] [Google Scholar]
- 13.Fleiss JL. Statistical methods for rates and proportions. 2nd Edition John Wiley & Sons; New York: 1981. [Google Scholar]
- 14.Kotecha S, Chan B, Azam N, Silverman M, Shaw RJ. Increase in interleukin-8 and soluble intercellular adhesion molecule-1 in bronchoalveolar lavage fluid from premature infants who develop chronic lung disease. Arch Dis Child. 1995;72:F90–F96. doi: 10.1136/fn.72.2.f90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones CA, Cayabyab RG, Kwong KYC, Stotts C, Wong B, Hamdan H, Minoo P, deLemos RA. Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a developmental basis for predisposition to chronic lung inflammation in preterm newborns. Pediatr Res. 1996;39:966–975. doi: 10.1203/00006450-199606000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimotake TK, Izhar FM, Rumilla K, Jing L, Tan A, Page K, Brasier AR, Schreiber MD, Hershenson MB. Interleukin (IL)-1b in tracheal aspirates from premature infants induces airway epithelial cell IL-8 expression via an NF-kB dependent pathway. Pediatr Res. 2004;56:907–913. doi: 10.1203/01.PDR.0000145274.47221.10. [DOI] [PubMed] [Google Scholar]
- 17.Bry K, Whitsett JA, Lappalainen U. IL-1b disrupts postnatal lung morphogenesis in the mouse. Am J Respir Cell Mol Biol. 2007;36:32–42. doi: 10.1165/rcmb.2006-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: Evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 19.Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1068–1078. doi: 10.1152/ajplung.00093.2006. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson B, Li YH, Noack G, Brauner A, Tullus K. Downregulatory cytokines in tracheobronchial aspirate fluid from infants with chronic lung disease of prematurity. Acta Paediatr. 2000;89:1375–1380. doi: 10.1080/080352500300002606. [DOI] [PubMed] [Google Scholar]
- 21.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286S–289. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 22.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Ann Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 23.Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Törnell J, Heath JK, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- 24.Lindahl P, Karlsson L, Hellstrom M, Gebre-Medhin S, Willetts K, Heath J, Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124:3943–3953. doi: 10.1242/dev.124.20.3943. [DOI] [PubMed] [Google Scholar]
- 25.Besnard V, Wert SE, Stahlman MT, Postle AD, Xu Y, Ikegami M, Whitsett JA. Deletion of Scap in alveolar type II cells influences lung lipid homeostasis and identifies a compensatory role for pulmonary lipofibroblasts. J Biol Chem. 2009;284:4018–4030. doi: 10.1074/jbc.M805388200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K, National Institutes of Child Health and Human Development Neonatal Research Network Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 27.Davis PG, Thorpe K, Roberts R, Schmidt B, Doyle LW, Kirpalani H. Evaluating “old” definitions for the “new” bronchopulmonary dysplasia. J Pediatr. 2002;140:555–560. doi: 10.1067/mpd.2002.123291. [DOI] [PubMed] [Google Scholar]
- 28.Been JV, Zimmermann LJI. Histological chorioamnionitis and respiratory outcome in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2009;94:F218–F225. doi: 10.1136/adc.2008.150458. [DOI] [PubMed] [Google Scholar]
- 29.Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng P-N, Traas J, Schugar R, Deasy BM, Badylak S, Buhring H-J, Giacobino J-P, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 30.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 31.Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]