Abstract
Objective
Determine sildenafil exposure and hemodynamic effect in children after Fontan single-ventricle surgery.
Design
Prospective, dose-escalation trial.
Setting
Single-center, pediatric catheterization laboratory.
Patients
9 children post Fontan single-ventricle surgical palliation and undergoing elective cardiac catheterization: Median (range) age and weight: 5.2 years (2.5–9.4) and 16.3 kg (9.5–28.1). Five children (55%) were male, and 6/9 (67%) had a systemic right ventricle.
Interventions
Catheterization and echocardiography performed before and immediately after single-dose intravenous sildenafil (0.25, 0.35, or 0.45 mg/kg over 20 minutes).
Measurements
Peak sildenafil and des-methyl sildenafil concentration, change in hemodynamic parameters measured by cardiac catheterization and echocardiography.
Main Results
Maximum sildenafil concentrations ranged from 124–646 ng/ml and were above the in vitro threshold needed for 77% phosphodiesterase type-5 (PDE-5) inhibition in 8/9 children and 90% inhibition in 7/7 of children with doses ≥0.35 mg/kg. Sildenafil improved stroke volume (+22%, p=0.05) and cardiac output (+10%, p=0.01) with no significant change in heart rate in 8/9 children. Sildenafil also lowered systemic (-16%, p=0.01) and pulmonary vascular resistance index (PVRI) in all 9 children (median baseline PVRI 2.4 [range: 1.3, 3.7]; decreased to 1.9 [0.8, 2.7] WU x m2; p=0.01) with no dose-response effect. Pulmonary arterial pressures decreased (−10%, p=0.02) and pulmonary blood flow increased (9%, p=0.02). There was no change in myocardial performance index and no adverse events.
Conclusions
After Fontan surgery, sildenafil infusion acutely improves cardiopulmonary hemodynamics, increasing cardiac index. For the range of doses studied, exposure was within the acute safety range reported in adult subjects.
Keywords: single ventricle, sildenafil, Fontan, pulmonary hypertension, pulmonary vascular resistance, pharmacokinetics
INTRODUCTION
Staged surgical palliation, culminating in the Fontan procedure, has markedly improved outcomes for children with single-ventricle heart defects.1 Despite success of this surgical strategy, circulatory physiology remains abnormal and results in an ongoing mortality risk and significant long-term morbidities.2–5,6 In this unique circulation, the two principal determinants of long-term outcome are low pulmonary vascular resistance (PVR) and adequate single-ventricle myocardial function.2,5–7 Drugs that lower PVR and/or improve myocardial performance could optimize circulatory efficiency and potentially improve outcomes.
Sildenafil is a selective phosphodiesterase type-5 (PDE-5) inhibitor that increases cyclic guanosine monophosphate and produces vascular smooth muscle relaxation.8 PDE-5 is highly expressed in the pulmonary vasculature, and sildenafil lowers PVR in adults with pulmonary arterial hypertension.9 Although not normally expressed in the myocardium, PDE-5 is up-regulated in states of chronic cardiomyopathy, and PDE-5 inhibition improves systolic and diastolic performance in both animal models and adults with cardiomyopathy.10,11 Clinicians have extrapolated these effects to the single-ventricle population, leading to much enthusiasm for its use within the field.12–14 However, there are limited data in these patients to support this enthusiasm. Studies to date have focused on exercise performance in older single-ventricle patients with conflicting results. No studies have evaluated the acute hemodynamic effect of PDE-5 inhibition after single-ventricle palliation, particularly in younger patients. Furthermore, no studies have evaluated sildenafil exposure in these patients where chronic hepatic congestion may alter drug metabolism.
The aim of the present study was to determine intravenous (IV), single-dose sildenafil pharmacokinetic and hemodynamic effect in children with single-ventricle heart defects. We previously reported improved PVR but little effect on systemic hemodynamics in patients after stage II surgery (in press, Pediatric Critical Care Medicine). In this analysis, we focus on those children who have completed Fontan (stage III) surgical palliation. We hypothesized that sildenafil would acutely improve cardiopulmonary hemodynamics by lowering PVR and improving global myocardial performance and that improvements in cardiopulmonary hemodynamics would be related to drug exposure.
MATERIALS AND METHODS
The study was an open-label, prospective, dose-escalation trial. The institutional review board of Duke University Medical Center (Durham, North Carolina) approved the study protocol. Children ≥6 months and ≤10 years of age with single-ventricle heart defects post Fontan surgical palliation and undergoing cardiac catheterization as a part of their routine clinical care were eligible for participation. Written informed consent was obtained for all study participants. Exclusion criteria included: 1) history of serious side effects to prior sildenafil therapy; 2) history of sildenafil exposure 96 hours prior to the study; 3) pulmonary venous obstruction; 4) known or suspected pulmonary arterial or cavopulmonary anastomosis obstruction (catheterization gradient ≥1 mm Hg); 5) known or suspected coarctation of the aorta (catheterization gradient ≥10 mm Hg); 6) current treatment with nitrates or alpha blockade medications; and 7) significant renal failure (serum creatinine >2 times higher than the upper limit of normal), thrombocytopenia (platelet count <50,000 x 106/L), or leukopenia (white blood cell count <2500 grams/dL). The original planned enrollment was for 12 children post Fontan surgical palliation. However, during the study, the U.S. Food and Drug Administration (FDA) released a safety advisory recommending against sildenafil therapy in children secondary to concerns for long-term safety. Following this advisory, we stopped enrollment in the present study.
Study protocol
All children were studied under general anesthesia with mechanical ventilation and a fraction of inspired oxygen of 0.21. A standardized general anesthetic approach was utilized. Induction was achieved with inhalational anesthetics (sevoflurane, 40% oxygen, 60% nitrous oxide). After induction, tracheal intubation was facilitated with rocuronium (1 mg/kg). Anesthesia was maintained with approximately 1 minimum alveolar concentration of isoflurane in room air, and fentanyl (1mcg/kg) was administered prior to arterial and venous catheter insertions. The depth of anesthesia and ventilation status were kept stable throughout the hemodynamic portion of the study. Baseline transthoracic echocardiogram and hemodynamic cardiac catheterization were performed after induction of anesthesia. Following the initial collection of hemodynamic data, patients were given a single, pre-determined dose of IV sildenafil (0.25 mg/kg, 0.35 mg/kg, or 0.45 mg/kg) over 20 minutes. The initial two patients enrolled in the study were assigned to the lowest dose category. After reviewing for adverse events at this dosing level, subsequent children were assigned to progressively higher dosing groups. Immediately after completion of the sildenafil infusion, the hemodynamic catheterization and transthoracic echocardiograms were repeated.
For echocardiograms, a single sonographer performed all studies using a standard cardiac ultrasound system (Vivid 7®, GE-Vingmed, Horten, Norway). Images were obtained in the parasternal long, parasternal short, apical, and suprasternal views. Measurements were performed to assess: 2D systolic ventricular function (fraction of area change [FAC] for systemic right ventricles, single-plane modified Simpson’s rule ejection fraction [EF] for systemic left ventricles), speckle tracking systolic function (strain, strain rate), and global myocardial performance (myocardial performance index).15,16 Speckle tracking analysis was performed offline using Vector Velocity Imaging™ (Siemens Medical Solutions, Mountain View, CA). Two physicians associated with the study independently performed all interpretations of the echocardiographic data. Inter-rater reliability was evaluated using Spearman’s correlation coefficient with r2 values of 0.58, 0.76, and 0.92 (p<0.05 for all measures) for FAC, strain rate, and myocardial performance index, respectively.
Catheterization assessment included measurement of saturations by co-oximetry including mixed venous, Fontan tunnel, pulmonary arterial, and systemic arterial oxygen saturations, as well as pressure measurements in the Fontan tunnel, branch pulmonary arteries, pulmonary capillary wedge pressures, single ventricle, ascending aorta, and descending aorta. Pressures were obtained in the standard fashion using fluid-filled catheters. Systemic vascular resistance (SVR) index, PVR, pulmonary blood flow, and cardiac output were calculated using the Fick method. Oxygen consumption was estimated based on age and heart rate. Flows and resistances were indexed to body surface area. For repeat calculations, we used the same estimated oxygen consumption as was used for baseline measurements, and saturations used for calculations were always sampled from the same locations as those used at baseline.
Pharmacokinetic sampling and analysis
Plasma sildenafil levels were collected immediately following completion of the infusion and saline flush (t=20 minutes after initiation of drug infusion). Samples were immediately transferred into heparinized polypropylene tubes and centrifuged for 5 minutes at 3500 RPM. Samples were then stored in dry ice and transferred to a −70°C freezer. Sildenafil and des-methyl sildenafil concentrations were determined using a commercially available assay. The quantitative analysis was performed by Covance Laboratories Inc. (Madison, WI) using high-performance liquid chromatography with tandem mass spectrometry. The assay lower limit of quantitation for sildenafil and des-methyl sildenafil detection was 1 ng/mL. The inter-day error (CV%) for the assay ranged from 1–3.6% across a range of quality control concentrations from 3.0–350 ng/mL.
Data analysis
The primary hemodynamic outcome measures were change in indexed PVR and change in indexed cardiac output. Secondary outcome measures included other hemodynamic variables, as well as echocardiographic measures and exposure response. Statistical analysis included all patients receiving sildenafil. Demographic data were summarized using descriptive statistics expressed as median (range). Change in clinical parameters was expressed as median percent change. Hemodynamic and echocardiographic data were calculated and presented by dosage group. Standard graphing and screening tests were used to assess for outliers and to determine data distribution. A Wilcoxon rank sum test was used to compare hemodynamics and echocardiographic measurements at baseline and after sildenafil administration. Bivariate linear regression was used to examine the correlation between sildenafil exposure and hemodynamic outcome measures. Statistical analysis was performed using SPSS v. 19.0 software package. A 2-tailed p value <0.05 was considered significant.
RESULTS
Between March 2011 and October 2012, 9 children were enrolled. Median (range) age and weight were 5.2 years (2.5–9.4) and 16.3 kg (9.5–28.1). Five children (55%) were male, and 6 (67%) children had a systemic right ventricle. Table 1 summarizes demographic features and baseline hemodynamic parameters.
Table 1.
Demographics and baseline hemodynamics
| Age (years) | Weight (kg) | O2 saturations (%) | Fontan Pressure (mm Hg) | PCWP (mm Hg) | PVRI (WUxm2) | CI (L/min/m2) |
|---|---|---|---|---|---|---|
| 2.5 | 9.5 | 92 | 11 | 6 | 2.5 | 2.0 |
| 3.8 | 12.6 | 88 | 14 | 5 | 2.4 | 3.7 |
| 4.5 | 16.2 | 92 | 16 | 6 | 3.7 | 3.1 |
| 5.2 | 16.0 | 91 | 17 | 11 | 2.8 | 2.5 |
| 5.2 | 18.1 | 95 | 9 | 4 | 1.3 | 4.2 |
| 5.3 | 19.5 | 98 | 12 | 7 | 1.6 | 3.1 |
| 6.0 | 16.3 | 91 | 11 | 6 | 2.3 | 2.4 |
| 9.3 | 23.4 | 98 | 16 | 7 | 2.3 | 3.9 |
| 9.4 | 28.1 | 96 | 12 | 7 | 2.3 | 2.2 |
|
| ||||||
| 5.2 (2.5–9.4) | 16.3 (9.5–28.1) | 92 (88–98) | 12 (9–17) | 6.5 (4–11) | 2.4 (1.3–3.7) | 2.2 (2.0–4.2) |
Summary statistics represent median (range); PCWP: pulmonary capillary wedge pressure, PVRI: pulmonary vascular resistance index, CI: cardiac index
Hemodynamic effect
Sildenafil effect on hemodynamic parameters immediately following completion of the 20-minute infusion is shown in Table 2. Because of the small numbers in each group, data were pooled for assessment of hemodynamic parameters. Sildenafil lowered pulmonary artery (PA) pressures (−10%, p=0.02), trans-pulmonary gradient (−20%, p=0.01), and indexed PVR (−44%, p=0.01). Pulmonary blood flow increased (9%, p=0.02) and ventilation improved (CO2 decreased 5%, p=0.04) (Figure 1). Sildenafil affected systemic hemodynamics by lowering diastolic blood pressure (−10%, p=0.01) and SVR (−16%, p=0.01) but had no effect on systolic or mean blood pressure. Stroke volume increased (+22%, p=0.05) in 8/9 children, resulting in improved cardiac index (+10%, p=0.01) (Figure 2). There was no overall effect on heart rate (p=0.5).
Table 2.
Sildenafil effect on hemodynamics (n=9)
| Baseline median (range) | Sildenafil median (range) | p | |
|---|---|---|---|
| Co-oximetry | |||
|
| |||
| CO2 (%) | 42 (36,50) | 36 (34,48) | 0.04 |
| SAO2 (%) | 92 (88,98) | 94 (90,97) | 0.32 |
| Mixed venous saturation (%) | 64 (50,76) | 70 (60,76) | 0.03 |
|
| |||
| Pressures | |||
|
| |||
| Fontan pressure (mm Hg) | 12 (9,17) | 11 (11,15) | 0.02 |
| PCWP (mm Hg) | 7 (4,11) | 7 (4,12) | 0.4 |
| Trans-pulmonary gradient (mm Hg) | 5 (5,10) | 4 (3,9) | 0.01 |
| End diastolic pressure (mm Hg) | 6 (4,9) | 6 (4,9) | 0.2 |
| Systolic BP (mm Hg) | 79 (64,98) | 78 (62,91) | 0.1 |
| Diastolic BP (mm Hg) | 50 (38,58) | 45 (35,55) | 0.01 |
| Mean BP (mm Hg) | 64 (52,80) | 64 (50,68) | 0.1 |
|
| |||
| Calculations | |||
|
| |||
| Qp (L/min/m2) | 2.7 (1.8,3.9) | 3.4 (2.2,4.2) | 0.02 |
| Qs (L/min/m2) | 3.1 (2.0,4.2) | 3.4 (2.4,4.4) | 0.01 |
| PVRI (WU x m2) | 2.4 (1.3,3.7) | 1.9 (0.8,2.7) | 0.01 |
| SVRI (WU x m2) | 17 (13,23) | 16 (9,19) | 0.01 |
|
| |||
| Echocardiographic assessment | |||
|
| |||
| Fractional Area Change | 0.36 (0.29, 0.46) | 0.40 (0.36, 0.48) | 0.2 |
| Strain rate (sec−1) | −1.0 (−1.9, −0.6) | −1.0 (−1.8, −0.6) | 0.7 |
| Myocardial Performance Index | 0.56 (0.3, 1.1) | 0.61 (0.3,1.2) | 0.6 |
SAO2: systemic oxygen saturation, PCWP: pulmonary capillary wedge pressure, Qp: pulmonary blood flow, Qs: systemic blood flow, PVRI: Pulmonary vascular resistance index, SVRI: Systemic vascular resistance index
Figure 1.
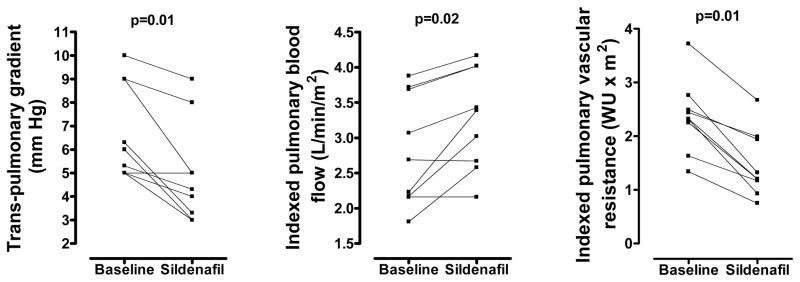
Sildenafil effect on trans-pulmonary gradient, pulmonary blood flow and pulmonary vascular resistance. WU: Wood Units
Figure 2.
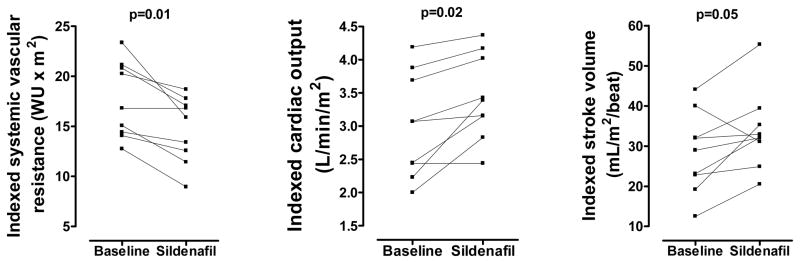
Sildenafil effect on systemic vascular resistance, indexed cardiac output and stroke volume. WU: Wood Units
Sildenafil did not affect echocardiographic measures of systolic function (FAC/EF, strain rate) or global myocardial performance (myocardial performance index). Sildenafil infusion was well tolerated by all 9 children with no adverse events.
Drug exposure data and effect on hemodynamic response
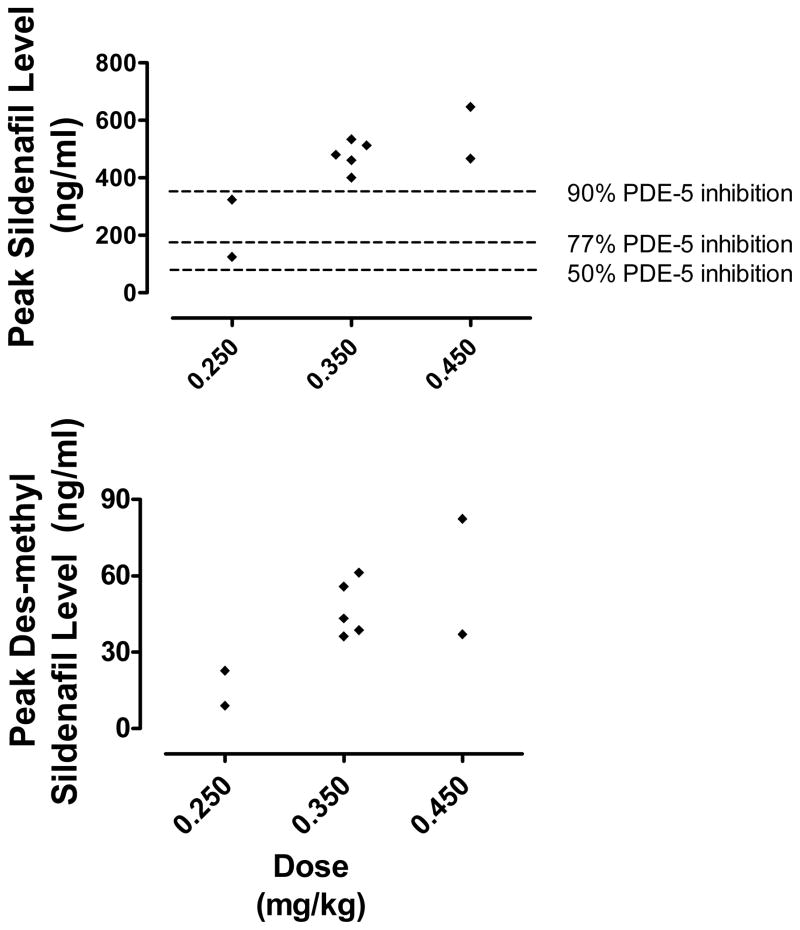
Sildenafil exposure data are summarized in Figure 3. Sildenafil levels peaked immediately after infusion completion, with peak levels ranging from 124–646 ng/mL. Peak drug concentrations were above the in vitro threshold needed for 77% PDE-5 inhibition in 8/9 of children and 90% inhibition in 7/7 of children with doses ≥0.35 mg/kg.17 Peak levels for the active metabolite, des-methyl sildenafil, ranged from 9–82 ng/mL and were seen later, between 26 and 138 minutes after infusion completion. There was high inter-individual variability (coefficient of variation = 34% for peak sildenafil levels). Linear regression analysis demonstrated no association between sildenafil exposure and improvement in hemodynamic parameters, including PVR (r=0.33, p=0.4), SVR (r=0.02, p=0.98), and indexed cardiac output (r=0.69, p=0.31).
Figure 3.
Peak sildenafil and des-methyl sildenafil levels by dosage group. Peak sildenafil levels predictive of varying degrees of PDE-5 inhibition (from in vitro data) are indicated. PDE-5: phosphodiesterase type-5.
DISCUSSION
This is the first prospective study evaluating the hemodynamic effect of IV sildenafil in children with single-ventricle heart defects following Fontan surgical palliation. Sildenafil lowered systemic and pulmonary vascular resistance and increased pulmonary blood flow as well as cardiac output. We hypothesize that improved cardiac output was directly related to pulmonary and/or systemic endothelial modulation as there was no direct effect on myocardial performance and no change in heart rate.
While studies in animal models and in adult subjects with a variety of cardiomyopathic conditions demonstrate improved hemodynamics with sildenafil therapy,11,15,18–24 this is the first prospective study to evaluate hemodynamic effect in children with palliated single-ventricle heart defects. These patients have unique physiology with passive, non-pulsatile pulmonary blood flow and a single pumping chamber supporting both the pulmonary and systemic circulations. Given this physiology, it was previously unclear whether PDE-5 inhibition would have hemodynamic effects similar to those seen in adult patients with right heart myopathy and/or pulmonary hypertension. These data demonstrate that, despite the unique pulmonary physiology, PDE-5 inhibition has a similar effect on the pulmonary vasculature, lowering PA pressures and PVR.
In the absence of a pulmonary pump, low PVR is critically important to the single-ventricle circulation. While the range of baseline indexed PVR in the current study was not remarkably abnormal (1.3–3.7 WU x m2), reduction in PVR was seen in all 9 study children and confirms our prior conclusion from studies after stage II surgery where we demonstrated that there was no “floor” effect on the capacity of PDE-5 inhibitors to lower PVR. Importantly, these improvements in pulmonary hemodynamics translate to improved pulmonary blood flow.
PDE-5 inhibition also acutely increased cardiac output by improving stroke volume. This effect was seen in 8/9 study children. These data are encouraging as low cardiac output is an important contributor to short- and long-term morbidity and mortality in this patient population.6 Strategies to improve cardiac output may have clinical utility in the acute post-operative period and also over the long term to improve functional status.
From a physiologic standpoint, there are three potential mechanisms that might explain the noted improvement in cardiac output: 1) improved contractility; 2) increased preload; or 3) decreased afterload.25 Improved contractility has been demonstrated with PDE-5 inhibition in animal models of the pressure-loaded and hypertrophied right ventricle.11 Furthermore, Goldberg at al. previously demonstrated improved myocardial performance index in an adolescent single-ventricle population with three-week sildenafil therapy.26 However, our data do not support a direct myocardial effect as there was no improvement in multiple echocardiographic measures of systolic function, including the myocardial performance index. Potentially, we did not see a similar effect to that described previously because we evaluated acute as opposed to chronic ventricular response, or alternatively because the ventricle is less responsive to PDE-5 inhibition in younger children. It is also possible that the sample size was too small to detect a small effect on myocardial performance. However, we did not see an effect in our prior analysis of 12 children post stage II surgery, and, even when pooling the two cohorts (unpublished data), there was no significant change.
In the absence of improved myocardial contractility, improved cardiac output might result from improved loading conditions. Preload would be expected to increase with the noted improved pulmonary blood flow. In addition, cardiac index and stroke volume might be augmented by decreased afterload (lowered SVR). There may be long-term benefit to sustained reduced afterload in the single-ventricle population as single-ventricular dysfunction and atrioventricular valve regurgitation are both important contributors to long-term failure of surgical palliation strategies. Tempering this enthusiasm, it should be noted that studies of afterload reduction with angiotensin-converting enzyme inhibitors have consistently failed to show improvement in important surrogate outcome measures such as growth or biomarker indices.27
In this small study, sildenafil infusion was acutely well tolerated with no adverse events. With doses up to 0.45 mg/kg, peak exposure was in the 400–600 ng/mL range, similar to what we have previously reported in younger single-ventricle patients (in press, Pediatric Critical Care Medicine) and similar to the range of exposures reported in adults with standard dosing regimens.28 In general, sildenafil is believed to have a wide acute safety margin, and exposures up to 1800 ng/mL have been well tolerated in adults.29 However, in a previous post-operative study in children, bolus IV sildenafil administration was associated with acute hypotension.30 We did not have any hypotensive events in this cohort with infusions administered over 20 minutes.
The safety of chronic (3–5-year) sildenafil therapy has recently been questioned after long-term follow-up of children enrolled in the Sildenafil in Treatment-naive Children, Aged 1–17 Years, with Pulmonary Arterial Hypertension (STARTS) trial demonstrated an increased three-year mortality risk in children with idiopathic pulmonary arterial hypertension treated with high-dose sildenafil when compared to low doses.31 These data resulted in an FDA safety advisory recommending against sildenafil use in children with pulmonary arterial hypertension.32 However, this recommendation has been widely debated, and experts in the pulmonary hypertension field have recently called for further study of sildenafil efficacy and safety.33 It should also be noted that the European Medicines Agency reviewed the STARTS data and reached a different conclusion than that of the FDA, deciding that “the data support the use of sildenafil in children.”34 These conflicting recommendations and opinions emphasize the importance of population-specific studies, such as the present study, that evaluate drug exposure and physiologic effects. As our data demonstrate, there is acute hemodynamic benefit to sildenafil therapy in single-ventricle patients. These pilot data support further study, potentially in the immediate post-operative period, to determine if the acute hemodynamic effects of sildenafil are sustained and translate into improved outcomes for this patient population.
There are several important limitations to the current study. The study was a small, single-center, open-label study with multiple dosing groups and no control group. This approach was warranted to optimize safety and because one of the objectives of this study was to provide pilot data to help justify and optimize design and conduct of future larger scale clinical trials. By using linear regression to analyze the exposure-response relationship, we were able to overcome some of the limitations intorduced with mutliple dosing groups in a small study cohort. However exposure-response is a less clinically meaningful outcome measure than dose-response. There were also multiple comparisons considering the small number of patients enrolled, however we identified a priori the primary study end points of PVR and cardiac output. Furthermore, the changes observed make physiologic sense. For example, it is logical that lowering of PA pressures might be associated with lowering of PVR and trans-pulmonary gradient. In addition, each patient served as his or her own control, which improves the study power and limits confounding. For example, assumptions used in hemodynamic calculations (i.e. estimation of oxygen consumption), and the effect of other hemodynamic factors (i.e. anesthetic agents, mechanical ventilation etc.) are expected to be constant for baseline and post-sildenafil assessments and therefore would not affect assessment of subject response to therapy. Sildenafil was the only pulmonary vasodilator medication administered during this study, so we cannot compare sildenafil effect to that of selective pulmonary vasodilators such as inhaled nitric oxide or oxygen. Also only acute response after a single dose was assessed and further study is needed to determine whether these effects translate to long-term benefit. Although our original study design called for enrollment of 12 post-Fontan study children, we decided to halt study enrollment and analyze the data after the FDA safety advisory was issued. Echocardiographic image acquisition was challenging, as children were stationary and under sterile drapes for the catheterization procedure. To limit subjectivity of interpretation of the echocardiographic data, we used two independent physicians to review all study images and perform all associated measurements.
In conclusion, single-dose IV sildenafil improves systemic and pulmonary hemodynamics by increasing stroke volume and indexed cardiac output and by lowering indexed PVR, PA pressure, and trans-pulmonary gradient in a small cohort of children with single-ventricle heart defects following Fontan surgical palliation. These data are encouraging and provide proof of concept in support of larger-scale clinical trials to determine if there is sustained benefit and improvement in clinical end points in this patient population.
Acknowledgments
Funding: This study was partially funded by an investigator-initiated research grant from Pfizer Incorporated, with additional funding provided by the Duke University Department of Pediatrics.
Footnotes
Clinicaltrials.gov identifier: NCT01169519
No reprints will be ordered
References
- 1.O’Leary PW. Prevalence, clinical presentation and natural history of patients with single ventricle. Prog Pediatr Cardiol. 2002;16:31–8. [Google Scholar]
- 2.Gentles TL, Gauvreau K, Mayer JE, Jr, et al. Functional outcome after the Fontan operation: factors influencing late morbidity. J Thorac Cardiovasc Surg. 1997;114:392–403. doi: 10.1016/s0022-5223(97)70184-3. discussion 4–5. [DOI] [PubMed] [Google Scholar]
- 3.Gersony WM. Fontan operation after 3 decades: what we have learned. Circulation. 2008;117:13–5. doi: 10.1161/CIRCULATIONAHA.107.748566. [DOI] [PubMed] [Google Scholar]
- 4.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fixler DE, Nembhard WN, Salemi JL, Ethen MK, Canfield MA. Mortality in first 5 years in infants with functional single ventricle born in Texas, 1996 to 2003. Circulation. 2010;121:644–50. doi: 10.1161/CIRCULATIONAHA.109.881904. [DOI] [PubMed] [Google Scholar]
- 6.Khairy P, Fernandes SM, Mayer JE, Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 7.Hosein RB, Clarke AJ, McGuirk SP, et al. Factors influencing early and late outcome following the Fontan procedure in the current era. The ‘Two Commandments’? Eur J Cardiothorac Surg. 2007;31:344–52. doi: 10.1016/j.ejcts.2006.11.043. discussion 53. [DOI] [PubMed] [Google Scholar]
- 8.Boolell M, Allen MJ, Ballard SA, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47–52. [PubMed] [Google Scholar]
- 9.Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361:1864–71. doi: 10.1056/NEJMct0904473. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz BG, Levine LA, Comstock G, Stecher VJ, Kloner RA. Cardiac uses of phosphodiesterase-5 inhibitors. J Am Coll Cardiol. 2012;59:9–15. doi: 10.1016/j.jacc.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 11.Nagendran J, Archer SL, Soliman D, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–48. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 12.Beghetti M. Fontan and the pulmonary circulation: a potential role for new pulmonary hypertension therapies. Heart. 2010;96:911–6. doi: 10.1136/hrt.2010.193912. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg DJ, Shaddy RE, Ravishankar C, Rychik J. The failing Fontan: etiology, diagnosis and management. Expert Rev Cardiovasc Ther. 2011;9:785–93. doi: 10.1586/erc.11.75. [DOI] [PubMed] [Google Scholar]
- 14.Reinhardt Z, Uzun O, Bhole V, et al. Sildenafil in the management of the failing Fontan circulation. Cardiol Young. 2010;20:522–5. doi: 10.1017/S1047951110000648. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg DJ, French B, Szwast AL, et al. Impact of sildenafil on echocardiographic indices of myocardial performance after the Fontan operation. Pediatr Cardiol. 2012;33:689–96. doi: 10.1007/s00246-012-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tei C, Nishimura RA, Seward JB, Tajik AJ. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10:169–78. doi: 10.1016/s0894-7317(97)70090-7. [DOI] [PubMed] [Google Scholar]
- 17.Walker DK, Ackland MJ, James GC, et al. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29:297–310. doi: 10.1080/004982599238687. [DOI] [PubMed] [Google Scholar]
- 18.Webster LJ, Michelakis ED, Davis T, Archer SL. Use of sildenafil for safe improvement of erectile function and quality of life in men with New York Heart Association classes II and III congestive heart failure: a prospective, placebo-controlled, double-blind crossover trial. Arch Intern Med. 2004;164:514–20. doi: 10.1001/archinte.164.5.514. [DOI] [PubMed] [Google Scholar]
- 19.Lindman BR, Zajarias A, Madrazo JA, et al. Effects of phosphodiesterase type 5 inhibition on systemic and pulmonary hemodynamics and ventricular function in patients with severe symptomatic aortic stenosis. Circulation. 2012;125:2353–62. doi: 10.1161/CIRCULATIONAHA.111.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis GD, Shah R, Shahzad K, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–62. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 21.Koka S, Das A, Zhu SG, Durrant D, Xi L, Kukreja RC. Long-acting phosphodiesterase-5 inhibitor tadalafil attenuates doxorubicin-induced cardiomyopathy without interfering with chemotherapeutic effect. J Pharmacol Exp Ther. 2010;334:1023–30. doi: 10.1124/jpet.110.170191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirata K, Adji A, Vlachopoulos C, O’Rourke MF. Effect of sildenafil on cardiac performance in patients with heart failure. Am J Cardiol. 2005;96:1436–40. doi: 10.1016/j.amjcard.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 23.Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50:2136–44. doi: 10.1016/j.jacc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 24.Bocchi EA, Guimaraes G, Mocelin A, Bacal F, Bellotti G, Ramires JF. Sildenafil effects on exercise, neurohormonal activation, and erectile dysfunction in congestive heart failure: a double-blind, placebo-controlled, randomized study followed by a prospective treatment for erectile dysfunction. Circulation. 2002;106:1097–103. doi: 10.1161/01.cir.0000027149.83473.b6. [DOI] [PubMed] [Google Scholar]
- 25.Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T. Cardiac system bioenergetics: metabolic basis of the Frank-Starling law. J Physiol. 2006;571:253–73. doi: 10.1113/jphysiol.2005.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg DJ, French B, McBride MG, et al. Impact of oral sildenafil on exercise performance in children and young adults after the fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation. 2011;123:1185–93. doi: 10.1161/CIRCULATIONAHA.110.981746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu DT, Zak V, Mahony L, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–40. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vachiery JL, Huez S, Gillies H, et al. Safety, tolerability and pharmacokinetics of an intravenous bolus of sildenafil in patients with pulmonary arterial hypertension. Br J Clin Pharmacol. 2011;71:289–92. doi: 10.1111/j.1365-2125.2010.03831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol. 1999;83:13C–20C. doi: 10.1016/s0002-9149(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 30.Stocker C, Penny DJ, Brizard CP, Cochrane AD, Soto R, Shekerdemian LS. Intravenous sildenafil and inhaled nitric oxide: a randomised trial in infants after cardiac surgery. Intensive Care Med. 2003;29:1996–2003. doi: 10.1007/s00134-003-2016-4. [DOI] [PubMed] [Google Scholar]
- 31.Barst RJ, Ivy DD, Gaitan G, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012;125:324–34. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 32.FDA Drug Safety Communication. FDA recommends against use of Revatio (sildenafil) in children with pulmonary hypertension. US Food and Drug Administration; 2012. [Accessed March 3, 2013]. at www.fda.gov/Drugs/DrugSafety/ucm317123.htm. [Google Scholar]
- 33.Abman SH, Kinsella JP, Rosenzweig EB, et al. Implications of the FDA Warning Against the Use of Sildenafil for the Treatment of Pediatric Pulmonary Hypertension. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201210-1928PP. [DOI] [PubMed] [Google Scholar]
- 34.Assessment report for Revatio (International Non-proprietary Name: sildenafil) European Medicines Agency; 2011. [Accessed March 3, 2013]. at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000638/WC500107804.pdf. [Google Scholar]