Abstract
Background: Vocal cord paralysis (VCP) caused by recurrent laryngeal nerve (RLN) damage during thyroidectomy commonly results in serious medico-legal problems. The purpose of this study was to evaluate the usefulness of an asymmetrically porous polycaprolactone (PCL)/Pluronic F127 nerve guide conduit (NGC) for functional regeneration in a RLN injury animal model.
Methods: A biodegradable, asymmetrically porous PCL/F127 NGC with selective permeability was fabricated for use in this study. A 10-mm segment of left RLN was resected in 28 New Zealand white rabbits, and then an asymmetrically porous NGC or a nonporous silicone tube was interposed between both stumps and securely fixed. Vocal cord mobility was endoscopically evaluated at one, four, and eight weeks postoperatively. Nerve growth through NGCs was assessed by toluidine blue staining, and thyroarytenoid (TA) muscle atrophy was evaluated by hematoxylin and eosin staining. Immunohistochemical stainings for acetylcholinesterase (AchE), anti-neurofilament (NF), and anti-S100 protein were also conducted, and transmission electron microscopy (TEM) was used to evaluate functional nerve regeneration.
Results: At eight weeks postoperatively, endoscopic evaluations showed significantly better recovery from VCP in the asymmetrically porous PCL/F127 NGC group (6 of 10 rabbits) than in the silicone tube group (1 of 10 rabbits). Continued nerve growth on the damaged nerve endings was observed with time in the asymmetrically porous PCL/F127 NGC-interposed RLNs. TA muscle dimensions and AchE expressions in TA muscle were significantly greater in the asymmetrically porous PCL/F127 NGC group than in the silicone tube group. Furthermore, immunohistochemical staining revealed the expression of NF and S100 protein in the regenerated nerves in the asymmetrically porous PCL/F127 NGC group at eight weeks postoperatively, and at this time, TEM imaging showed myelinated axons in the regenerated RLNs.
Conclusion: The study shows that asymmetrically porous PCL/F127 NGC provides a favorable environment for RLN regeneration and that it has therapeutic potential for the regeneration of RLN damage.
Introduction
The recurrent laryngeal nerve (RLN) can be damaged or resected during thyroid surgery, and the resultant vocal cord paralysis (VCP) commonly results in severe voice changes, dyspnea, dysphagia, and sometimes life-threatening aspiration (1). Furthermore, in addition to a profound effect on quality of life, RLN can impose enormous psychosocial and economic burdens. For these reasons, RLN damage results in the most significant clinical and legal problems after thyroid surgery (2). However, although awareness of the pathophysiology of nerve damage has improved, no satisfactory surgical treatment has been devised to facilitate functional recovery in patients with VCP.
RLN is one of the most difficult peripheral nerves in which to achieve functional regeneration, especially when it is severed. When a RLN is invaded by a tumor throughout its course, its sacrifice is inevitable. The surgical options for a resected RLN are the end-to-end anastomosis of the transected nerve stumps for a short resection gap or an autologous nerve graft when the gap between stumps is large. However, although the immediate anastomosis of nerve stumps may induce nerve connection and sometimes prevent denervation muscle atrophy, it does not necessarily result in the functional recovery of vocal cord mobility. The main reason for intransient VCP after surgical anastomosis is believed to be due to the atrophy of the denervated muscle or the misdirection of the regenerating nerve fibers. In addition, autologous nerve graft transplantation has several disadvantages, such as the need for another surgical step for harvesting the donor nerve, donor morbidity, the limited lengths of available grafts, three-dimensional structural mismatches between the defect nerve and graft, and the failure of end-organ innervation (3–5).
The development of new strategies to overcome surgical limitations and to facilitate regenerative processes in the context of tissue engineering has become an attractive research field (6,7). Recently, an artificial nerve guide conduit (NGC) between resected nerve stumps was devised to guide axonal sprouting from proximal to distal stumps, and is widely accepted as an alternative treatment option (8,9). For successful nerve regeneration using NGCs, the material must meet several essential criteria, such as the structural stability required for nerve growth, biodegradability to avoid surgery for secondary removal, and easy application to the surgical process (10,11). A variety of NGCs based on biological tissues and polymers have been devised to meet these requirements, but RLN regeneration using NGCs has received little attention to date.
In a previous study, we developed an asymmetrically porous polycaprolactone (PCL)/Pluronic F127 tube (inner surface, nano-sized pores; outer surface, micro-sized pores) with selective permeability, that is, it prevents fibrous scar tissue infiltration but allows the permeation of nutrients/oxygen, which is critical for effective nerve regeneration through a NGC (12). Furthermore, our studies in a rat sciatic nerve defect model showed that PCL/F127 NGC provides a favorable environment for peripheral nerve regeneration. Accordingly, the main aim of this study was to evaluate the potential of asymmetrically porous PCL/F127 NGC for the recovery of vocal cord movement by promoting RLN regeneration and preventing atrophy of intrinsic laryngeal muscles in a RLN injury animal model.
Materials and Methods
Fabrication of an asymmetrically porous nerve guide conduit
Asymmetrically porous PCL/F127 NGCs with selective permeability were prepared by rolling an asymmetrically porous sheet fabricated using an immersion precipitation method, as previously described (12). Briefly, to prepare an asymmetrically porous PCL/F127 sheet (nano- and micro-pores on both surfaces), PCL pellets were dissolved in tetraglycol (12 wt%; Sigma Aldrich, St Louis, MO) at 90°C, and then Pluronic F127 powder (BASF, Ludwigshafen, Germany) was added in the PCL solution (5 wt%, PCL base). The PCL/F127 solution was filled in a mold (50 mm×50 mm×0.4 mm) and immersed in excess water for one hour at room temperature. The precipitated PCL/F127 sheet was washed in excess water to remove the residual tetraglycol. The prepared sheet (thickness ∼0.4 mm) was vacuum-dried. To prepare the NGC using the asymmetrically porous PCL/F127 sheet, the sheet was rolled into a tube using a 1.5-mm diameter metal mandrel (inside of the tube, smaller pore side) and the edge fold of the sheet was fixed using a tissue adhesive (Histoacryl®; B. Braun, Melsungen, Germany). The prepared asymmetrically porous NGCs had an inner diameter of ∼1.5 mm and a length of ∼12 mm.
Animal studies
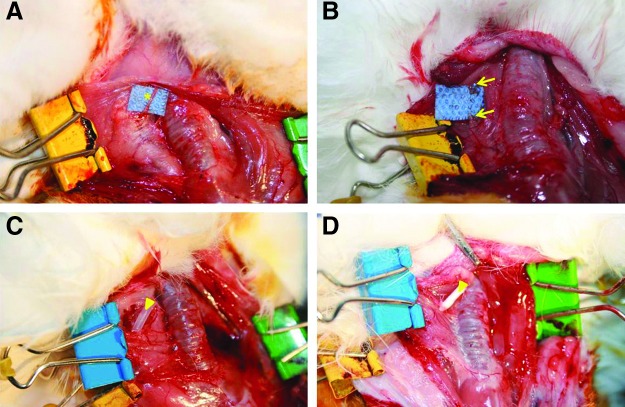
This study was approved by the Animal Ethics Committee of Inha University Hospital, and the animals were cared for in accordance with established institutional guidelines. Twenty-eight female New Zealand white rabbits weighing 3.0–3.5 kg were used in the experiments. Animals were maintained in a temperature, humidity, and light-controlled environment in an animal house with free access to water and a standard rabbit diet. Rabbits were assigned randomly to two groups: the silicone tube (Silastic®; Dow Corning, Midland, MI) interposed control group (n=14) or the PCL/F127 NGC tube interposed experimental group (n=14). Both groups were then divided into three subgroups based on time of sacrifice, which was conducted at one (n=2), four (n=2), or eight (n=10) weeks postimplantation. The animals were subcutaneously premedicated with 0.05 mg/kg glycopyrrolate and 5 mg/kg xylazine, and then anesthetized with an intramuscular injection of 15 mg/kg zolazepam, with all efforts made to minimize suffering. A vertical skin incision was made, followed by division of the platysma and strap musculature. The left RLN was carefully exposed and dissected circumferentially under an operating microscope (Fig. 1A). A 10-mm segment of the RLN was transected using microscissors (Fig. 1B). NGCs were interposed between the proximal and distal stumps using two sutures (7-0 vicryl; Ethicon, Somerville, NJ) at each junction. The animals were then subjected to one of the following two procedures: (i) nerve resection and silicone tube interposition (Fig. 1C), or (ii) nerve resection and PCL/F127 NGC tube interposition (Fig. 1D). Following the implantation, the muscle incision was closed using a 5-0 chromic catgut suture (Ethicon), and the skin was closed using a 4-0 nylon suture (Ethicon).
FIG. 1.
Interposition of the nerve guide conduits (NGCs) in a recurrent laryngeal nerve (RLN) injury animal model. (A) Dissected left RLN, (B) segmental resection of the RLN (10 mm), (C) the silicone tube implant, and (D) the PCL/F127 NGC implant. *, RLN; arrow, proximal and distal stumps of a resected RLN; arrowheads, silicone and PCL/F127 NGCs.
Functional evaluation of recurrent laryngeal nerve regeneration
Laryngeal endoscopy was performed to confirm normal motion of the larynx before and after RLN resection. Vocal cord movements were evaluated and recorded using an endoscopy recording system at one, four, and eight weeks postimplantation. Briefly, an anesthetized rabbit was secured to an operating platform in the supine position. First, the left side of the neck was explored to detect the interposed tube. After exposure of the interposed tube, a rigid laryngeal endoscope (Storz, Tuttlingen, Germany) was inserted orally and fixed in position to provide the best view of the larynx. Vocal cord movements were induced by stimulating the proximal stump of the damaged RLN using a nerve stimulator (Maplewood; WR Medical Electronics Co., Saint Paul, MN) and recorded. Vocal cord movements were evaluated by analyzing the recorded images. Two frames of the vocal cord pictures obtained during nerve stimulation were selected and captured using a computer: one frame in the fully adducted position and the other in the fully abducted position. Traces of vocal cord movements were obtained by calculating the triangular areas in the abducted and adducted positions using Image J (NIH, Bethesda, MD). Vocal cord function was evaluated by measuring the areas of vocal cord movements of the injured left side and the normal right side as a ratio (relative gap ratio).
Histological examination
After evaluating vocal cord movement, the animals were euthanized, the RLNs between the proximal and distal stumps were harvested to evaluate nerve growth, and the larynges were excised to assess TA muscle status. RLNs and larynxes were immediately placed in 4% paraformaldehyde at room temperature, processed, embedded in paraffin, and sectioned at 4 μm (RLNs were sectioned longitudinally, and larynxes were sectioned axially). For evaluation, the RLNs were stained with toluidine blue and hematoxylin and eosin, and the larynges were stained with hematoxylin and eosin. The thyroarytenoid (TA) muscle was evaluated at the level of the vocal process. Muscle sections were observed under a light microscope (Nikon, Tokyo, Japan), and under blinded conditions, a single observer measured the total cross-sectional areas of the muscles. The cross-sectional areas of the TA muscles were measured using Image J software by tracing the outlines of the microscopic images. Histologic changes in TA muscles were evaluated by calculating the ratios of the areas of denervated TA muscles and the contralateral normal sides (relative area ratios).
Immunohistochemical analysis
The sections were washed in phosphate buffered saline (PBS; pH ∼7.4) and pre-incubated for one hour in a blocking solution containing 5% normal goat serum in PBS. After a brief PBS wash, primary antibodies for neurofilament (NF) and S100 protein (Merck Millipore, Darmstadt, Germany) diluted in blocking solution were applied. Sections were then incubated overnight at 4°C, in secondary antisera (Zymed Laboratories Inc., San Francisco, CA) for 90 minutes at room temperature, and then treated with avidin–biotin–peroxidase solution. The peroxidase label was visualized using diaminobenzidine as the chromogen (Vector Laboratories, Burlingame, CA). Sections were then washed, allowed to air dry, coverslipped, and processed for observation under a confocal microscope (IX81, Olympus, Center Valley, PA). To evaluate the nerve endplates of the regenerated nerves, acetylcholinesterase (AchE) immunohistochemical staining was performed. Briefly, sections were hydrated in water, placed in sodium sulfate solution (20%) for five minutes, rinsed in water, and incubated in acetylcholinesterase solution for up to three hours at 37°C until bright blue endplates could be clearly distinguished (13). The endplates of the regenerated nerves were evaluated by calculating the ratio of the numbers of injured left and normal right nerve endings.
Transmission electron microscopy evaluation
The regenerating RLNs within NGCs were immersed for two hours in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), washed in 0.1 M cacodylate buffer (pH 7.4), and postfixed for 90 min in 1% osmium tetroxide containing 0.8% potassium ferrocyanide and 5 nM calcium chloride in 0.1 M cacodylate buffer (pH 7.4). Segments were then washed in 0.1 M cacodylate buffer (pH 7.4), stained in 1% uranyl acetate, dehydrated using an acetone series, infiltrated with Poly/Bed 812 resin, and polymerized at 60°C for two days. Transverse sections (70 nm thick) were obtained using an ultramicrotome (MT-6000-XL-RMC, Boeckeler Instruments, Inc., Tucson, AZ), placed on copper grids, treated with 5% uranyl acetate and 1% lead citrate, and examined under a transmission electron microscope operated at 80 kV (Zeiss, Oberkochen, Germany).
Statistical analysis
All analyses were performed using GraphPad Prism 5.01 (GraphPad Software, Inc., San Diego, CA). The Mann–Whitney test was used to compare the vocal cord movements, the cross-sectional areas of TA muscles, and the numbers of nerve endings in the PCL/F127 NGC and silicone tube groups. Statistical significance was accepted for p-values <0.05. All results are expressed as means±standard deviations (SD).
Results
Functional recurrent laryngeal nerve regeneration
Endoscopic observations of larynges before RLN transection showed normal vocal cord movement during RLN stimulation in all rabbits. Restoration of vocal cord movement was not found at one and four weeks after the operation in both the silicone tube and the PCL/F127 NGC groups. However, the restoration of vocal cord movement was seen in 6 of 10 (60%) rabbits in the PCL/F127 NGC group and 1 of 10 (10%) in the silicone tube group at eight weeks postimplantation, although the amplitudes of the vocal cord movements varied. The mean relative gap ratio between vocal cord adduction and abduction of the injured vocal cord to the normal side was measured (Fig. 2A and B). The gap ratios improved to 55.53±17.69% in the PCL/F127 NGC group as compared with 10.2±10.20% in the silicone tube group at eight weeks, with this restoration of vocal cord movement significantly better in the PCL/F127 NGC group (p<0.05; Fig. 2C).
FIG. 2.
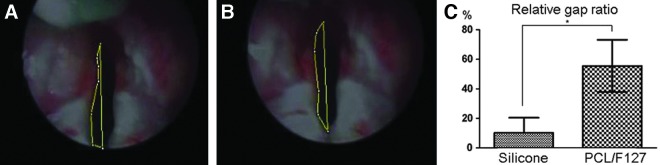
Endoscopic examinations of vocal cord movement. Captured images of (A) adducted position after nerve stimulation and (B) abducted position. (C) Mean gap ratio during vocal cord movements were significantly different between the PCL/F127 NGC group (55.53±17.69%) and the silicone tube group (10.20±10.20%; n=20; *p<0.05).
Histologic findings
Nerve growth and vasculogenesis
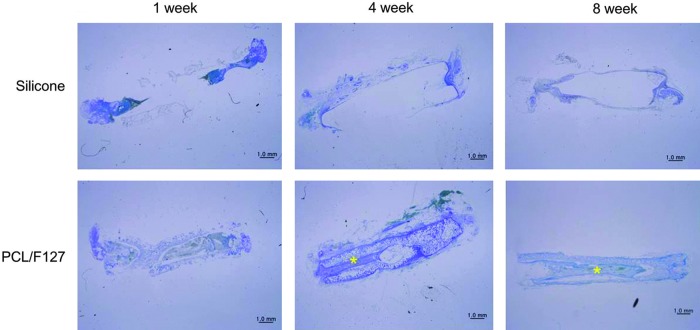
Figure 3 shows the longitudinal sections along with RLNs within NGCs at one, four, and eight weeks postimplantation. At one week, no nerve regeneration was detected in either group. At four weeks, a regenerated segment was found in the PCL/F127 NGC group but not in the silicone tube group. At eight weeks, the gross appearances of regenerated nerves in the PCL/F127 NGC group were comparable to normal, whereas poor neural growth was observed in the silicone tube group. The porous nature of PCL/F127 NGCs also allowed vascular ingrowth into the tube walls through the columnar pores in their outer surfaces (Fig. 4).
FIG. 3.
Histologic evaluation of regenerated RLNs at one, four, and eight weeks postimplantation in the PCL/F127 NGC and silicone tube groups. No nerve regeneration was detected in the silicone tube group over the eight-week study period. By contrast, continued nerve regeneration of the resected segment was observed at four weeks in the PCL/F127 NGC group, and the regenerated nerve looked structurally normal in the PCL/F127 NGC group at eight weeks (toluidine blue stained;×12.5; *, regenerated RLNs). Color images available online at www.liebertpub.com/thy
FIG. 4.
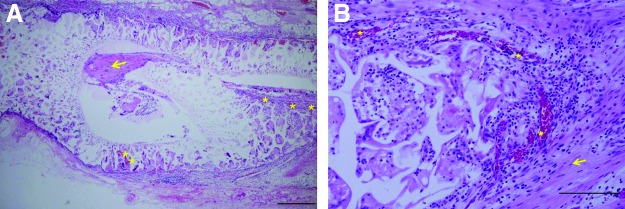
Histologic evaluation of vasculogenesis through the PCL/F127 NGC group at eight weeks postimplantation. Histologic evaluation revealed that the walls of PCL/F127 NGCs were infiltrated by blood vessels. (A) H&E stained, ×40; bar, 500 μm. (B) H&E stained, ×200; bar, 200 μm. *, blood vessels; arrow, regenerated RLNs. Color images available online at www.liebertpub.com/thy
Thyroarytenoid muscle atrophy
Representative cross-sectional findings of TA muscle are shown in Figure 5. In the silicone tube group, marked atrophy of TA muscle was observed on the operated sides at eight weeks postimplantation (Fig. 5A), whereas in the PCL/F127 NGC group, TA muscles showed mild deformity (Fig. 5B). Area ratios of TA muscle decreased to 86.5% in the PCL/F127 NGC group and to 77.3% in the silicone tube group, which represented a significant difference (p=0.034; Fig. 5C).
FIG. 5.
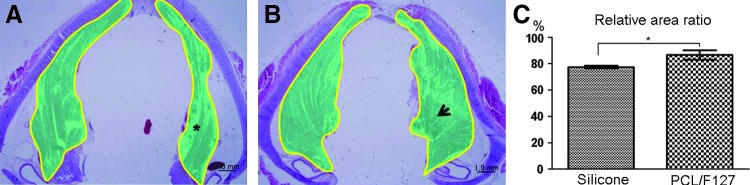
Histologic evaluation of thyroarytenoid (TA) muscle atrophy at the level of the vocal process. (A) The cross-sectional areas of TA muscles in the silicone tube group. (B) The cross-sectional areas of TA muscles in the PCL/F127 NGC group. The cross-sectional areas of TA muscles were measured using Image J by tracing their outlines on microscopic images. *, atrophied TA muscle due to denervation of RLN in the silicone tube group; arrow, compensated TA muscle due to reinnervation of the RLN in the PCL/F127 NGC group. (C) The mean area ratio of the denervated TA muscles to the control side was significantly greater in the PCL/F127 NGC group (86.51±3.59%) than in the silicone tube group (77.34±1.03%; n=20; *p<0.05).
Immunohistochemistry
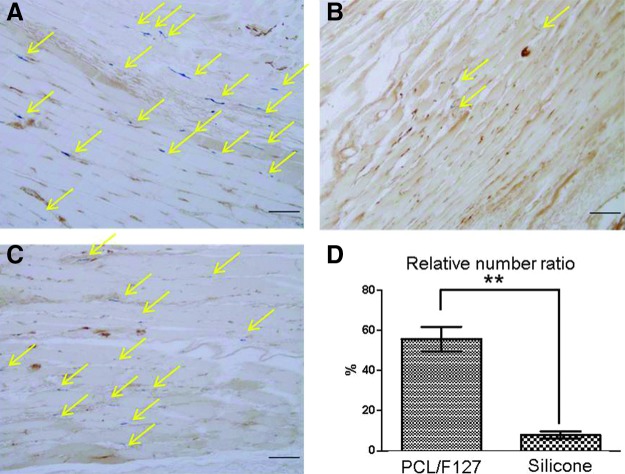
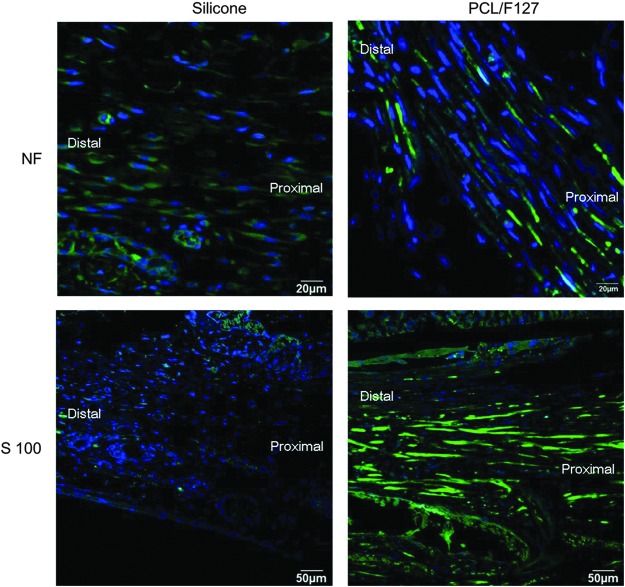
The numbers of nerve endings were compared eight weeks after implantation. Many more nerve endings were observed in the normal sides in comparison to the injured sides. The numbers of nerve endings in the PCL/F127 NGC group were found to be much greater than those observed in the silicone tube group (p=0.001; Fig. 6). Figure 7 shows the expression of NF and S100 proteins at eight weeks after implantation. Both proteins were abundantly expressed in the PCL/F127 NGC group, but were not detected in the silicone tube group.
FIG. 6.
Numbers of nerve endings as determined by AchE immunostaining at eight weeks postimplantation. (A) Normal TA muscle; (B) TA muscle in the silicone tube group; (C) TA muscle in the PCL/F127 NGC group; and (D) the calculated mean number ratios of nerve endings in the denervated TA muscles to those in the normal sides. The mean number ratios were 7.83±1.83% (PCL/F127 NGC group) and 55.75±6.13% (silicone tube group), which were significantly different. Arrow, nerve ending; n=20; bar, 100 μm; *p<0.05).
FIG. 7.
The expression of NF and S100 protein at eight weeks. Blue, DAPI; green, NF or S100 protein. Both NF and S100 protein were abundantly expressed in the PCL/F127 NGC group, but were not detected in the silicone tube group.
Transmission electron microscopy images of regenerated nerves
Transmission electron microscopy (TEM) of the mid-portion of the regenerative nerves in the PCL/F127 NGC group at eight weeks after implantation showed the formation of regenerated myelinated fibers. The structure of the myelinated fibers of the regenerated nerves in the PCL/F127 NGC group was compact and well organized with surrounding Schwann cells. The axons contained neurofilaments, which are essential for axonal transport (Fig. 8).
FIG. 8.
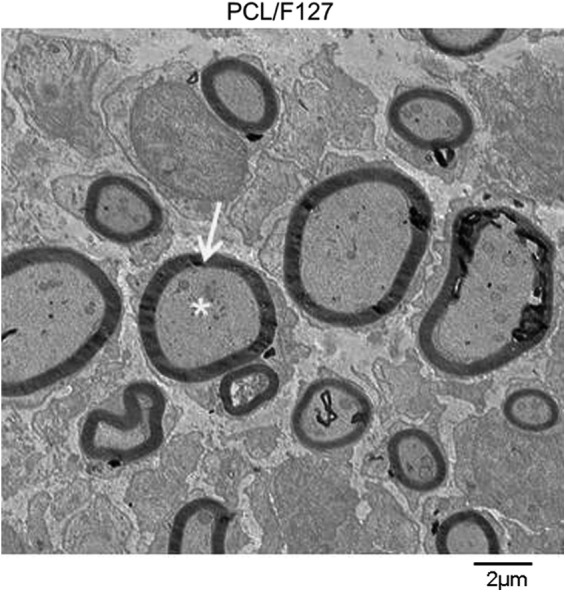
Transmission electron microscopy findings at eight weeks. The PCL/F127 NGC group showed the formation of regenerated myelinated fibers. The structure of the myelinated fibers of the regenerated nerves was compact and well organized with the surrounding Schwann cells (×6000; bar, 2 μm; asterisk, axon; arrow, myelin).
Discussion
In this study, we sought to determine whether asymmetrically porous PCL/F127 NGCs could be used to regenerate resected RLNs. We found that the asymmetrically porous PCL/F127 NGC tubes facilitated nerve regeneration as compared with nonporous silicone tubes. At eight weeks postimplantation, the PCL/F127 NGC group showed more growing neural tissue and more rapid bridging of the 10-mm nerve gap than what was observed in the silicone tube group. Our results show that NGC sheathing provides an environment that stimulates nerve growth, which is consistent with previous reports (14,15). In particular, the asymmetrically porous PCL/F127 NGCs enabled vascular ingrowth into tube walls through its unique asymmetric column shape porous structure, which might deliver nutrients/oxygen into the tube more effectively, thus allowing faster nerve regeneration. Azzam et al. reported that tube permeability plays an important role in nerve regeneration (16), particularly during the early stages.
After RLN injury, the distal portions of axons separate from the soma and degenerate in a series of steps called Wallerian degeneration (17). Thereafter, the axons extend from the proximal to distal stump via the proliferation of Schwann cells, and finally reinnervate their distal targets, possibly restoring function. Thus, PCL/F127 NGCs may play the role of artificial Schwann cells and enable stable nerve growth in a controlled microenvironment. Furthermore, the distal position of a NGC is maintained as free space, thus allowing growing axons unimpeded access to their targets in the distal stump free from interference by fibrous tissue scars. In the present study, we estimated the nerve growth rate through PCL/F127 NGC tubes to be 0.18 mm/day, which is similar to previously reported results (18).
When a resected RLN is anastomosed by autologous nerve grafting, functional recovery of vocal cord mobility is hardly ever achieved (19–21). This is believed to be due to incomplete RLN regeneration at the site of injury and the progressive atrophy of laryngeal muscles. In particular, the TA muscle is believed to be most affected after resection of the RLN, undergoing severe degeneration (22). Accordingly, we compared TA muscle atrophy in the two study groups and found that RLN reinnervation in the PCL/F127 NGC group led to improved vocal cord movement and less denervation-induced muscle atrophy. In addition, we also counted the numbers of nerve endings (AchE) because of the likely relation between this and the functional restoration of the neuromuscular junction. The results obtained suggest that PCL/F127 NGCs facilitated not only the regeneration of transected RLNs, but also the restoration of laryngeal function by preventing denervation-induced atrophy of laryngeal muscles.
The misdirection of regenerating nerve fibers is another known cause of persistent vocal cord immobilization after nerve grafting (23). Previous studies on the subject have shown that after repair of the RLN, regenerating nerve fibers frequently connect with the wrong laryngeal muscles. For example, adductor fibers have been found to receive input from abductor motor neurons and vice versa (24,25). Because it is difficult to investigate paradoxical movements caused by RLN fiber misdirection to laryngeal muscles, topographic electrophysiological studies are needed to determine whether reinnervation using NGCs reduces this phenomenon.
Furthermore, the present study confirms the expressions of NF and S100 proteins in regenerated nerve fibers. NF is specifically expressed in neurons and supports normal axonal radial growth, whereas S100 is preferentially expressed in myelin-forming Schwann cells (26). In the present study, TEM was used to obtain a detailed view of regenerating axons in tubes, and the PCL/F127 NGC group was found to exhibit a more organized neural structure and more myelination than the control group. The expression of these proteins could be considered evidence of the regeneration of a normal neural structure.
In recent years, vocal fold medialization procedures have been widely performed during voice rehabilitation for patients with RLN paralysis, including medialization thyroplasty, arytenoid adduction, and injection laryngoplasty (27). The main goal of these treatments is to facilitate glottic closure and improve voice quality by medializing the paralyzed vocal cord (28). A number of studies have attempted to confirm the improvements in voice and aspiration after vocal fold medicalization, and the results suggest that they can be a good treatment option for post-thyroidectomy VCP. However, no satisfactory surgical techniques to recover permanent RLN injury have been developed to date.
In this study, we investigated the potential use of asymmetrically porous PCL/F127 NGC for promoting the regeneration of RLNs by different morphologic and functional evaluations over the first eight weeks postimplantation in a denervated rabbit model. To the best of our knowledge, this is the first study to describe the therapeutic potential of asymmetrically porous PCL/F127 NGC tubes for RLN regeneration. However, this study has some limitations that bear consideration. First, the length of the severed RLN in our study was 10-mm, but the maximum length at which NGC can be clinically applied needs to be determined. In recent studies, the maximum length of the peripheral nerve defect that can be regenerated through NGC was found to be around 30 mm (29). Furthermore, NGC combined with nerve growth factors, Schwann cells, or stem cells deserve further investigation for long-gap nerve defects. Second, this study was conducted over eight weeks in a small number of rabbits, and thus larger-scale, longer-term studies are needed to confirm our findings. Lastly, we used immunohistochemistry to document the expression of NF and S100 protein within nerve fibers, and despite the value of this approach for detecting specific proteins in tissues, studies supported by more quantitative techniques such as protein and gene analysis should be conducted to confirm and expand our results.
Conclusion
In this in vivo RLN injury animal model, asymmetrically porous PCL/F127 NGCs were found to be better at promoting nerve regeneration and alleviating TA muscle atrophy than nonporous silicone tubes. We believe that these differences were caused by the improved access due to the sufficient mechanical strength of the tube, the effective prevention of fibrous scar tissue invasion, and the suitable permeation of nutrients and oxygen, thus avoiding delays in axonal sprouting and the misdirection of regenerating nerve fibers. We conclude that the asymmetrically porous PCL/F127 NGC tube appears to be a good alternative option for the repair and regeneration of RNL damage during surgery.
Acknowledgment
This research was supported by an Inha University Research Grant.
Author Disclosure Statement
The authors have no potential conflict of interest to declare.
References
- 1.Randolph GW.2010The importance of pre- and postoperative laryngeal examination for thyroid surgery. Thyroid 20:453–458 [DOI] [PubMed] [Google Scholar]
- 2.Gardner GM, Smith MM, Yaremchuk KL, Peterson EL.2013The cost of vocal fold paralysis after thyroidectomy. Laryngoscope 123:1455–1463 [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Cai Q, Hou J, Bei J, Zhang T, Yang J, Wan Y.2003Acceleration effect of basic fibroblast growth factor on the regeneration of peripheral nerve through a 15-mm gap. J Biomed Mater Res A 66:522–531 [DOI] [PubMed] [Google Scholar]
- 4.Kiyotani T, Teramachi M, Takimoto Y, Nakamura T, Shimizu Y, Endo K.1996Nerve regeneration across a 25-mm gap bridged by a polyglycolic acid-collagen tube: a histological and electrophysiological evaluation of regenerated nerves. Brain Res 740:66–74 [DOI] [PubMed] [Google Scholar]
- 5.Widmer MS, Gupta PK, Lu L, Meszlenyi RK, Evans GR, Brandt K, Savel T, Gurlek A, Patrick CW, Jr, Mikos AG.1998Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials 19:1945–1955 [DOI] [PubMed] [Google Scholar]
- 6.Evans GR.2001Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec 263:396–404 [DOI] [PubMed] [Google Scholar]
- 7.Pettersson J, McGrath A, Kalbermatten DF, Novikova LN, Wiberg M, Kingham PJ, Novikov LN.2011Muscle recovery after repair of short and long peripheral nerve gaps using fibrin conduits. Neurosci Lett 500:41–46 [DOI] [PubMed] [Google Scholar]
- 8.Maquet V, Martin D, Malgrange B, Franzen R, Schoenen J, Moonen G, Jerome R.2000Peripheral nerve regeneration using bioresorbable macroporous polylactide scaffolds. J Biomed Mater Res 52:639–651 [DOI] [PubMed] [Google Scholar]
- 9.Heath CA, Rutkowski GE.1998The development of bioartificial nerve grafts for peripheral-nerve regeneration. Trends Biotechnol 16:163–168 [DOI] [PubMed] [Google Scholar]
- 10.Mackinnon SE, Dellon AL, Hudson AR, Hunter DA.1984Chronic nerve compression—an experimental model in the rat. Ann Plast Surg 13:112–120 [DOI] [PubMed] [Google Scholar]
- 11.Keilhoff G, Stang F, Wolf G, Fansa H.2003Bio-compatibility of type I/III collagen matrix for peripheral nerve reconstruction. Biomaterials 24:2779–2787 [DOI] [PubMed] [Google Scholar]
- 12.Oh SH, Kim JR, Kwon GB, Namgung U, Song KS, Lee JH.2012Effect of surface pore structure of nerve guide conduit on peripheral nerve regeneration. Tissue Eng Part C Methods 19:233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin AD, Hogikyan ND, Oh A, Feldman EL.2012Potential for promoting recurrent laryngeal nerve regeneration by remote delivery of viral gene therapy. Laryngoscope 122:349–355 [DOI] [PubMed] [Google Scholar]
- 14.Navarro X, Rodriguez FJ, Labrador RO, Buti M, Ceballos D, Gomez N, Cuadras J, Perego G.1996Peripheral nerve regeneration through bioresorbable and durable nerve guides. J Peripher Nerv Syst 1:53–64 [PubMed] [Google Scholar]
- 15.Chen MH, Chen PR, Hsieh ST, Huang JS, Lin FH.2006An in vivo study of tricalcium phosphate and glutaraldehyde crosslinking gelatin conduits in peripheral nerve repair. J Biomed Mater Res B Appl Biomater 77:89–97 [DOI] [PubMed] [Google Scholar]
- 16.Azzam NA, Zalewski AA, Williams LR, Azzam RN.1991Nerve cables formed in silicone chambers reconstitute a perineurial but not a vascular endoneurial permeability barrier. J Comp Neurol 314:807–819 [DOI] [PubMed] [Google Scholar]
- 17.Pan YA, Misgeld T, Lichtman JW, Sanes JR.2003Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo. J Neurosci 23:11479–11488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SC, Oh SH, Seo TB, Namgung U, Kim JM, Lee JH.2010Ultrasound-stimulated peripheral nerve regeneration within asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. J Biomed Mater Res B Appl Biomater 94:359–366 [DOI] [PubMed] [Google Scholar]
- 19.Crumley RL.1991Update: ansa cervicalis to recurrent laryngeal nerve anastomosis for unilateral laryngeal paralysis. Laryngoscope 101:384–387; discussion 388 [DOI] [PubMed] [Google Scholar]
- 20.Lee WT, Milstein C, Hicks D, Akst LM, Esclamado RM.2007Results of ansa to recurrent laryngeal nerve reinnervation. Otolaryngol Head Neck Surg 136:450–454 [DOI] [PubMed] [Google Scholar]
- 21.Zheng H, Li Z, Zhou S, Cuan Y, Wen W.1996Update: laryngeal reinnervation for unilateral VCP with the ansa cervicalis. Laryngoscope 106:1522–1527 [DOI] [PubMed] [Google Scholar]
- 22.Iizuka T.1966. [Experimental studies on the nerve interception and atrophy of the intrinsic muscles of the larynx]. Nihon Jibiinkoka Gakkai Kaiho 69:176–195 [DOI] [PubMed] [Google Scholar]
- 23.Crumley RL.2000Laryngeal synkinesis revisited. Ann Otol Rhinol Laryngol 109:365–371 [DOI] [PubMed] [Google Scholar]
- 24.Nahm I, Shin T, Watanabe H, Maeyama T.1993Misdirected regeneration of injured recurrent laryngeal nerve in the cat. Am J Otolaryngol 14:43–48 [DOI] [PubMed] [Google Scholar]
- 25.Nahm I, Shin T, Chiba T.1990Regeneration of the recurrent laryngeal nerve in the guinea pig: reorganization of moneurons after freezing injury. Am J Otolaryngol 11:90–98 [DOI] [PubMed] [Google Scholar]
- 26.Mata M, Alessi D, Fink DJ.1990S100 is preferentially distributed in myelin-forming Schwann cells. J Neurocytol 19:432–442 [DOI] [PubMed] [Google Scholar]
- 27.Lee SW, Kim JW, Chung CH, Mok JO, Shim SS, Koh YW, Choi EC.2010Utility of injection laryngoplasty in the management of post-thyroidectomy vocal cord paralysis. Thyroid 20:513–517 [DOI] [PubMed] [Google Scholar]
- 28.Rubin AD, Sataloff RT.2008Vocal fold paresis and paralysis: what the thyroid surgeon should know. Surg Oncol Clin N Am 17:175–196 [DOI] [PubMed] [Google Scholar]
- 29.Alluin O, Wittmann C, Marqueste T, Chabas JF, Garcia S, Lavaut MN, Guinard D, Feron F, Decherchi P.2009Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials 30:363–373 [DOI] [PubMed] [Google Scholar]