Abstract
We report here an efficient and expeditious microwave-assisted synthesis of cyclopentadiene ring-fused tetrahydroquinolines using the three-component Povarov reaction catalyzed by indium (III) chloride. This method has an advantage of shorter reaction time (10 – 15 min) with high and reproducible yields (up to 90%) and is suitable for parallel library synthesis. The optimization process is reported and the results from the microwave route are compared with those of the conventional synthetic route. In almost all cases, the microwave acceleration consistently provided improved yields favoring the cis-diastereomer.
Keywords: Tetrahydroquinolines, Cyclopentene ring-fused tetrahydroquinolines, Microwave-assisted synthesis, Parallel synthesis, Povarov reaction
1. Introduction
The tetrahydroquinoline derivatives have attracted considerable attention because of their important biological activities and occurrence in natural products.1 Those containing fused cyclopentene ring have been found to exhibit a variety of pharmacological activities like agonism at BKCa receptor (1),2 GPR30 estrogen receptor (2)3,4 and allosteric modulation at the α7 nicotinic acetylcholine receptors (nAChRs)5–7 as well as antitubercular activity against M. tuberculosis (Figure 1).8 One such representative tetrahydroquinoline derivative is TQS (3; 4-(1-naphthyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide), a potent type II positive allosteric modulator (PAM) of α7 nAChRs.7,9,5,10 Such type II PAMs have been preclinically shown to be useful in improving memory, cognition and in treating neuropathic pain. Therefore, synthesis of new tetrahydroquinolines still holds great interest.11,1 A variety of synthetic methods have been developed for constructing such tetrahydroquinolines and among them the three-component Povarov reaction has been most empowering and versatile.12,11,1,13 This reaction which is also known as aza-Diels Alder reaction can be carried out in a one-pot fashion using an aniline, an aldehyde and an electron-rich dienophile in the presence of various Brønsted as well as Lewis acids.11,14 Although the original Povarov reaction was carried out with cyclopentadiene as a dienophile, most of the later optimization and scope expansion of this reaction was focused on shelf-stable dienophiles such as pyran, furan etc.14,11 Synthesis of cyclopentene ring-fused tetrahydroquinolines has been relatively low yielding7,6,14,11 due to thermal instability of cyclopentadiene leading to its spontaneous dimerization at room temperature and gradual polymerization even at low temperature.15 Various Brønsted and Lewis acids have been utilized to accelerate and improve reaction times, but have led to only limited success.16–21,2,22,23 Microwave-assisted organic synthesis has become an important tool in organic synthesis for rate enhancement, improving reaction yields, and reducing thermal degradation byproducts.24 To the best of our knowledge use of microwave acceleration for the Povarov reaction has been limited to thermally-stable N-vinylpyrrolidin-2-one25, pyran26, furan26, 2-methoxyacrylate27 and acrylamides27 dienophiles. In view of this, we report here a microwave-assisted synthesis of cyclopentene ring-fused tetrahydroquinolines using InCl3 as a Lewis acid, which provides excellent results in terms of yield, reaction time and diasteroselectivity.
Figure 1.
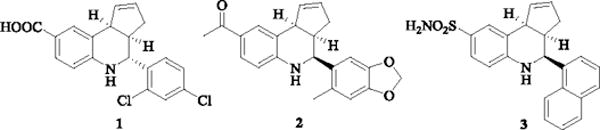
Representative biologically-active cyclopentene ring-fused tetrahydroquinolines.
2. Chemistry and Discussion
As significant pharmacological work has been done around the α7 nAChR allosteric modulator TQS (3), we selected it’s synthesis as a model reaction. To date, it has been synthesized in a maximum of 22% yield with a reaction time of 24 hrs at room temperature and using 20 mol% InCl3 as the catalyst.6
With an objective of improving this reaction yield and reducing reaction time, we carried out optimization under microwave irradiation by evaluating four different aspects of this reaction: a. reaction temperature; b. reaction time (Table 1); c. catalyst loading and d. cyclopentadiene loading (Table 2). We preferred InCl3 as a Lewis acid for our optimization because compared to the conventional Lewis acids, it has some advantages including its compatibility with both organic and aqueous media, recyclability, operational simplicity and a strong tolerance to oxygen- and nitrogen- containing substrates and functional groups.28,29 Additionally, it is a preferred catalyst in multicomponent reactions providing better regio- and diastero-selectivities.28
Table 1.
Influence of reaction temperature and time
| Entry | Temperature (°C) | Time (min) | Yield (%) |
|---|---|---|---|
| 1 | 100 | 15 | 60 |
| 2 | 75 | 15 | 60 |
| 3 | 50 | 15 | 35 |
| 4 | 75 | 5 | 49 |
| 5 | 75 | 10 | 60 |
| 6 | 120 | 5 | 55 |
Table 2.
Effect of catalyst and cyclopentadiene loading
| Entry | InCl3 (mol%) | Cyclopentadiene (equiv.) | Yield (%) |
|---|---|---|---|
| 1 | 10 | 3 | 41 |
| 2 | 15 | 3 | 50 |
| 3 | 20 | 3 | 60 |
| 4 | 25 | 3 | 60 |
| 5 | 20 | 1 | 43 |
| 6 | 20 | 2 | 45 |
We began our optimization by executing the TQS synthesis (Scheme 1) under microwave irradiation at 100°C and utilizing 20 mol% of InCl3 and 3 equivalents of cyclopentadiene in acetonitrile as a solvent. The reaction was complete within 15 minutes, giving TQS in 60% yield (Table 1). The reaction was very clean and no chromatographic purification was required as the product was obtained in greater than 99% purity (by HPLC and 1H-NMR). TQS has three chiral centers with the cyclopentene and 1- naphthyl rings oriented cis-to each other. We observed that these reaction conditions favored the formation of the cis-diastereomer. The relative orientation of cyclopentene ring and 1-naphthyl ring was cis as determined from the coupling constants and NOE measurements (Figure 2). In the 1H NMR spectra of compound 3, the two doublets at δ 5.45 and 4.25 were attributed to protons H-4 and H-9b respectively. The coupling constants between protons H-3a and H-4 (J3a,4 = 3.5Hz) in 1H NMR confirmed the cis- relationship of these protons.
Scheme 1.
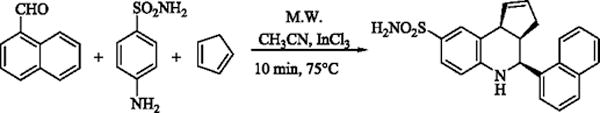
Microwave acclerated synthesis of TQS.
Figure 2.
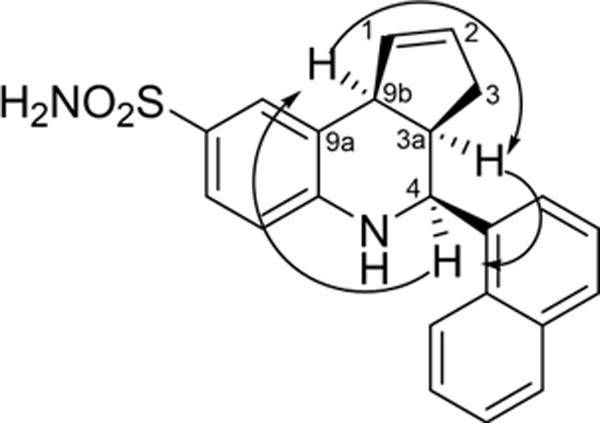
Characteristic NOEs of TQS (3)
Although the above experiment gave the desired product in good yield, in order to find optimal reaction conditions, we executed this reaction under conditions vary ing in temperature and time (Table 1). We got similar yields (i.e. 60%) for a shorter period of time (10 min) and at a lower temperature (75 °C). Temperatures and reaction times higher or lower than this did not improve reaction yields. In subsequent experiments, the amounts of InCl3 and cyclopentadiene were varied only to confirm that any reduction in these quantities led to reduced yields (entries 1, 2, 5 and 6; Table 2). Also increasing the quantities of InCl3 and cyclopentadiene did not improve reaction yields. Thus, in our hands, 3 equivalents of cyclopentadiene with 20 mol% of InCl3 at 75°C for 10 minutes in a Biotage Microwave Synthesizer was the most efficient procedure for the synthesis of TQS.30 We then extended the scope of this reaction to various substituted aldehydes and anilines and compared their yields with those reported under standard conditions (Table 3). As seen, microwave-assisted reaction conditions provided significantly improved reaction yields for almost all analogs synthesized compared to those obtained under standard conditions. In all cases, except for compound 8, we observed formation of only the cis-diastereomer under our reaction conditions. Compound 8 was obtained as a 72:28 mixture of cis: trans diastereomers.
Table 3.
Comparison of microwave-assisted synthesis of tetrahydroquinoline under standard reaction conditions.
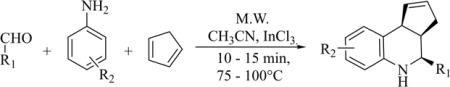
| ||||||
|---|---|---|---|---|---|---|
| Compound | R1 | R2 | Microwave Heating | Conventional heating | ||
| Time | Yield (%) | Time | Yield (%) | |||
| 3 |
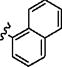
|
p-SO2NH2 | 10 min | 60 | 48 h | 22 (ref:7) |
| 4 |
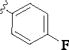
|
p-SO2NH2 | 15 min | 66 | 24 h | 2 (ref:7) |
| 5 |
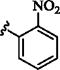
|
p-SO2NH2 | 15 min | 90 | 24 h | 20 (ref:7) |
| 6 |
|
p-SO2NH2 | 10 min | 61 | 48 h | 19 (ref:7) |
| 7 |
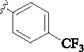
|
p-SO2NH2 | 15 min | 67 | 24 h | 30 (ref:6) |
| 8 |
|
p-SO2NH2 | 15 min | 66 | 12 h | 13 (ref:7) |
| 9 |
|
p-SO2NH2 | 15 min | 67 | – | – |
| 10 |
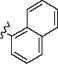
|
o-SO2NH2 | 15 min | 55 | – | – |
| 11 |
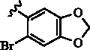
|
p-COCH3 | 15 min | 74 | 2 h | 98 (ref: 3) |
| 12 |
|
p-SO2NH2 | 15 min | 40 | – | – |
| 13 |
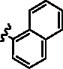
|
p-OMe | 10 min | 58 | – | – |
| 14 |
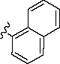
|
p-Br | 10 min | 56 | – | – |
| 15 | –H | p-SO2NH2 | 15 min | 48 | – | – |
| 16 |
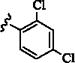
|
p-COOH | 15 min | 65 | – | – |
In summary, we have developed a microwave-assisted, rapid and high yielding one-pot Povarov reaction for the synthesis of cyclopentene ring-fused tetrahydroquinoline derivatives. In general, the reactions for various substrates were rapid, clean and in most cases no chromatographic purification was necessary, produced superior yields with high diastereoselectivities and could be scaled up to gram quantities.
Acknowledgments
This work supported by grant from National Institute on Drug Abuse (DA027113 to GAT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sridharan V, Suryavanshi PA, Menendez JC. Chem Rev. 2011;111:7157–7259. doi: 10.1021/cr100307m. [DOI] [PubMed] [Google Scholar]
- 2.Gore VK, Ma VV, Yin R, Ligutti J, Immke D, Doherty EM, Norman MH. Bioorg Med Chem Lett. 2010;20:3573–3578. doi: 10.1016/j.bmcl.2010.04.125. [DOI] [PubMed] [Google Scholar]
- 3.Burai R, Ramesh C, Shorty M, Curpan R, Bologa C, Sklar LA, Oprea T, Prossnitz ER, Arterburn JB. Org Biomol Chem. 2010;8:2252–2259. doi: 10.1039/c001307b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramesh C, Nayak TK, Burai R, Dennis MK, Hathaway HJ, Sklar LA, Prossnitz ER, Arterburn JB. J Med Chem. 2010;53:1004–1014. doi: 10.1021/jm9011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gronlien JH, Hakerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. Mol Pharmacol. 2007;72:715–724. doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
- 6.Gill JK, Dhankher P, Sheppard TD, Sher E, Millar NS. Mol Pharmacol. 2012;81:710–718. doi: 10.1124/mol.111.076026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker C, Comstock JW, Michne WF, Murphy M, Phillips E, Rosamond JD, Simpson TR. US Patent 2007/0179172 A1
- 8.Kumar A, Srivastava S, Gupta G, Chaturvedi V, Sinha S, Srivastava R. ACS Comb Sci. 2011;13:65–71. doi: 10.1021/co100022h. [DOI] [PubMed] [Google Scholar]
- 9.Faghih R, Gopalakrishnan M, Briggs CA. J Med Chem. 2008;51:701–712. doi: 10.1021/jm070256g. [DOI] [PubMed] [Google Scholar]
- 10.Gill JK, Savolainen M, Young GT, Zwart R, Sher E, Millar NS. Proc Natl Acad Sci U S A. 2011;108:5867–5872. doi: 10.1073/pnas.1017975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouznetsov VV. Tetrahedron. 2009;65:2721–2750. [Google Scholar]
- 12.Smith CD, Gavrilyuk JI, Lough AJ, Batey R. J Org Chem. 2010;75:702–715. doi: 10.1021/jo9021106. [DOI] [PubMed] [Google Scholar]
- 13.Michael JP. Natural Product Reports. 2008;25:166–187. doi: 10.1039/b612168n. [DOI] [PubMed] [Google Scholar]
- 14.Glushkov VA, Tolstikov AG. Russ Chem Rev. 2008;77:137–159. [Google Scholar]
- 15.Krueger AC, Malachowski WP. Encyclopedia of Reagents for Organic Synthesis. Wiley Online Library; 2008. [Google Scholar]
- 16.Povarov LS. Russ Chem Rev. 1967;36:656–670. [Google Scholar]
- 17.Grieco PA, Bahsas A. Tetrahedron Lett. 1988;29:5855–5858. [Google Scholar]
- 18.Kiselyov AS, Smith L, Armstrong RW. Tetrahedron. 1998;54:5089–5096. [Google Scholar]
- 19.Babu G, Perumal PT. Tetrahedron. 1998;54:1627–1638. [Google Scholar]
- 20.Nagarajan R, Chitra S, Perumal PT. Tetrahedron. 2001;57:3419–3423. [Google Scholar]
- 21.Nagarajan R, Perumal PT. Synth Commun. 2001;31:1733–1736. [Google Scholar]
- 22.Nagarajan R, Magesh CJ, Perumal PT. Synthesis. 2004:69–74. [Google Scholar]
- 23.Kumar RS, Nagarajan R, Perumal PT. Synthesis. 2004:949–959. [Google Scholar]
- 24.Kappe CO, Dallinger D. Nat Rev Drug Disc. 2006;5:51–63. doi: 10.1038/nrd1926. [DOI] [PubMed] [Google Scholar]
- 25.Astudillo LS, Gutierrez M, Gaete H, Kouznetsov VV, Melendez CM, Palenzuela JA, Vallejos G. Lett Org Chem. 2009;6:208–212. [Google Scholar]
- 26.Xing XL, Wu JL, Dai WM. Tetrahedron. 2006;62:11200–11206. [Google Scholar]
- 27.Duvelleroy D, Perrio U, Parisel O, Lasne MC. Org & Biomol Chem. 2005;3:3794–3804. doi: 10.1039/b509400c. [DOI] [PubMed] [Google Scholar]
- 28.Singh MS, Raghuvanshi K. Tetrahedron. 2012;68:8683–8697. [Google Scholar]
- 29.Auge J, Lubin-Germain N, Uziel J. Synthesis. 2007:1739–1764. [Google Scholar]
- 30.Experimental procedure for the synthesis of TQS (3) : In a microwave vial, cyclopentadiene (254 mg, 3.84 mmol, 3 equiv.) was added to a suspension of 1-naphthaldehyde (200 mg, 1.28 mmol), 4-aminosulfonamide (220 mg, 1.28 mmol) and indium (III) chloride (55.6 mg, 0.256 mmol, 0.2 equiv.) in acetonitrile (5 mL). The reaction vial was placed in a Biotage Initiator microwave synthesizer and heated to 100°C for 15 min. The contents were added to 10% aqueous Na2CO3 solution (0.1 M; 10 mL) and extracted with chloroform (3×20 mL). The combined organic layer was washed with brine (15 mL), dried (Na2SO4) and concentrated under reduced pressure. The residue was treated with hexane/dichloromethane to precipitate out compound 3 as an off white solid (290 mg; yield 60%).7 Compounds 7, 8, 10, 11, 13, and 14 were purified by column chromatography (EtOAc/Hexane = 10/90 → 50/50) to yield the desired product. The rest were crystallized from dichloromethane/hexane. All compounds were >95% pure by HPLC and 1H NMR.Compound 9: 1H NMR (500 MHz, DMSO-d6) δ 7.34 (d, J = 2.0 Hz, 1H), 7.26 (dd, J = 8.5 Hz, 2.0 Hz, 1H), 6.89 (s, 2H), 6.80 (d, J = 9.0 Hz, 1H), 5.87 – 5.81 (m, 1H), 5.70 – 5.65 (m, 1H), 5.62 (s, 1H), 3.89 (d, J = 9.0 Hz, 1H), 3.02 (dd, J = 8.5 Hz, 2.0 Hz, 1H), 2.33 – 2.23 (m, 1H), 2.22 – 2,14 (m,2H), 1.88 – 1.60 (m, 4H), 1.34 – 1.10 (m, 4H), 1.16 – 0.88 (m, 2H); m/z = 333.79 [M+H]+. Compound 10: 1H NMR (500 MHz, CDCl3) δ 8.07 (d, J = 8.0 Hz, 1H), 7.90 (dd, J = 7.5 Hz, 1.5 Hz, 1H), 7.80 (d, J = 7.5 Hz, 1H), 7.74 (d, J = 7.5 Hz, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.57 – 7.44 (m, 3H), 7.27 (d, J = 7.5 Hz, 1H), 6.78 (t, J = 7.5 Hz, 1H), 5.99 (s, 1H), 5.84 – 5.79 (m, 1H), 5.65 – 5.59 (m, 1H), 5.52 (d, J = 3.0 Hz, 1H), 4.90 (s, 2H), 4.28 (d, J = 9.0 Hz, 1H), 3.33 (dq, J = 9.0 Hz, 2.0 Hz, 1H), 2.56 (ddd, J = 14.5 Hz, 9.0 Hz, 2.0 Hz, 1H), 1.66 (ddd, J = 15.5 Hz, 9.0 Hz, 1.5 Hz, 1H); m/z = 377.84 [M+H]+. Compound 12: 1H NMR (500 MHz, DMSO-d6) δ 7.69 (brs, 1H), 7.66 (dd as t, J = 1.5 Hz, 1H), 7.42 (d, J = 1.5 Hz, 1H), 7.32 (dd, J = 8.5 Hz, 2.0 Hz, 1H), 6.95 (s, 2H), 6.77 (d, J = 8.5 Hz, 1H), 6.59 (d, J = 1.5 Hz, 1H), 6.20 (s, 1H), 5.88 – 5.82 (m,1H), 5.67 – 5.63 (m, 1H), 4.51 (d, J = 2.0 Hz, 1H), 4.04 (d, J = 9.0 Hz, 1H), 2.99 (dq, J = 8.5 Hz, 3.5 Hz, 1H), 2.38 (ddd, J = 16.5 Hz, 9.5 Hz, 2.5 Hz, 1H), 1.98 (dd, J = 16 Hz, 9 Hz, 1H); m/z = 317.64 [M+H]+. Compound 13: 1H NMR (500 MHz, CDCl3) δ 8.12 (d, J = 8.5 Hz, 1H), 7.90 (dd, J = 7.0 Hz, 2.0 Hz, 1H), 7.82 (d, J = 7.0 Hz, 1H), 7.80 (d, J = 9.0 Hz, 1H), 7.57 – 7.46 (m, 3H), 6.72 (s, 1H), 6.64 (d, J = 1.5 Hz, 2H), 5.86 – 5.79 (m, 1H), 5.68 – 5.60 (m, 1H), 5.35 (d, J = 3.0 Hz, 1H), 4.25 – 4.18 (m, 1H), 3.78 (s, 3H), 3.54 (s, 1H), 3.30 (dq, J = 9.0 Hz, 2.5 Hz, 1H), 2.66 (qdd, J = 17.0 Hz, 9.0 Hz, 2.5 Hz, 1H), 1.64 (tdd, J = 16.5 Hz, 9.0 Hz, 2.0 Hz, 1H); m/z = 328.36 [M+H]+. Compound 14: 1H NMR (500 MHz, CDCl3) δ 8.10 (d, J = 8.0 Hz, 1H), 7.90 (dd, J = 8.0 Hz, 2.0 Hz, 1H) 7.81 (d, J = 8.0 Hz, 1H), 7.77 (d, J = 6.5 Hz, 1H), 7.58–7.48 (m, 3H), 7.23 (dd, J = 2.5 Hz, 1.0 Hz, 1H), 7.10 (dd, J = 8.5 Hz, 2.5 Hz, 1H), 5.82 – 5.79 (m, 1H), 5.68 – 5.62 (m, 1H), 5.40 (d, J = 3.0 Hz, 1H), 4.20 (d, J = 9.0 Hz, 1H), 3.75 (s, 1H), 3.30 (dq, J = 8.5 Hz, 3.0 Hz, 1H), 2.62 (qdd, J = 17.0 Hz, 9.5 Hz, 2.5 Hz, 1H), 1.64 (qdd, J = 16.0 Hz, 8.5 Hz, 1.5 Hz, 1H); m/z = 377.16 [M+H]+. Compound 15: 1H NMR (500 MHz, DMSO-d6) δ 7.51 (d, J = 1.5 Hz, 1H), 7.32 (dd, J = 8.5 Hz, 2.0 Hz, 1H), 6.91 (s, 2H), 6.61 (d, J = 8.5 Hz, 1H), 6.31 (d, J = 1.5 Hz, 1H), 5.82 – 5.76 (m, 1H), 5.74 – 5.68 (m, 1H), 3.82 (s, 1H), 3.12 – 3.02 (m, 1H), 2.70 – 2.54 (m, 3H), 2.09 (dd, J = 9.0 Hz, 2.0 Hz, 1H); m/z = 250.99 [M+H]+. Compound 16: 1H NMR (500 MHz, DMSO-d6) δ 12.21 (s, 1H), 7.66 (s, 1H), 7.65 (d, J = 10.0 Hz, 1H), 7.52 (dt, J = 8.0 Hz, 2.0 Hz, 2H), 6.75 (d, J = 8.5 Hz, 2.0 Hz, 1H), 6.39 (s, 1H), 5.98 – 5.90 (m, 1H), 5.64 – 5.56 (m, 1H), 4.89 (d, J = 2.5 Hz, 1H), 4.08 (d, J = 8.5 Hz, 1H), 3.06 (ddq, J = 8.5 Hz, 3.5 Hz, 1.5 Hz, 1H), 2.36 (ddd, J = 15.5 Hz, 10.0 Hz, 2.0 Hz, 1H), 1.61 (dd, J = 15.0 Hz, 9.0 Hz, 1H); m/z = 361.23 [M+H]+.


