Abstract
Importance
Lubricin may be an important barrier to the development of corneal and conjunctival epitheliopathies that may occur in dry eye disease and contact lens wear.
Objective
To test the hypotheses that lubricin (ie, proteoglycan 4 [PRG4]), a boundary lubricant, is produced by ocular surface epithelia and acts to protect the cornea and conjunctiva against significant shear forces generated during an eyelid blink and that lubricin deficiency increases shear stress on the ocular surface and promotes corneal damage.
Design, Setting, and Participants
Human, porcine, and mouse tissues and cells were processed for molecular biological, immunohistochemical, and tribological studies, and wild-type and PRG4 knockout mice were evaluated for corneal damage.
Results
Our findings demonstrate that lubricin is transcribed and translated by corneal and conjunctival epithelial cells. Lubricin messenger RNA is also present in lacrimal and meibomian glands, as well as in a number of other tissues. Absence of lubricin in PRG4 knockout mice is associated with a significant increase in corneal fluorescein staining. Our studies also show that lubricin functions as an effective friction-lowering boundary lubricant at the human cornea-eyelid interface. This effect is specific and cannot be duplicated by the use of hyaluronate or bovine serum albumin solutions.
Conclusions and Relevance
Our results show that lubricin is transcribed, translated, and expressed by ocular surface epithelia. Moreover, our findings demonstrate that lubricin presence significantly reduces friction between the cornea and conjunctiva and that lubricin deficiency may play a role in promoting corneal damage.
Lubricin plays a critical role as a boundary lubricant in articulating joints.1–4 This amphiphilic glycoprotein, which is also called superficial zone protein, is a product of the proteoglycan 4 (PRG4) gene, contains a 1404–amino acid core, is extensively O-glycosylated with β(1–3)Gal-GalNac oligosaccharides, and is partially capped with NeuAc.3 Lubricin is synthesized and secreted by chondrocytes and synoviocytes, adheres to cartilaginous surfaces, and protects articular cartilage against frictional forces, cell adhesion, and protein deposition.1–6 Lubricin deficiency in the joint leads to loss of joint lubrication, increased shear stress, cartilage degradation, and significant pain.2–4,6–8 Exogenous application of natural or recombinant lubricin to the joint, in turn, may retard these sequelae.2,8–12
We hypothesize that lubricin serves an analogous role on the ocular surface and protects the cornea and conjunctiva against significant shear forces generated during the frequent, physiological, and spontaneous eyelid blinks. More specifically, we hypothesize that lubricin is transcribed and translated in ocular surface and adnexal epithelial cells, secreted onto, and adsorbed to, the cornea and conjunctiva, and thereupon acts to reduce friction and prevent shear stress between these tissues. Conversely, we also hypothesize that lubricin deficiency will increase shear stress on the ocular surface and promote corneal damage.
If our hypotheses are correct, then lubricin may be an important barrier to the development of corneal and conjunctival epitheliopathies. Damage to the ocular surface is a prevalent complication of Sjögren syndrome, graft vs host disease, Stevens-Johnson syndrome, refractive surgery, contact lens wear, and dry eye disease. Dry eye disease, in particular, affects tens of millions of Americans and as many as 20% to 50% of elderly populations in Asia.13–15 A central feature of these anterior segment disorders is believed to be an increase in shear stress that drives a destructive cycle of inflammation and wear, which may possibly be prevented by a therapeutic supplementation with lubricin.
The purpose of this study was to begin to test our hypotheses. For comparative purposes, we examined whether lubricin messenger RNA (mRNA) is transcribed in murine ocular, as well as in nonocular, tissues. Our results demonstrate that lubricin is produced by ocular surface epithelia, as well as other tissues, and acts as a boundary lubricant at the human cornea-eyelid biointerface. In contrast, lubricin deficiency promotes corneal damage.
METHODS
Human studies in Boston, Massachusetts, and Calgary, Alberta, Canada, were approved by the institutional review boards of the Massachusetts Eye and Ear Infirmary, the Schepens Eye Research Institute, and the University of Calgary Conjoint Health Research Ethics Board. All research involving humans followed the tenets of the Declaration of Helsinki. All studies with mice were approved by the Institutional Animal Care and Use Committee of the Schepens Eye Research Institute and adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
ANALYSIS OF LUBRICIN mRNA EXPRESSION IN HUMAN AND MOUSE TISSUES AND CELLS
To determine whether lubricin mRNA exists in ocular surface and adnexal epithelia, human tissues and cells were obtained and/or cultured and then processed for molecular biological procedures. For comparative purposes, we also examined whether lubricin mRNA is transcribed in murine ocular, as well as in nonocular, tissues.
Corneoscleral rims (n=10) and bulbar conjunctivae (n=6) were obtained from the National Disease Research Interchange and/or clinicians at the Massachusetts Eye and Ear Infirmary. All tissues were deidentified prior to our use, according to Health Insurance Portability and Accountability Act regulations. Primary human corneal cell cultures were generated and maintained by previously published methods.16 Human conjunctival impression cytology complementary DNA samples were a gift from Pablo Argueso, PhD, and Abha Gulati, MD. Immortalized human corneal17 and conjunctival18 epithelial cells were gifts from Ilene K. Gipson, PhD, and James Jester, PhD. Immortalized human meibomian gland epithelial cells were generated in our laboratory.19 Human articular cartilage and synovial membrane complementary DNA samples were a gift from Roman Krawetz, PhD. Human uterine, cervical, bladder, liver, and prostate RNA samples were obtained from Ambion. Cornea and conjunctival RNA preparations from young adult, male and/or female C57BL/6 and BALB/c mice were a gift from Zahra Sadrai, MD. Adult BALB/c and non-obese diabetic NOD/LtJ mice were purchased from Taconic Laboratories and Jackson Laboratory, and mice with a 129Sv/Ev genetic background were obtained from Yajun Cui, PhD. Mice were killed by carbon dioxide inhalation and exorbital, lacrimal, meibomian, submandibular, parotid and/or sublingual glands, and bladder and vaginal/cervical tissue and/or seminal vesicles were removed.
Reverse transcriptase–polymerase chain reaction (PCR) was used to analyze tissue and cell samples for lubricin gene expression. Total RNA was first extracted by using TRIzol reagent (Invitrogen); samples were then exposed to RNase-Free DNase (Invitrogen) and evaluated on an RNA 6000 Nano Lab-Chip with an Agilent 2100 Bioanalyzer (Agilent Technologies) to confirm RNA integrity. Complementary DNAs were transcribed by using oligo dT or random hexamer primers (Promega), deoxyribonucleotide triphosphates (Invitrogen), and SuperScript III Reverse Transcriptase (Invitrogen) according to Invitrogen’s protocol. Species-specific oligonucleotide primers (Sigma-Aldrich) were generated to amplify 526– and 367–base pair fragments of the human and mouse lubricin coding regions, respectively (Table).20 The lubricin primers spanned more than 1 kilobase pair of intron sequences to distinguish between the amplification of target complementary DNA and contaminating chromosomal DNA. The identity of lubricin PCR products was verified by DNA sequencing. Amplicon samples, as well as sense and antisense primers, were sequenced at the DNA Sequencing Center for Vision Research at the Massachusetts Eye and Ear Infirmary. Tracing data were collected with 3100 Data Collection Software version 1.1 (Applied Biosystems) and resulting chromatograms were downloaded to FinchTV (www.geospiza.com) or CLC Sequence Viewer 6.0.2 (CLC bio) software. Text files of these data were used to conduct Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi) searches of GenBank databases.
Table.
Oligonucleotide Primers Designed for Reverse Transcriptase–Polymerase Chain Reaction Analysis of Lubricin (PRG4) Messenger RNA
| Species | Orientation | Nucleotide Sequence (5′ → 3′) | Exons | Amplicon Size, base pairs |
|---|---|---|---|---|
| Human | Sense | GATGCAGGGTACCCCAAA | 9–12 | 526 |
| Antisense | CAGACTTTGGATAAGGTCTGCC | |||
| Mouse | Sense | TGTCAAAGGCTTTGGAGGAC | 9–11 | 367 |
| Antisense | TCGCTGAAGTAACAACATTTG |
EVALUATION OF LUBRICIN PROTEIN EXPRESSION IN HUMAN AND PORCINE OCULAR SURFACE TISSUES
For histological evaluation, human tissues, including the cornea, conjunctiva, and eyelid, were obtained from a human female cadaver (81 years of age) that was cold-stored prior to para-formaldehyde fixation.21 The body donor had given informed consent prior to death, and her ocular surface was macroscopically normal. Articular cartilage from a 74-year-old human female knee was generously provided by Prof M. Dietel and Dr M. von Laffert (Department of Pathology, Charite-Universitatsmedizin Berlin).
Paraffin-embedded tissues were sectioned serially (10 μm), placed on glass slides, and rehydrated in a graded alcohol series. To facilitate antigen retrieval, sections were incubated in boiling buffer (either citrate buffer pH 6.0 or TRIS-EDTA buffer pH 9.0) for 20 minutes, allowed to cool to room temperature, and then transferred to phosphate buffer. Sections were overlaid with an aliquot of affinity-purified rabbit polyclonal antibody to human lubricin (Thermo Fisher Scientific) (4 μg/mL in a commercial diluent [Medac Vir-Dil])22 or appropriate control solutions. Control preparations included (1) diluent buffer; (2) an irrelevant rabbit monoclonal antibody to CD8 (clone SP16, 100-fold dilution; Thermo Fisher Scientific); (3) normal rabbit IgG (100-fold dilution; Santa Cruz Biotechnology Inc); and (4) rabbit antihuman lubricin antibody, after antibody preincubation for 2 hours at room temperature with either sense (LPNIRKPDGYDYYAFSKDQ) or random (MFQSYNTDVILPALRGEAK) peptides (120 μg/mL; Bio Basic Canada). The sense competitor corresponds to residues 1356 to 1374 of human lubricin. The random peptide has no reactivity with the rabbit antilubricin antibody. The optimal competitor concentration was predetermined experimentally by dot blot analyses.23 Following incubation first with antibody or control solutions overnight at approximately 4°C, sections were incubated with a polymer-based secondary antibody system containing purified F(ab)2 fragment of goat antirabbit IgG (Nichirei Histofine Simple Stain Max PO; Medac) for 30 minutes at room temperature, followed by diaminobenzidine (Hoechst) and hydrogen peroxide. Sections were rinsed, dehydrated, and placed under a coverslip with Entellan mounting medium (Merck), examined with a light microscope (DMRB; Leica), and photographed with a digital camera (SPOT Imaging Solutions, a division of Diagnostic Instruments Inc) with SPOT software version 4.5.
To examine whether porcine corneal epithelial cells contain lubricin protein, fresh porcine corneas were obtained from an abattoir. Corneal surfaces from 20 pigs were scraped repeatedly with a scalpel. Scrapings were then homogenized in ice-cold RIPA buffer (Sigma-Aldrich), pipetted repeatedly through a syringe needle, and centrifuged for 30 minutes at 14 000 × g at 4°C. The supernatant proteins were separated by anion exchange chromatography, as previously described.23 The 0.15M sodium chloride eluent was subject to 3% to 8% TRIS-acetate sodium dodecyl sulfate–polyacrylamide gel electrophoresis using the NuPAGE system (Invitrogen) and evaluated by Western blotting.22,24 Protein bands were identified with SimplyBlue SafeStain (Life Technologies) and excised and processed for tandem mass spectrometry analysis (Southern Alberta Mass Spectrometry Centre for Proteomics, University of Calgary).22 To serve as a positive control for lubricin, bovine synovial fluid was obtained from Animal Technologies Inc.
To determine whether human corneas express lubricin protein by Western blot, corneas were obtained from the Lions Eye Bank through the Southern Alberta Organ and Tissue Donation Program. Corneas from 92- and 100-year-old donors were stored in Optisol-GS (Bausch & Lomb) at 4°C for 22 days. The corneal epithelia were then removed, suspended in sodium dodecyl sulfate buffer, centrifuged at 3000 × g for 3 minutes, and then processed for electrophoresis and blotting. Similarly, to assess whether mouse corneas contain lubricin protein, 26 corneas from 13 adult 129Sv/Ev mice were homogenized, pipetted repeatedly, centrifuged, concentrated, and processed for a Western blot.
INFLUENCE OF LUBRICIN ABSENCE ON MURINE OCULAR SURFACE TISSUES
For histological evaluation of mouse ocular tissues, central vertical plane sections (3 μm) of methacrylate-embedded samples were stained with hematoxylin-eosin or periodic acid–Schiff. Photographs were taken at different magnifications with a digital camera (Eclipse 80i; Nikon Corp) and SPOT imaging software.
To determine whether lubricin absence promotes ocular surface damage, lubricin knockout (KO) and wild-type (WT) control mice were evaluated for fluorescein staining patterns in a randomized and masked fashion. Adult PRG4 heterozygous mice were generously provided by Yajun Cui. These mice, which have a 129Sv/Ev genetic background, were briefly quarantined at Charles River Laboratories and then mated in the Animal Facilities of the Schepens Eye Research Institute. Lubricin WT, heterozygous, and KO mice were identified with a PCR-based genotyping protocol.6 All genotype identifications were confirmed, before conducting experimental eye evaluations, by collecting and analyzing murine ear punch samples. Animals were maintained in constant-temperature rooms with fixed light/ dark intervals of 12-hour durations.
Fluorescein staining of mouse corneas was performed by micropipetting 0.5 μL of fluorescein, 5%, into the inferior conjunctival sac of both eyes. Approximately 3 minutes after the fluorescein instillation, the corneas were examined with a slit-lamp biomicroscope using cobalt blue light 3. Punctate staining was recorded in a masked fashion by using a standardized grading system of 0 to 3 for each of 5 areas of the corneal surface.25 Corneas were photographed with a Topcon SL-D7 high-resolution slitlamp imaging system with a Sony SR52 camera.
ANALYSIS OF BOUNDARY LUBRICATION ON THE HUMAN OCULAR SURFACE
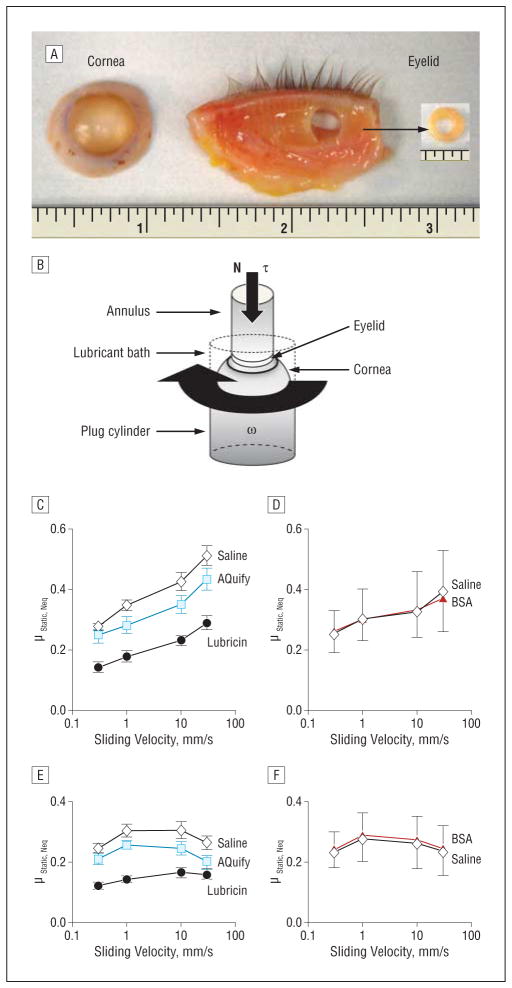
For studies of lubricin function at a human cornea-eyelid interface, human corneas were obtained from the Lions Eye Bank through the Southern Alberta Organ and Tissue Donation Program. Corneas were stored in Optisol-GS at 4°C and used within 2 weeks of collection. Human eyelids were procured from the University of Calgary Body Donation Program within 3 days after death and either used immediately or stored at −20°C in saline for at most 2 weeks. Lubricin was purified from culture media that had been conditioned by articular cartilage disks from mature bovine stifle joints, according to reported methods.1,26
Briefly, human corneas and eyelids were mounted on a BOSE ELF 3200 biomechanical testing machine, forming a cornea-eyelid interface. Tissue surfaces were articulated against each other at effective sliding velocities ranging from 0.3 to 30 mm/s under physiological loads of approximately 15 to 20 kPa following preconditioning.26
Ocular tissues (n = 6) were tested serially in lubricant baths of Sensitive Eyes Plus saline solution (Bausch & Lomb), AQuify Long-Lasting Comfort Drops (which contain hyaluronic acid, 0.1%; CIBA Vision), and bovine lubricin (at 300 μg/mL). Additional tissues (n = 3) were tested in saline followed by bovine serum albumin (300 μg/mL; Sigma-Aldrich) dissolved in saline. Both static and kinetic friction coefficients were calculated.
STATISTICAL ANALYSES
Statistical analysis of the corneal fluorescein staining data between 2 groups was performed by using a t test. The effects of test lubricant and effective sliding velocity (veff) (as repeated factors) on the friction coefficients were assessed by analysis of variance. Tukey post hoc testing was then used to determine statistically significant differences (P < .05) at individual veff.
RESULTS
ANALYSIS OF LUBRICIN mRNA EXPRESSION IN HUMAN AND MOUSE TISSUES AND CELLS
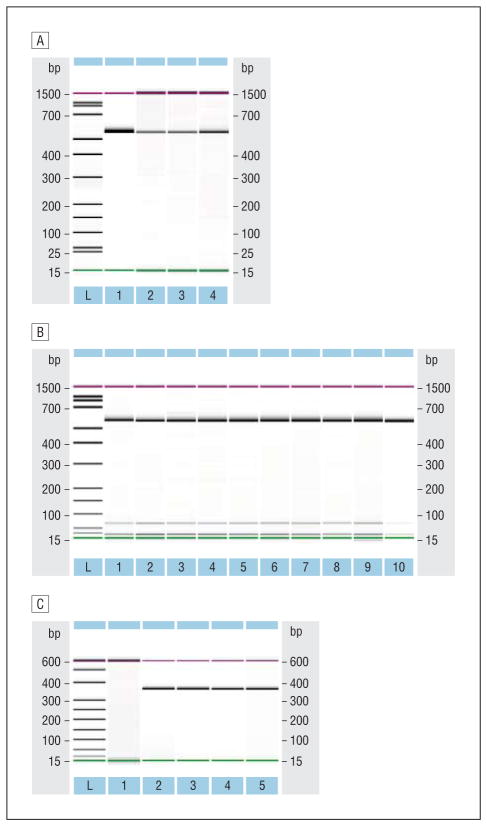
Our reverse transcriptase–PCR results demonstrate that lubricin mRNA is present in human corneal and conjunctival epithelial cells, corneoscleral rims, conjunctival tissues, and conjunctival impression cytology samples (Figure 1A and B). The identity of lubricin PCR products in the cornea and conjunctiva was confirmed by DNA sequence analysis, with all forward and reverse sequences returning greater than 96% alignment to human PRG4 (RefSeq NM_005807.2) on Basic Local Alignment Search Tool searches of GenBank databases (eTable 1, http://www.jamaophth.com). We also discovered lubricin mRNA in human meibomian gland epithelial cells, human uterine, cervical, bladder, and prostatic samples, and human articular cartilage and synovial membranes (eFigure 1), as well as in mouse lacrimal and meibomian glands (Figure 1C), mouse cornea and conjunctiva (eFigure 1), and mouse submandibular, parotid, sublingual, bladder, vaginal/cervical, and seminal vesicle tissues. The identity of most of these lubricin PCR products was evaluated and verified by DNA sequence analysis, again showing greater than 93.8% homology (eTable 2). Alignments for each human sample were identical to lubricin (PRG4) transcript variants A (RefSeq NM_005807.3), B (RefSeq NM_001127708.1), C (RefSeq NM_001127709.1), and D (RefSeqNM_001127710.1), while alignments for each mouse sample were identical to lubricin (PRG4) transcripts 1 (RefSeqNM_021400.3) and 2 (Ref-Seq NM_001110146.1).
Figure 1.
Identification of lubricin messenger RNA (mRNA) in human and mouse ocular tissues and cells by reverse transcriptase–polymerase chain reaction. A, RNA samples in lanes 1 through 4 are a human liver standard, a corneoscleral rim of a 24-year-old woman, a corneoscleral rim of a 51-year-old woman, and immortalized human conjunctival epithelial cells. The anticipated amplicon size of the human lubricin transcript was 526 base pairs (bp), and all bands fell within the 10% error of the Bioanalyzer (2100; Agilent Technologies) when using a 1000-bp sizing kit (internal controls = 15 and 1500 bp). Samples were sequenced and shown to be lubricin. Analogous bands were also found in RNAs prepared from the corneoscleral rim of a 64-year old man, pools of 3 male and 3 female conjunctival tissues, primary cultures of human male and female corneal epithelial cells, and immortalized human corneal and meibomian gland epithelial cells (data not shown). L indicates ladder. B, Presence of lubricin mRNA in impression cytology samples from the human ocular surface. Samples in lanes 1 through 9 are from a 29-year-old man, 31-year-old man, 59-year-old woman, 36-year-old woman, 24-year-old woman, 29-year-old woman, 37-year-old woman, 27-year-old man, and 35-year-old woman, respectively. The RNA in lane 10 was from the conjunctival epithelial cells shown in part A and was used here as a positive control. C, Identification of lubricin mRNA in mouse lacrimal and meibomian glands. Samples in lanes 1 through 5 are a no template control, female NOD/LtJ mouse lacrimal glands, male NOD/LtJ mouse lacrimal glands, female BALB/c mouse meibomian glands, and male BALB/c mouse meibomian glands. The bands in these lanes are equivalent to the anticipated 367-bp size. The NOD/LtJ mouse lacrimal glands were obtained from 5 adult mice of each sex (ie, 10 glands per sample), and the BALB/c mouse meibomian glands were isolated from the eyelids of 7 adult mice of each sex (ie, 28 eyelids/sample).
EVALUATION OF LUBRICIN PROTEIN EXPRESSION IN HUMAN OCULAR SURFACE TISSUES
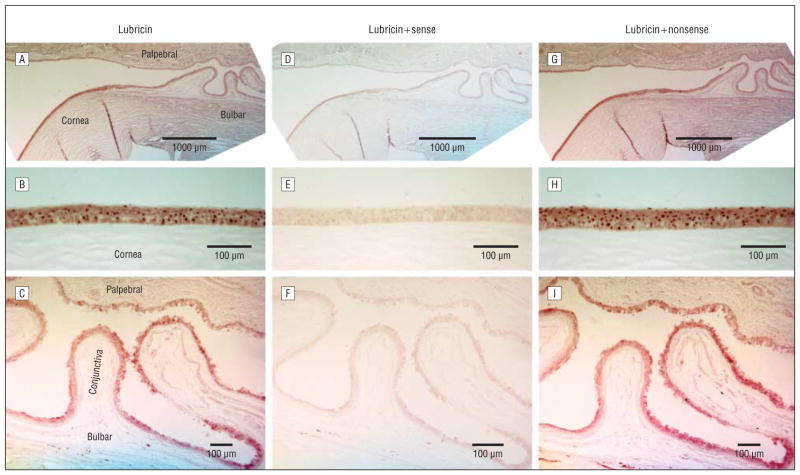
Our results demonstrate that lubricin protein is produced and expressed by human corneal and conjunctival epithelial cells (Figure 2A–C). Staining was strongest in the corneal epithelium (Figure 2B) and slightly less intense along the complete bulbar and palpebral conjunctiva (Figure 2C). The epithelium of the cornea and conjunctiva stained throughout all epithelial layers (insets in Figure 2B and C). In the conjunctiva, the goblet cells showed less intense or absent staining and hence appeared as bright spots (arrowhead in Figure 2C, inset). The lubricin staining intensity at the ocular surface was similar to that observed in the positive control cartilage (Figure 2M–O). Staining at the bone-cartilage junction occurs in particular in the tidemark region between the mineralized and hyaline cartilage.
Figure 2.
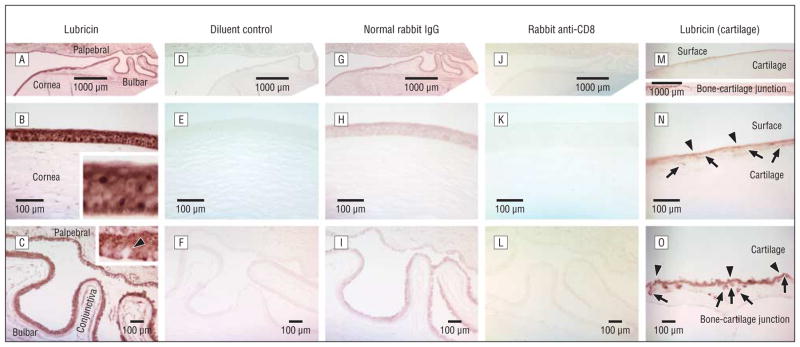
Lubricin protein expression in human ocular surface epithelia and cartilage. Immunohistochemical evaluation for lubricin (A–C) shows a strong staining of the ocular surface epithelia of the cornea and the palpebral (eyelid) and bulbar (eyeball) conjunctivae. In the conjunctiva, the goblet cells showed less intense or absent staining and hence appeared as bright spots (arrowhead in C, inset). Negative controls with omission of antibody (diluent control) (D–F) are completely negative and normal rabbit IgG is almost completely negative (G–I). An irrelevant rabbit antibody (anti-CD8) (J–L) shows no specific lubricin staining. A, D, G, and J, Overview of the ocular surface with the cornea and palpebral and bulbar conjunctiva (original magnification ×2.5). B, E, H, and K, Cornea (original magnification ×20). C, F, I, and L, Conjunctiva (original magnification ×10). Strong staining similar to that at the ocular surface is seen in the positive control tissue (cartilage) with a known presence of lubricin protein (M–O) (same enlargements). Lubricin staining is seen at the surface of the cartilage and at the bone-cartilage junction. Chondrocytes are marked by arrows and the lubricin depositions in the cartilage matrix are marked by arrowheads (N and O). The size markers indicate either 1000 μm (A, D, G, J, and M) or 100 μm.
Omission of the primary antibody (diluent buffer control) (Figure 2D–F) led to a complete lack of staining. Use of a normal rabbit IgG as the first antibody resulted in an almost complete absence of staining (Figure 2G–I). Similarly, replacement of the antilubricin antibody with an irrelevant rabbit antibody (to CD8) (Figure 2J–L) yielded no specific staining in ocular surface epithelial cells. However, as anticipated, this anti–T-cell antibody did react with lymphocytes in the conjunctival lamina propria and epithelium (not shown).
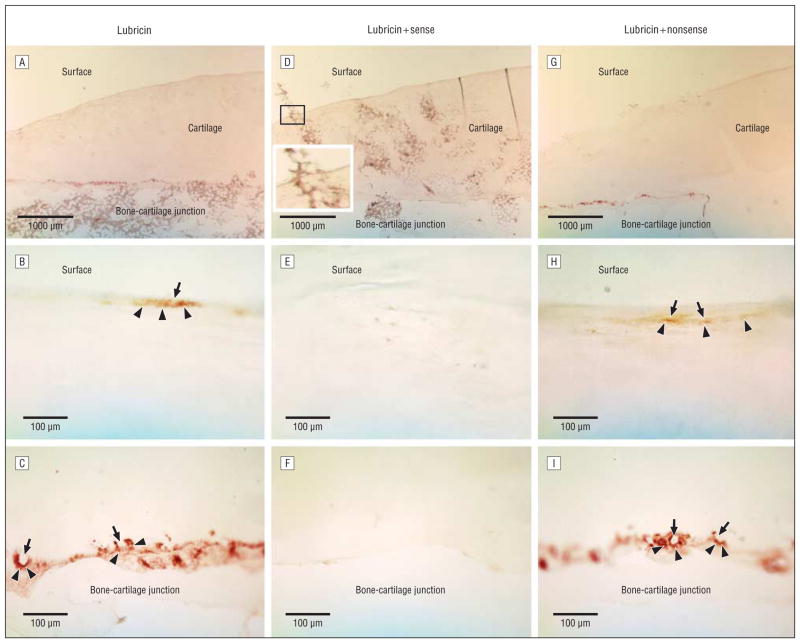
Further controls were performed by preabsorbing the antilubricin antibody with sense or nonsense peptides. As compared with the strong staining of antilubricin (Figure 3A–C), preincubation of the antibody with a sense peptide, which had served to generate the antibody, almost completely inhibited immunohistochemical staining (Figure 3D–F). In contrast, preexposure to a random, nonreactive peptide did not interfere with lubricin staining in the ocular surface tissue samples (Figure 3G–I) or the positive control cartilage sections (Figure 4).
Figure 3.
Specificity of lubricin staining in human ocular surface epithelia. The strong lubricin staining (A–C) in the cornea and conjunctiva was distinctly reduced after preincubation of the lubricin antibody with a sense peptide (D–F). In contrast, preadsorption to a random peptide (nonsense) did not affect the staining (G–I). The apparent staining of artificial folds in the cornea (A, D, and G) is a technical artifact, due to the entrapment of reagents. A, D, and G, Overview of the ocular surface with the cornea and palpebral and bulbar conjunctivae (original magnification ×2.5). B, E, and H, Cornea (original magnification ×20). C, F, and I, Conjunctiva (original magnification ×10). The size markers indicate either 1000 μm (A, D, and G) or 100 μm.
Figure 4.
Specificity of lubricin staining in human cartilage. A section of articular cartilage is seen in low magnification (A, D, and G) as well as in higher magnification at the surface (B, E, and H) and bone-cartilage junction (C, F, and I). Lubricin staining (A–C) was blocked by antibody preincubation with the sense (D–F), but not a random (G–I), peptide. Lubricin staining at the cartilage surface forms a thin surface layer (arrowheads), and narrow flattened chrondrocytes may be seen (arrows). At the bone-cartilage junction, chondrocyte lacunae (arrows) occur as bright spaces that are encircled by lubricin (arrowheads). The overview image for the sense peptide preincubation (D) has superimposed areas of bone marrow that spilled over the section during the preparation process. Magnification of the area indicated by a rectangle shows that this material is equally present over and outside the surface and the staining is not specific. Size markers indicate 1000 μm (A, D, and G) or 100 μm.
During preliminary experiments on human tissue sections processed without antigen retrieval or stored in Optisol, lubricin staining was either greatly reduced (no antigen retrieval) or undetectable (Optisol storage). Optisol is known to be associated with a marked loss of epithelial cells and extensive epithelial cell damage.27
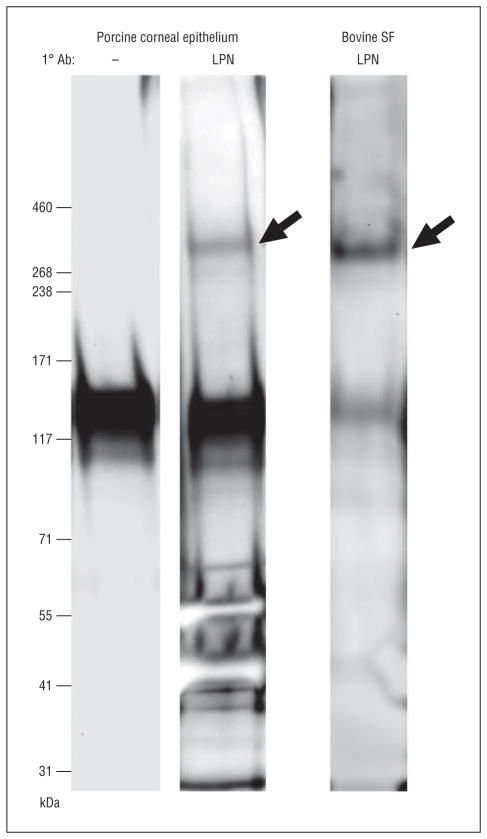
To examine whether lubricin protein is expressed in porcine corneal epithelia, samples were collected and processed for Western blot staining. A high-molecular-weight, approximately 345-kDa immunoreactive lubricin species was observed in the cornea extract (Figure 5). This approximately 345-kDa band was analogous to that found in bovine synovial fluid and was not present if the primary antilubricin antibody was replaced with diluent. Analysis of tandem mass spectrometry data confirmed that the approximately 345-kDa band was lubricin (data not shown). Additional studies identified lubricin protein in Western blots of human (eFigure 2) and mouse (eFigure 3) corneas.
Figure 5.
Identification of lubricin protein in porcine corneal epithelium and bovine synovial fluid (SF) by Western blot. Lanes contain molecular weight markers; blots shown with (LPN) and without (–) exposure to antilubricin antibody (Ab). Arrows indicate lubricin protein.
INFLUENCE OF LUBRICIN ABSENCE ON MURINE OCULAR SURFACE TISSUES
A significant increase in the corneal fluorescein staining of male and female lubricin KO mice (n = 20; mean [SE] age, 224 [8] days) as compared with their WT controls (n = 18; mean [SE] age, 224 [12] days) was observed (Figure 6A). This response was associated with 40% to 44% increases in the staining of the superior (mean [SE], WT = 1.0 [0.1] and KO = 1.4 [0.1]; P < .01) and medial (mean [SE], WT = 0.9 [0.1] and KO = 1.3 [0.1]; P < .05) regions of both corneas. Such staining differences were also observed if comparisons were limited to the worst zones of either eye (superior: mean [SE], WT = 1.3 [0.1] and KO = 1.7 [0.2]; P < .05; medial: mean [SE], WT = 1.2 [0.1] and KO = 1.6 [0.2]; P < .05). As shown in Figure 6B, fluorescein staining of this WT mouse cornea revealed relatively little damage, marked by superficial stippling and occasional micropunctate staining. By contrast, Figure 5C shows significant fluorescein staining in the superior, medial, and lateral regions of this KO mouse cornea. Numerous macropunctate coalescent areas and patches of contiguous staining were evident.
Figure 6.
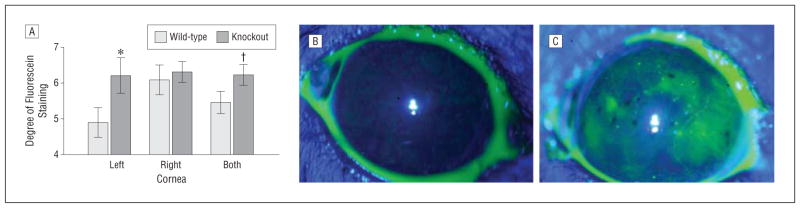
Effect of lubricin deficiency on the pattern of corneal fluorescein staining. A, Columns and vertical bars equal the mean (standard error) of the degree of staining on the left, right, or both corneas. Staining was significantly (*P < .05 and †P < .05, 1-tailed) greater in the knockout than the wild-type mice. B, Example of fluorescein staining of the wild-type mouse ocular surface. Relatively little damage is evident, marked by superficial stippling and occasional micropunctate staining. C, Example of fluorescein staining of the lubricin PRG4 −/− knockout mouse ocular surface. Numerous macropunctate coalescent areas and patches of contiguous staining are evident. Photographs (original magnification ×1.6) were taken after instillation of fluorescein drops into the inferior conjunctival sac. The degree of staining in these images of the superior, medial, inferior, lateral, and central regions of the cornea were all 1 in the wild-type mice (total score = 5) and 2, 3, 1, 2, and 1, respectively, in the knockout mice (total score = 9).
To examine whether lubricin deficiency alters the morphology of the anterior segment, tissues (4 per group, 2 males and 2 females per group) were processed for light microscopy. Our analyses (4 nonadjacent sections per tissue) showed no obvious differences between WT and KO mice in the overall histological evaluation of the cornea, conjunctiva, or eyelid (eFigure 4). The eyelids of 2 KO mice each contained a single, relatively large pocket of inflammation (eFigure 4). In contrast, only 1 small inflammatory focus was present in the eyelid of 1 WT mouse (eFigure 4).
FUNCTION OF LUBRICIN AS A BOUNDARY LUBRICANT AT THE HUMAN CORNEA-EYELID INTERFACE
As shown in Figure 7, lubricin functioned as an effective friction-lowering boundary lubricant at the human cornea-eyelid interface when compared with saline and AQuify. Values of static friction were greatest in saline (mean [SE], 0.27 [0.01] to 0.51 [0.03]), which were similar to the performance of AQuify (mean [SE], 0.25 [0.03] to 0.43 [0.04]) at all velocities (P = .12–.60). By contrast, lubricin exhibited significantly lower static friction (mean [SE], 0.14 [0.02] to 0.29 [0.02]) than both saline and AQuify at all velocities (P < .001–.05). Kinetic friction demonstrated similar trends, exhibiting the highest values in saline (mean [SE], 0.24 [0.02] to 0.31 [0.03]), which was not significantly different from AQuify (mean [SE], 0.20 [0.02] to 0.26 [0.01]) at all velocities (P = .10–.33). Lubricin values of kinetic friction (mean [SE], 0.12 [0.01] to 0.17 [0.02]) were significantly lower than saline at all velocities (P < .001– <.01) and from AQuify at the slower speeds 1 and 0.3 mm/s (both P < .01), where a boundary mode of lubrication is more operative, yet not at the faster speeds (P = .33 and .06, respectively), where hydrodynamic lubrication plays a more substantial role. The friction-lowering effect of lubricin appeared to be specific, because bovine serum albumin did not reduce friction compared with saline (Figure 7D and F).
Figure 7.
Effects of lubricin and sliding velocity on static and kinetic friction at a human cornea-eyelid interface. A, Tissue preparation. An annulus (outer radius = 3.2 mm, inner radius = 1.5 mm) was punched from the eyelid, then attached to a nonpermeable, rigid plastic annular cylinder. B, Test setup. The corneal ocular surface was fastened to the spherical end of an inert nonpermeable semirigid rubber plug cylinder (radius = 6 mm) by applying superglue to the sclera. This plug was attached to the rotational actuator of the BOSE ELF 3200 mechanical testing machine, thus forming the bottom articular surface. The annulus was attached to the linear actuator coupled with an axial load (N) and torsion (τ) load cell, thus forming the upper articulating surface. The lubricant bath was formed by securing an inert tube around the cylinder. C, Static friction of saline, AQuify Long-Lasting Comfort Drops (which contain hyaluronic acid, 0.1%; CIBA Vision), and lubricin. D, Static friction of saline and bovine serum albumin (BSA). E, Kinetic friction of saline, AQuify, and lubricin. F, Kinetic friction of saline and BSA. Lubricin and BSA were tested at 300 μg/mL. Values are the mean (standard error) of 6 (C and E) and 3 (D and F) tests. Values of μStatic, Neq were greatest in saline and similar in AQuify, whereas values in lubricin were statistically lower than those in both saline and AQuify at all effective sliding velocities (veff) (P < .001-P < .05). Values of μKinetic, Neq in lubricin were lowest and statistically different from saline at all veff (P <.001-P < .01) and from AQuify at the lower veff of 1 and 0.3 mm/s (both P < .01), where a boundary mode of lubrication is more operative, yet not at the higher veff of 30 and 10 mm/s (P = .33 and .06, respectively). The friction-lowering effect of lubricin appeared to be specific, because BSA did not reduce friction compared with saline.
DISCUSSION
Our study demonstrates that lubricin is transcribed, translated, and expressed by ocular surface epithelial cells. Our findings also demonstrate that lubricin deficiency promotes corneal damage and lubricin presence significantly reduces friction between the cornea and conjunctiva. These results indicate that lubricin functions as a natural boundary lubricant to reduce shear stress at the ocular surface. In addition, lubricin may play an analogous role at other sites throughout the body, given that we discovered the presence of lubricin mRNA in a number of exocrine and reproductive tissues.
The discovery of a molecule responsible for boundary mode lubrication at the ocular surface supports the hypothesis of Ehlers,28 who theorized that boundary lubrication must take place between the eyelid wiper region29 and the cornea.28 However, Ehlers’ hypothesis was posited without data and was not supported by other investigators.30 Thus, researchers were unable to identify boundary lubricants in secreted mucins from the lacrimal gland and speculated that the cornea-conjunctiva interface is lubricated by a hydrodynamic mechanism.30,31 They further theorized that maximum eyelid velocities generate shear rates that far exceed the boundary lubricant range but are favorable to a hydrodynamic mode of lubrication.30 Recent studies echo this proposal31 and are in line with an elastohydrodynamic model to predict the shear stresses acting between the eyelid and ocular surface.32 These stresses are purportedly reduced by the mucous layer of the tear film.33 Our data, though, indicate that lubricin contributes significantly to the friction reduction mechanism of the tear film and protects the cornea and conjunctiva against the significant shear forces generated during an eyelid blink.
While hydrodynamic lubrication surely contributes to the normal tribology of the ocular surface, in pathologies such as dry eye disease where there is little or no tear film covering parts of the cornea between blinks, boundary lubrication involving lubricin may be a primary method of friction reduction. Boundary lubrication may also be dominant at the cornea–contact lens interface where there is a relative lack of aqueous tear.
The absence of lubricin also promotes ocular surface damage. Corneas of lubricin KO mice had significantly increased fluorescein staining, as compared with their WT controls. This heightened staining occurred predominantly in the superior and medial regions of both corneas. Increased fluorescein staining is commonly associated with dry eye disease34 and is often found in the medial corneal area.35 Loss or downregulation of lubricin likely increases shear stress at the ocular surface, which, in turn may lead to inflammation, stimulation of corneal nerves, and accumulation of inflammatory mediators (eg, interleukin 1α [IL-1α], IL-6, and tumor necrosis factor α) and proteases (eg, metalloproteinase 9 and neutrophil elastase) in the tear film, as well as symptoms of discomfort.36,37
An increased level of inflammatory mediators and proteolytic enzymes in the tear film of patients with dry eye disease could have a particularly deleterious effect on the production and expression of ocular surface lubricin. Cytokines such as IL-1, IL-6, and tumor necrosis factor α are known to cause a significant decrease in the synthesis and/or secretion of lubricin.5,23,38,39 Similarly, proteases like neutrophil elastase degrade lubricin.40,41 Progressive lubricin loss would lead to a further reduction in lubrication, a continued increase in excessive shear stress, greater inflammation, and, potentially, a more severe disease.
Our research demonstrates that exogenous lubricin functions as an effective friction-lowering boundary lubricant at the cornea-eyelid interface. This effect appears to be specific and is not duplicated by the use of hyaluronate-containing solutions or bovine serum albumin. This lubricating ability, which dramatically reduces friction, is a key attribute of lubricin in articulating joints.1–4 It is possible that lubricin’s friction-lowering effect on the ocular surface may even be stronger. Our experiments were performed with eyelid tissues that were obtained up to 3 days after donor death. Consequently, conjunctival changes may have occurred during this period and limited the efficacy of lubricin. The inability of hyaluronate to replicate lubricating activity in the boundary mode was not surprising, given that hyaluronate may often function by providing hydrodynamic viscosity.3
As part of our studies, we discovered lubricin mRNA in mouse submandibular, parotid, sublingual, bladder, vaginal/cervical, and seminal vesicle tissues and in human uterine, cervical, bladder, and prostatic samples. The identity of a majority of these lubricin PCR products was evaluated and verified by DNA sequence analysis. The presence of lubricin in these different tissues should not be considered aberrant. Aside from cartilage, other investigators have also found lubricin in the liver, heart, lung, bone, tendon, kidney, brain, fat, muscle, testis, small intestine, and the trabecular meshwork.20,42–44 Lubricin may be expressed in multiple isoforms, and these splice variants may show tissue and regional specificity and possess different functions.20,45 For example, various lubricin domains may modulate cell proliferation and adhesion, promote matrix binding, stimulate megakaryocytes, influence parathyroid hormone action, and regulate the complement system.3,6,20,46,47 In addition, species differences exist in the molecular weight, amino acid content, and carbohydrate composition of lubricin.20,48
Our finding of lubricin in salivary glands would help explain the identification of boundary lubricant properties in human parotid and submandibular-sublingual saliva49 and their possible activity in enamel-to-enamel lubrication.50,51 It is also possible that lubricin could play a role in multiple hyaluronate functions, given that lubricin and hyaluronate often act in synergy.1,26 Such hyaluronate functions include those related to prostate tumor formation, prostate cancer radiotherapy, bladder disease treatment (eg, recurrent urinary tract infection, chemical or radiation cystitis, and painful bladder syndrome/interstitial cystitis), seminal vesicle homeostasis, uterine cervix healing, cervical relaxation for artificial insemination, endometriotic lesion suppression, pregnancy maintenance, vaginal antimicrobial defense, and therapy for postmenopausal vaginal atrophy.52–63
Acknowledgments
Funding/Support: This research was supported by National Institutes of Health grant R01EY05612, the Margaret S. Sinon Scholar in Ocular Surface Research fund, and funding from the Natural Sciences and Engineering Research Council of Canada, the Canadian Arthritis Network, and the Faculty of Kinesiology and the Schulich School of Engineering Centre for Bioengineering Research and Education at the University of Calgary. We acknowledge use of tissues procured by the National Disease Research Interchange with support from National Institutes of Health grant 5 U42 RR006042.
Footnotes
Author Contributions: Drs Schmidt and D. A. Sullivan are co–first authors.
Conflict of Interest Disclosures: Drs Schmidt, D. A. Sullivan, and B. D. Sullivan are cofounders of a company, Singularis, that was designed to develop a recombinant human lubricin protein for the treatment of dry eye disease and other health care conditions. Singularis has merged with Lubris, which retains the same objectives. A series of patents have been awarded or filed around this technology. The intellectual property for the dry eye application is owned by the Schepens Eye Research Institute/Massachusetts Eye and Ear Infirmary and the University of California, San Diego.
Online-Only Material: The eTables and eFigures are available at http://www.jamaophth.com.
Additional Contributions: We express our appreciation to Zahra Sadrai, MD (Boston, Massachusetts), for mouse cornea and conjunctival RNA preparations; Roman Krawetz, PhD (Calgary, Alberta, Canada), for human articular cartilage and synovial membrane complementary DNA samples; Pablo Argueso, PhD, and Abha Gulati, MD (Boston), for human conjunctival impression cytology complementary DNA samples; Ilene K. Gipson, PhD, and Sandra Michaud, MS (Boston), for immortalized human conjunctival epithelial cells; James Jester, PhD (Irvine, California), for immortalized human corneal epithelial cells; Matthew L. Warman, MD (Boston), and Yajun Cui, PhD (Cleveland, Ohio), for making available breeding pairs of mice with a 129Sv/Ev genetic background and Ms Stefanie DeLuca (Wilmington, Massachusetts) for help with these animals; Prof M. Dietel and Dr M. von Laffert (Berlin, Germany) for providing human articular tissues; the Lions Eye Bank through the Southern Alberta Organ and Tissue Donation Program (Calgary) for providing human corneas; and the University of Calgary Body Donation Program (Calgary) for providing human eyelids.
References
- 1.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56(3):882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 2.Jones AR, Gleghorn JP, Hughes CE, et al. Binding and localization of recombinant lubricin to articular cartilage surfaces. J Orthop Res. 2007;25(3):283–292. doi: 10.1002/jor.20325. [DOI] [PubMed] [Google Scholar]
- 3.Jay DJ. Lubricin and surfacing of articular joints. Curr Opin Orthop. 2004;15(5):355–359. [Google Scholar]
- 4.Bao JP, Chen WP, Wu LD. Lubricin: a novel potential biotherapeutic approaches for the treatment of osteoarthritis. Mol Biol Rep. 2011;38(5):2879–2885. doi: 10.1007/s11033-010-9949-9. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt TA, Schumacher BL, Klein TJ, Voegtline MS, Sah RL. Synthesis of proteoglycan 4 by chondrocyte subpopulations in cartilage explants, monolayer cultures, and resurfaced cartilage cultures. Arthritis Rheum. 2004;50(9):2849–2857. doi: 10.1002/art.20480. [DOI] [PubMed] [Google Scholar]
- 6.Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115(3):622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jay GD, Torres JR, Rhee DK, et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007;56(11):3662–3669. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsaid KA, Jay GD, Chichester CO. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 2007;56(1):108–116. doi: 10.1002/art.22321. [DOI] [PubMed] [Google Scholar]
- 9.Teeple E, Elsaid KA, Jay GD, et al. Effects of supplemental intra-articular lubricin and hyaluronic acid on the progression of posttraumatic arthritis in the anterior cruciate ligament-deficient rat knee. Am J Sports Med. 2011;39(1):164–172. doi: 10.1177/0363546510378088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jay GD, Elsaid KA, Kelly KA, et al. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2012;64(4):1162–1171. doi: 10.1002/art.33461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flannery CR, Zollner R, Corcoran C, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60(3):840–847. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 12.Flannery CR. Novel therapies in OA. Curr Drug Targets. 2010;11(5):614–619. doi: 10.2174/138945010791011884. [DOI] [PubMed] [Google Scholar]
- 13.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 14.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 15.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127(6):763–768. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki T, Sullivan DA. Comparative effects of estrogen on matrix metalloproteinases and cytokines in immortalized and primary human corneal epithelial cell cultures. Cornea. 2006;25(4):454–459. doi: 10.1097/01.ico.0000220777.70981.46. [DOI] [PubMed] [Google Scholar]
- 17.Robertson DM, Li L, Fisher S, et al. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46(2):470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- 18.Gipson IK, Spurr-Michaud S, Argüeso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44(6):2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Hatton MP, Khandelwal P, Sullivan DA. Culture, immortalization, and characterization of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2010;51(8):3993–4005. doi: 10.1167/iovs.09-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikegawa S, Sano M, Koshizuka Y, Nakamura Y. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet. 2000;90(3–4):291–297. doi: 10.1159/000056791. [DOI] [PubMed] [Google Scholar]
- 21.Knop N, Knop E. Conjunctiva-associated lymphoid tissue in the human eye. Invest Ophthalmol Vis Sci. 2000;41(6):1270–1279. [PubMed] [Google Scholar]
- 22.Schmidt TA, Plaas AH, Sandy JD. Disulfide-bonded multimers of proteoglycan 4 PRG4 are present in normal synovial fluids. Biochim Biophys Acta. 2009;1790(5):375–384. doi: 10.1016/j.bbagen.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt TA, Gastelum NS, Han EH, Nugent-Derfus GE, Schumacher BL, Sah RL. Differential regulation of proteoglycan 4 metabolism in cartilage by IL-1α, IGF-I, and TGF-β1. Osteoarthritis Cartilage. 2008;16(1):90–97. doi: 10.1016/j.joca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Steele BL, Alvarez-Veronesi MC, Schmidt TA. Molecular weight characterization of PRG4 proteins using multi-angle laser light scattering (MALLS) Osteoarthritis Cartilage. 2013;21(3):498–504. doi: 10.1016/j.joca.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Barabino S, Shen LL, Chen L, Rashid S, Rolando M, Dana MR. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005;46(8):2766–2771. doi: 10.1167/iovs.04-1326. [DOI] [PubMed] [Google Scholar]
- 26.Morrison S, Sullivan DA, Sullivan BD, Sheardown H, Schmidt TA. Dose-dependent and synergistic effects of proteoglycan 4 on boundary lubrication at a human cornea-polydimethylsiloxane biointerface. Eye Contact Lens. 2012;38(1):27–35. doi: 10.1097/ICL.0b013e31823f7041. [DOI] [PubMed] [Google Scholar]
- 27.Means TL, Geroski DH, L’Hernault N, Grossniklaus HE, Kim T, Edelhauser HF. The corneal epithelium after optisol-GS storage. Cornea. 1996;15(6):599–605. [PubMed] [Google Scholar]
- 28.Ehlers N. The precorneal film: biomicroscopical, histological and clinical investigations. Acta Ophthalmol Suppl. 1965;81:109–113. [PubMed] [Google Scholar]
- 29.Knop E, Knop N, Zhivov A, et al. The lid wiper and muco-cutaneous junction anatomy of the human eyelid margins: an in vivo confocal and histological study. J Anat. 2011;218(4):449–461. doi: 10.1111/j.1469-7580.2011.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jay GD, Hong BS. Characterization of a bovine synovial fluid lubricating factor, II: comparison with purified ocular and salivary mucin. Connect Tissue Res. 1992;28(1–2):89–98. doi: 10.3109/03008209209014229. [DOI] [PubMed] [Google Scholar]
- 31.Knop N, Korb DR, Blackie CA, Knop E. The lid wiper contains goblet cells and goblet cell crypts for ocular surface lubrication during the blink. Cornea. 2012;31(6):668–679. doi: 10.1097/ICO.0b013e31823f8d8c. [DOI] [PubMed] [Google Scholar]
- 32.Jones MB, Fulford GR, Please CP, McElwain DLS, Collins MJ. Elastohydrodynamics of the eyelid wiper. Bull Math Biol. 2008;70(2):323–343. doi: 10.1007/s11538-007-9252-7. [DOI] [PubMed] [Google Scholar]
- 33.Cher I. A new look at lubrication of the ocular surface: fluid mechanics behind the blinking eyelids. Ocul Surf. 2008;6(2):79–86. doi: 10.1016/s1542-0124(12)70271-9. [DOI] [PubMed] [Google Scholar]
- 34.Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):108–152. doi: 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 35.Schwallie JD, Mckenney CD, Long WD, Jr, Mcneil A. Corneal staining patterns in normal non-contact lens wearers. Optom Vis Sci. 1997;74(2):92–98. doi: 10.1097/00006324-199702000-00020. [DOI] [PubMed] [Google Scholar]
- 36.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan DA, Hammitt KM, Schaumberg DA, et al. Report of the TFOS/ARVO Symposium on global treatments for dry eye disease: an unmet need. Ocul Surf. 2012;10(2):108–116. doi: 10.1016/j.jtos.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Blewis ME, Lao BJ, Schumacher BL, Bugbee WD, Sah RL, Firestein GS. Interactive cytokine regulation of synoviocyte lubricant secretion. Tissue Eng Part A. 2010;16(4):1329–1337. doi: 10.1089/ten.tea.2009.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elsaid KA, Fleming BC, Oksendahl HL, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58(6):1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52(6):1746–1755. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 41.Jones ARC, Keohan C, Sheldon R, et al. Regulation of lubricin expression and metabolism in synovial joints. Eur Cell Mater. 2006;12(suppl 1):17. [Google Scholar]
- 42.Lee SY, Nakagawa T, Reddi AH. Induction of chondrogenesis and expression of superficial zone protein (SZP)/lubricin by mesenchymal progenitors in the infrapatellar fat pad of the knee joint treated with TGF-beta1 and BMP-7. Biochem Biophys Res Commun. 2008;376(1):148–153. doi: 10.1016/j.bbrc.2008.08.138. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y, Berger EJ, Zhao C, Jay GD, An KN, Amadio PC. Expression and mapping of lubricin in canine flexor tendon. J Orthop Res. 2006;24(9):1861–1868. doi: 10.1002/jor.20239. [DOI] [PubMed] [Google Scholar]
- 44.Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47(1):226–234. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Berger EJ, Zhao C, An KN, Amadio PC, Jay G. Mapping lubricin in canine musculoskeletal tissues. Connect Tissue Res. 2006;47(4):215–221. doi: 10.1080/03008200600846754. [DOI] [PubMed] [Google Scholar]
- 46.Flannery CR, Hughes CE, Schumacher BL, et al. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254(3):535–541. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 47.Novince CM, Koh AJ, Michalski MN, et al. Proteoglycan 4, a novel immunomodulatory factor, regulates parathyroid hormone actions on hematopoietic cells. Am J Pathol. 2011;179(5):2431–2442. doi: 10.1016/j.ajpath.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swann DA, Silver FH, Slayter HS, Stafford W, Shore E. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J. 1985;225(1):195–201. doi: 10.1042/bj2250195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aguirre A, Mendoza B, Levine MJ, Hatton MN, Douglas WH. In vitro characterization of human salivary lubrication. Arch Oral Biol. 1989;34(8):675–677. doi: 10.1016/0003-9969(89)90024-1. [DOI] [PubMed] [Google Scholar]
- 50.Reeh ES, Douglas WH, Levine MJ. Lubrication of human and bovine enamel compared in an artificial mouth. Arch Oral Biol. 1995;40(11):1063–1072. doi: 10.1016/0003-9969(95)00031-j. [DOI] [PubMed] [Google Scholar]
- 51.Reeh ES, Douglas WH, Levine MJ. Lubrication of saliva substitutes at enamel-to-enamel contacts in an artificial mouth. J Prosthet Dent. 1996;75(6):649–656. doi: 10.1016/s0022-3913(96)90251-6. [DOI] [PubMed] [Google Scholar]
- 52.Thompson CB, Shepard HM, O’Connor PM, et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther. 2010;9(11):3052–3064. doi: 10.1158/1535-7163.MCT-10-0470. [DOI] [PubMed] [Google Scholar]
- 53.Ghatak S, Hascall VC, Markwald RR, Misra S. Stromal hyaluronan interaction with epithelial CD44 variants promotes prostate cancer invasiveness by augmenting expression and function of hepatocyte growth factor and androgen receptor. J Biol Chem. 2010;285(26):19821–19832. doi: 10.1074/jbc.M110.104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sommariva ML, Sandri SD, Guerrer CS. Treatment of acute iatrogenic cystitis secondary to bladder chemo-immuno-instillation or pelvic radiotherapy [in Italian] Urologia. 2010;77(3):187–192. [PubMed] [Google Scholar]
- 55.Sakairi A, Tsukise A, Meyer W. Localization of hyaluronic acid in the seminal vesicles of the miniature pig. Anat Histol Embryol. 2007;36(1):4–9. doi: 10.1111/j.1439-0264.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 56.Markowska J, Madry R, Markowska A. The effect of hyaluronic acid (Cicatridine) on healing and regeneration of the uterine cervix and vagina and vulvar dystrophy therapy. Eur J Gynaecol Oncol. 2011;32(1):65–68. [PubMed] [Google Scholar]
- 57.Perry K, Haresign W, Wathes DC, Khalid M. Intracervical application of hyaluronan improves cervical relaxation in the ewe. Theriogenology. 2010;74(9):1685–1690. doi: 10.1016/j.theriogenology.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Hasegawa A, Yoshino O, Osuga Y, et al. Hyaluronic acid reagent suppressed endometriotic lesion formation in a mouse model. Fertil Steril. 2010;93(8):2757–2759. doi: 10.1016/j.fertnstert.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 59.Cordo-Russo R, Garcia MG, Barrientos G, et al. Murine abortion is associated with enhanced hyaluronan expression and abnormal localization at the fetomaternal interface. Placenta. 2009;30(1):88–95. doi: 10.1016/j.placenta.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 60.Dusio GF, Cardani D, Zanobbio L, et al. Stimulation of TLRs by LMW-HA induces self-defense mechanisms in vaginal epithelium. Immunol Cell Biol. 2011;89(5):630–639. doi: 10.1038/icb.2010.140. [DOI] [PubMed] [Google Scholar]
- 61.Costantino D, Guaraldi C. Effectiveness and safety of vaginal suppositories for the treatment of the vaginal atrophy in postmenopausal women: an open, non-controlled clinical trial. Eur Rev Med Pharmacol Sci. 2008;12(6):411–416. [PubMed] [Google Scholar]
- 62.Wilder RB, Barme GA, Gilbert RF, et al. Cross-linked hyaluronan gel improves the quality of life of prostate cancer patients undergoing radiotherapy. Brachytherapy. 2011;10(1):44–50. doi: 10.1016/j.brachy.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Damiano R, Cicione A. The role of sodium hyaluronate and sodium chondroitin sulphate in the management of bladder disease. Ther Adv Urol. 2011;3(5):223–232. doi: 10.1177/1756287211418723. [DOI] [PMC free article] [PubMed] [Google Scholar]