Abstract
Aims
To assess the clinical and demographic differences in patients with pre-gestational diabetes mellitus (PGDM) compared to those with gestational diabetes (GDM).
Methods
Using the 2001–2007 California Health Discharge Database, we identified 22,331 cases of PGDM and 147,097 cases of GDM via ICD-9-CM codes after excluding cases which were missing race or age data or with extremes of age. Data analyzed included demographics, pre-existing medical conditions, antepartum complications, and intrapartum complications. Logistic regression was used to adjust for potential confounders.
Results
Both PGDM and GDM incidences increased during the study period. Advancing age was associated with increased prevalence of both diseases. Although Asians were found to have the highest prevalence of GDM, they, along with Caucasians, were found have the lowest prevalence of PGDM.
Conditions with increased frequency in PGDM versus GDM included chronic hypertension, renal disease, thyroid dysfunction, fetal CNS malformation, fetal demise, pyelonephritis, and eclampsia. Subjects with PGDM were more likely than those with GDM to have a shoulder dystocia, failed induction of labor, or undergo cesarean delivery.
Conclusions
We have demonstrated clinical morbidities and demographic factors which differ in patients with PGDM compared to patients with GDM. Our findings suggest PGDM to be associated with significantly higher morbidity when compared to GDM. Our findings also suggest that races with the highest tendency for GDM during pregnancy may not necessarily have the highest tendency for PGDM outside of pregnancy.
Keywords: Demographics, Gestational diabetes, Pregestational diabetes, Risk factors
1. Introduction
Diabetes complicates approximately 6%–7% of pregnancies in the United States, with California demonstrating a similar prevalence of 7.6% (Lawrence, Contreras, Chen, & Sacks, 2008). Approximately 85% are attributed to gestational diabetes mellitus (GDM), while the remaining are due to pre-gestational diabetes mellitus (PGDM) (Wier, Witt, Burgess, & Elixhauser, 2006).
GDM is currently defined by the American Diabetes Association as “any degree of glucose intolerance with onset or first recognition during pregnancy”(Diagnosis & classification of diabetes mellitus, 2012). The pathogenesis is typically attributed to insulin resistance during pregnancy due to factors such as human placental lactogen and tumor necrosis factor alpha (Metzger et al., 2008; Vambergue et al., 2002). PGDM, on the other hand, includes both type I and type 2 diabetes mellitus (DM) occurring prior to pregnancy.
Previous studies have reported on morbidities of both PGDM and GDM in pregnancy which include fetal macrosomia, neonatal hypoglycemia, perinatal mortality, polyhydramnios, and increased risk of cesarean delivery (Gestational diabetes mellitus, 2004; Macintosh et al., 2006; Persson, Norman, & Hanson, 2009). However, few studies have looked at direct comparisons of morbidity between subjects with PGDM and GDM. Given PGDM’s ability to affect the maternal–fetal dyad at an earlier gestational age, we hypothesize that there will be increased morbidity of PGDM when compared to GDM in all periods of pregnancy (pre-pregnancy, antepartum, and delivery).We also postulate that there will be certain racial predilections towards developing GDM and PGDM. We hypothesize that our results will confirm advancing maternal age to be associated with an increased risk of both conditions. Finally, we believe that incidences of both diseases have increased over time.
The objective of this study was to compare the trends, demographic factors and maternal morbidity between women with GDM versus those with PGDM using a California population cohort.
2. Patients and methods
This is a retrospective study using health discharge data for all deliveries during 2001–2007 in California. The dataset, provided by the California Office of Statewide Health Planning and Development (OSHPD), is a publicly available dataset comprising cases where a patient is treated in a licensed general acute care hospital in California. Information regarding demographics, diagnoses, specific procedures undergone, and details regarding the patient’s stay, such as source of funding, length of stay are contained in the dataset. The local Institutional Review Board granted exempt approval because of the de-identified, retrospective design.
2.1. Inclusion/exclusion criteria
3,556,567 million deliveries were extracted from inpatient California discharge data using delivery codes. Cases of GDM were identified using ICD-9-CM (International Classification of Diseases, Ninth Revision, Clinical Modification) coding for “Abnormal glucose tolerance of mother complicating pregnancy childbirth or the puerperium” (648.80, 648.81, 648.82, 648.83). PGDM cases were identified using ICD-9-CM codes for “Diabetes mellitus complicating pregnancy childbirth or the puerperium” (648.00, 648.01, 648.02, 648.03) (ICD-9-CM, 2006). Pre-pregnancy, antepartum, and delivery comorbidities (e.g. hypertension, preterm delivery, postpartum hemorrhage) as well as procedures (e.g. operative vaginal delivery, cesarean delivery) were also identified using respective ICD-9-CM coding. A table listing all ICD-9 codes used for case identification is available as a supplemental file.
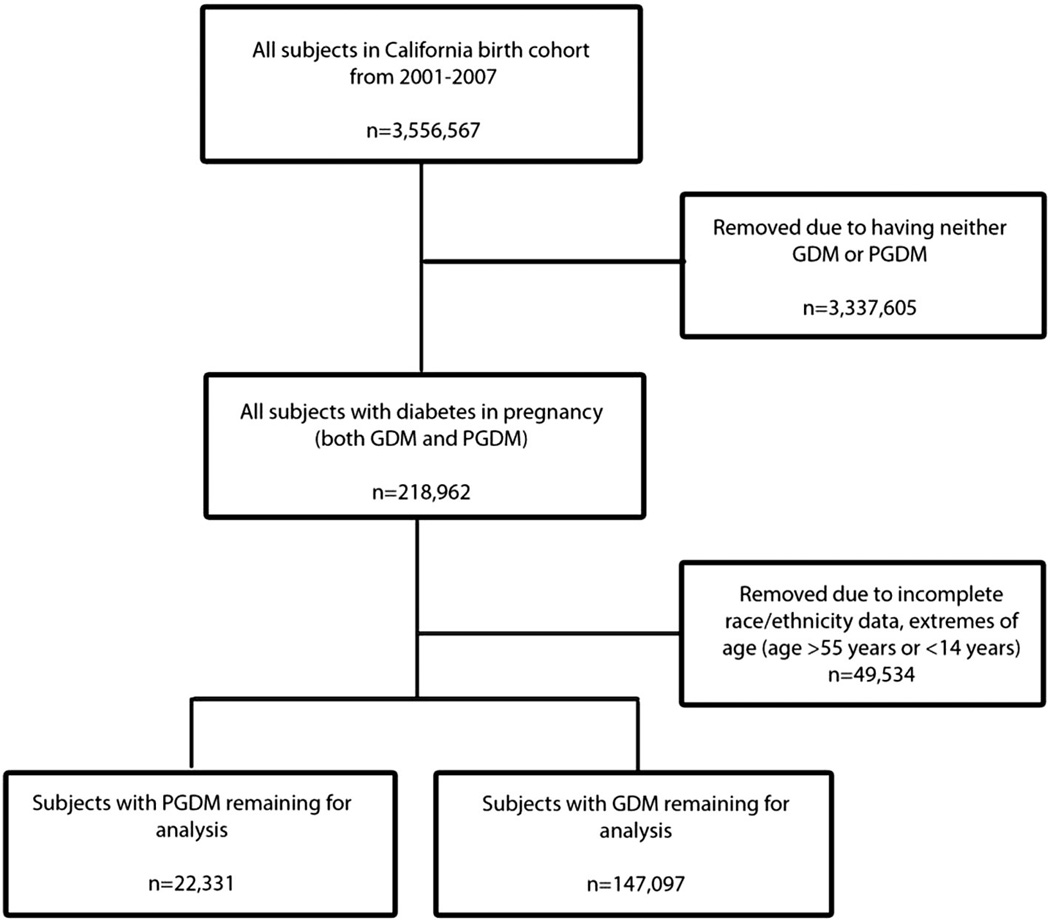
Using initial ICD-9-CM identification, there were 189,873 cases of GDM identified and 29,089 PGDM cases. Subjects missing age or race/ethnicity data as well as extremes of age (<15 years old and ≥55 years old) were excluded. A lower limit of age 15 was chosen because it is defined by the World Health Organization as the lower limit of reproductive age, while an upper limit of 55 years of age allowed for inclusion of very advanced maternal age mothers, while still minimizing cases with probable age coding errors (Women's Health Fact Sheet, 2009). Ultimately, 40 cases were deleted due to extremes of age, 19,022 due to missing age, and 30,472 due to missing race/ethnicity data. Ultimately, 147,097 cases of GDM and 22,331 cases of PGDM remaining for final analysis (see Fig. 1).
Fig. 1.
Subject selection criteria.
2.2. Variables/outcomes measured
Clinical outcome variables were grouped into three temporal categories: 1) pre-pregnancy 2) antepartum and 3) delivery. All outcome variables were assessed using univariate chi-square analysis to obtain an unadjusted odds ratio. They were then entered into a multivariate unconditional logistic regression model to obtain an adjusted odds ratio. Adjustments were made for age, race, ethnicity, insurance type, and presence of any hypertensive disease (mild preeclampsia, severe preeclampsia, eclampsia, chronic hypertension, and unspecified hypertension as defined by ICD-9-CM coding), unless the adjusted factor was the primary outcome itself.
2.3. Statistical analysis
Comparisons were made between the PGDM and GDM groups. Calculations were performed using student’s t-test for continuous variables, chi-square test for discrete variables, and logistic regression for adjustment of covariates. A power analysis was performed to assess the ability of the dataset to determine outcomes. Based on an alpha error of 0.05 and 80% power for detecting a difference between the two groups, the sample size far exceeded this requirement for detecting even a modest difference in most outcomes. For rarer outcomes such as stillbirth (which had a prevalence of 3.4 in 1000 in the GDM group in our cohort), we calculated that we would need at least 22,986 cases per arm in order to detect a 50% difference, to 5.1 in 1000 in the PGDM group.
Results were expressed in odds ratios (ORs) and 95% confidence intervals (CI). Calculations were performed in SPSS 18.0 (Chicago, IL). Significance was established at a 2-sided p < .003 after Bonferroni adjustment.
3. Results
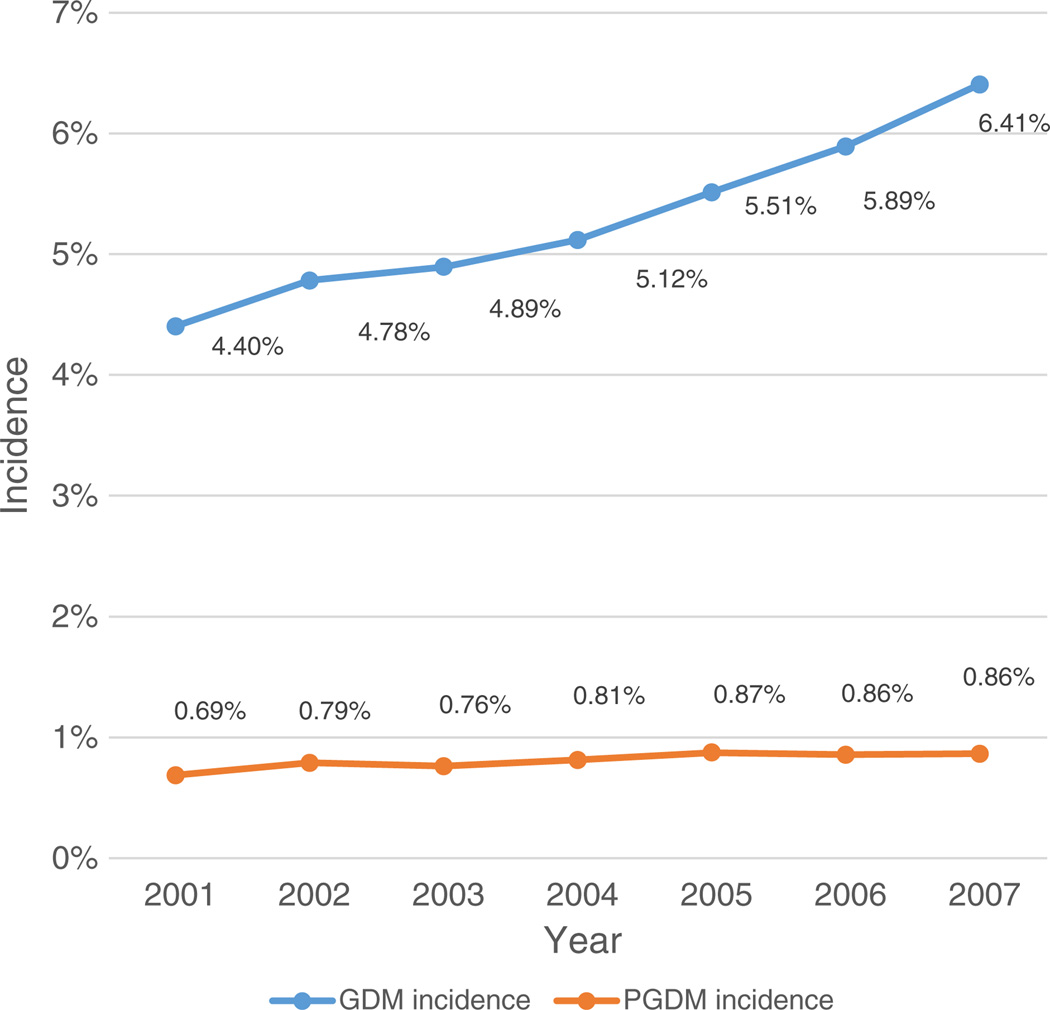
The prevalence of GDM was 5.34% while PGDM prevalence was 0.82% during the study period. As seen in Fig. 2, both conditions increased over time, even after age adjustment. PGDM increased from 0.69% in 2001 to 0.86% in 2007. GDM increased from 4.40% in 2001 to 6.41% in 2007. The mean maternal age of the entire study population was 31.14 ± 5.71 years standard deviation (SD), with 33.82% of all subjects being of advanced maternal age (age > 35 years).
Fig. 2.
Age-adjusted incidence of pre-gestational diabetes and gestational diabetes over time.
The baseline characteristics for the 169,428 diabetic pregnancies are shown in Table 1, divided into GDM and PGDM comparisons. Although there was no difference in mean age of subjects with PGDM compared to those with GDM (31.06 ± 6.12 vs. 31.15 ± 5.64, p = 0.09), there were differences in distribution of certain age ranges, advanced maternal age status, race/ethnicity, presence of hypertensive disease, and private insurance status.
Table 1.
Baseline Characteristics between women with pre-gestational diabetes and gestational diabetes in pregnancy.
| Baseline Characteristic | Subjects with PGDM (n = 22,331) |
Subjects with GDM (n = 147,097) |
p-value |
|---|---|---|---|
| n (prevalence) | n (prevalence) | ||
| Age (years) | <.001 | ||
| 15–19 | 545 (2.44%) | 2495 (1.70%) | |
| 20–24 | 2684 (12.02%) | 15,273 (10.38%) | |
| 25–29 | 4897 (21.93%) | 33,237 (22.60%) | |
| 30–34 | 6352 (28.44%) | 46,500 (31.61%) | |
| 35–39 | 5729 (25.65%) | 38,327 (26.06%) | |
| 40–44 | 1996 (8.94%) | 10,556 (7.18%) | |
| 45+ | 128 (0.57%) | 709 (0.48%) | |
| Advanced Maternal Age (≥35 years) | 7853 (35.17%) | 49,592 (33.71%) | <.001 |
| Race/Ethnicity | <.001 | ||
| Caucasian | 6791 (30.41%) | 45,348 (30.83%) | |
| Black | 1478 (6.62%) | 4815 (3.27%) | |
| Native American/Eskimo/Aleut | 61 (0.27%) | 196 (0.13%) | |
| Asian/Pacific Islander | 1574 (7.05%) | 20,814 (14.15%) | |
| Hispanic | 12,427 (55.65%) | 75,924 (51.61%) | |
| Hypertensive disease present | 4038 (18.08%) | 11,547 (7.85%) | <.001 |
| Private insurance | 10,045 (45.12%) | 77,095 (52.59%) | <.001 |
PGDM: Pre-gestational diabetes.
GDM: Gestational diabetes.
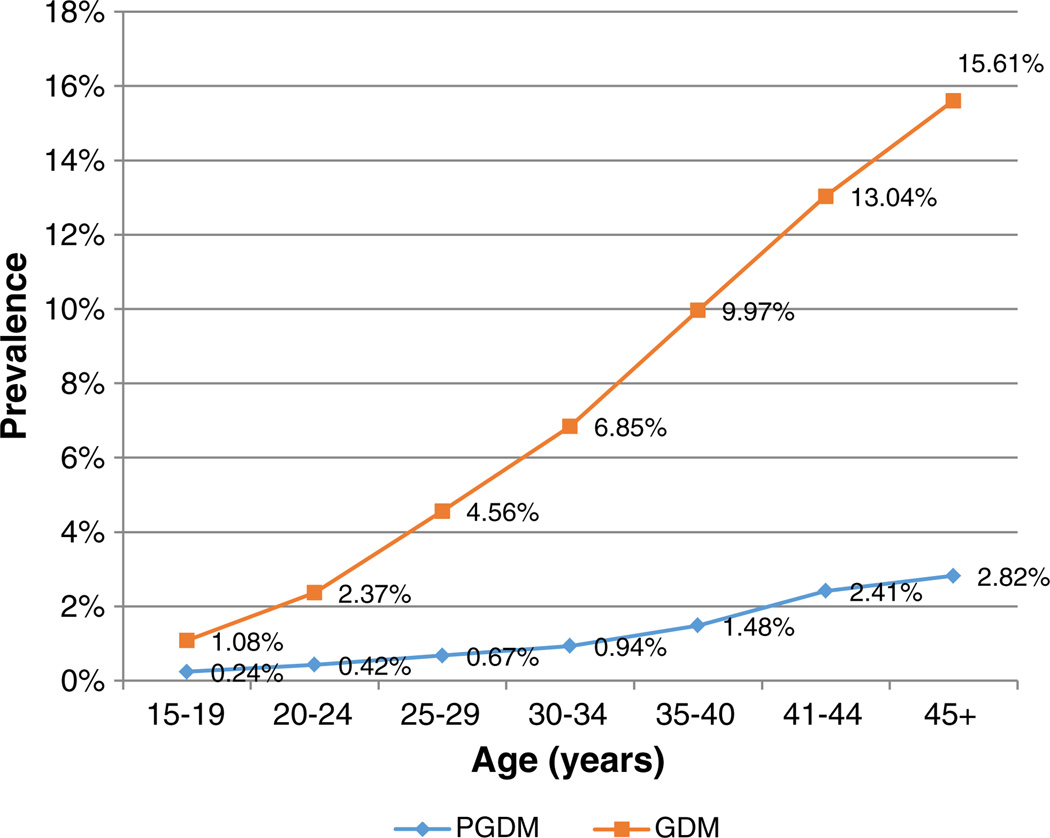
Fig. 3 demonstrates the prevalence of PGDM and GDM by age range. There was a continuous increase in prevalence with advancing age for both diseases. PGDM and GDM prevalences ranged from 0.24% and 1.08% repectively at age 15–19, to 2.82% and 15.61% respectively at age >45 years.
Fig. 3.
Prevalence of pre-gestational diabetes compared to gestational diabetes in pregnancy by age range.
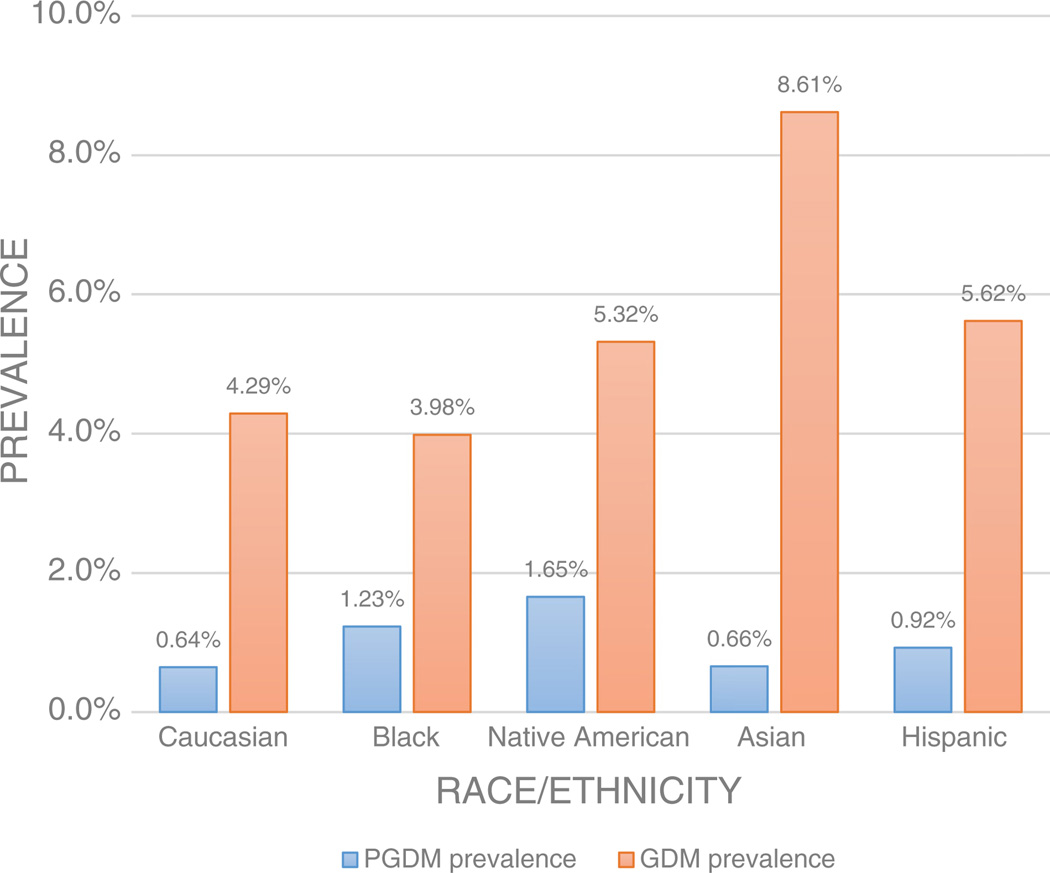
As seen in Fig. 4, when comparing different age-adjusted races/ethnicities and their respective prevalences of diabetic disease, GDM risk was highest in Asians (8.61%) followed by Hispanics (5.62%) and Native Americans (5.32%). PGDM prevalence was highest Native Americans (1.65%) followed by Blacks (1.23%). After covariate adjustment and using Caucasians as a referent group, there were increased odds of GDM in Asians, Native Americans, and Hispanics. On the other hand, PGDM was significantly increased the most in Native Americans, followed by Hispanics and Blacks in comparison to Caucasians (see Table 2).
Fig. 4.
Age-adjusted prevalence of PGDM and GDM by race/ethnicity.
Table 2.
Racial/ethnic odds of pre-gestational diabetes and gestational diabetes compared to Caucasians.
| Caucasian | Blacks | Asian | Hispanic | Native American | |
|---|---|---|---|---|---|
| Odds of GDM | 1 | 0.99 (0.96–1.02), p = 0.44 | 2.00 (1.97–2.04), p < .001 | 1.72 (1.70–1.74), p < .001 | 1.41 (1.22–1.63), p < .001 |
| Odds of PGDM | 1 | 1.65 (1.56–1.76), p < .001 | 1.00 (0.94–1.05), p = 0.86 | 1.67 (1.63–1.75), p < .001 | 2.35 (1.82–3.04), p < .001 |
All results expressed as odds ratio (95% CI), with Caucasian as referent group.
Adjusted for age, insurance type, and presence of hypertensive disease.
Several clinical co-morbidities were found to be significantly increased in PGDM when compared to GDM after covariate adjustment (see Table 3). Subjects with PGDM were more likely to be affected by chronic disease conditions such as thyroid dysfunction (OR 2.24, 95% CI 2.09–2.40) and chronic hypertension (OR 3.33, 95% CI 3.14–3.53). PGDM was also associated more with fetal CNS malformations (OR 4.52, 95% CI 3.33–6.14), intrauterine fetal demise (OR 3.18, 95% CI 2.73–3.71), pyelonephritis (OR 2.84, 95% CI 2.27–3.55), mild preeclampsia (OR 1.62, 95% CI 1.51–1.73), severe preeclampsia (OR 1.93, 95% CI 1.75–2.12), and eclampsia (OR 1.84, 95% CI 1.27– 2.67) when compared to those with GDM. Finally, in terms of delivery-related risks, subjects with PGDM had a higher chance of undergoing cesarean delivery (OR 1.31, 95% CI 1.28–1.35), a failed induction (OR 1.18, 95% CI 1.09–1.28), or shoulder dystocia (OR 1.14, 95% CI 1.04–1.26). They were less likely to undergo post-term delivery (OR 0.40, 95% CI 0.37–0.44) or operative vaginal delivery (OR 0.75, 95% CI 0.70–0.80). PGDM subjects had a slightly longer mean length of stay than those with GDM (3.73 ± 3.91 days [SD] vs. 3.07 ± 3.61 days [SD], p < .001).
Table 3.
Clinical differences between subjects with pre-gestational diabetes compared to those with gestational diabetes.
| Outcome variable | Subjects with PGDM (n = 22,331) |
Subjects with GDM (n = 147,097) |
Unadjusted odds ratio (95% confidence interval) |
p-value | Adjusted odds ratio (95% confidence interval) |
p-value |
|---|---|---|---|---|---|---|
| n (prevalence) | n (prevalence) | |||||
| Pre-pregnancy Conditions | ||||||
| Thyroid dysfunction | 1140 (5.11%) | 3511 (2.39%) | 2.20 (2.06–2.36) | <.001 | 2.24 (2.09–2.40) | <.001 |
| Chronic hypertension | 1794 (8.03%) | 3617 (2.46%) | 3.47 (3.27–3.67) | <.001 | 3.33 (3.14–3.53) | <.001 |
| Renal disease without hypertension | 102 (0.46%) | 269 (0.18%) | 2.51 (1.99–3.15) | <.001 | 2.51 (1.99–3.17) | <.001 |
| Antepartum Conditions | ||||||
| Mild preeclampsia | 1182 (5.29%) | 4656 (3.17%) | 1.71 (1.60–1.83) | <.001 | 1.62 (1.51–1.73) | <.001 |
| Severe preeclampsia | 568 (2.54%) | 1884 (1.28%) | 2.01 (1.83–2.21) | <.001 | 1.93 (1.75–2.12) | <.001 |
| Eclampsia | 37 (0.17%) | 123 (0.08%) | 1.98 (1.37–2.87) | <.001 | 1.84 (1.27–2.67) | .001 |
| Pyelonephritis | 118 (0.53%) | 253 (0.17%) | 3.08 (2.48–3.84) | <.001 | 2.84 (2.27–3.55) | <.001 |
| Preterm delivery | 3179 (14.24%) | 13,214 (8.98%) | 1.68 (1.61–1.75) | <.001 | 1.49 (1.42–1.55) | <.001 |
| Post-term delivery | 533 (2.39%) | 8972 (6.10%) | 0.38 (0.35–0.41) | <.001 | 0.40 (0.37–0.44) | <.001 |
| Fetal CNS malformation | 72 (0.32%) | 105 (0.07%) | 4.53 (3.35–6.11) | <.001 | 4.52 (3.33–6.14) | <.001 |
| Intrauterine fetal demise | 265 (1.19%) | 499 (0.34%) | 3.53 (3.04–4.10) | <.001 | 3.18 (2.73–3.71) | <.001 |
| Poor fetal growth | 391 (1.75%) | 2119 (1.44%) | 1.22 (1.09–1.36) | 0.001 | 1.11 (0.99–1.24) | .07 |
| Excess fetal growth | 1657 (7.42%) | 8942 (6.08%) | 1.24 (1.17–1.31) | <.001 | 1.21 (1.14–1.27) | <.001 |
| Peripartum Conditions | ||||||
| Failed induction | 766 (3.43%) | 3854 (2.62%) | 1.32 (1.22–1.43) | <.001 | 1.18 (1.09–1.28) | <.001 |
| Shoulder dystocia | 526 (2.36%) | 3069 (2.09%) | 1.13 (1.03–1.24) | .009 | 1.14 (1.04–1.26) | .003 |
| Cesarean delivery | 11,711 (52.44%) | 64,803 (44.05%) | 1.40 (1.36–1.44) | <.001 | 1.31 (1.28–1.35) | <.001 |
| Postpartum hemorrhage | 559 (2.50%) | 4327 (2.94%) | 0.85 (0.78–0.93) | <.001 | 0.82 (0.75–0.89) | <.001 |
| Third/Fourth degree lacsa | 353 (3.32%) | 3734 (4.54%) | 0.72 (0.65–0.81) | <.001 | 0.84 (0.75–0.94) | <.001 |
| Operative vaginal delivery | 1002 (4.49%) | 9468 (6.44%) | 0.68 (0.64–0.73) | <.001 | 0.75 (0.70–0.80) | <.001 |
All outcomes adjusted for age, race/ethnicity, insurance type, and presence of hypertensive disease (including mild preeclampsia, severe preeclampsia, eclampsia, chronic hypertension, unspecified hypertension) unless the variable being adjusted for is the outcome itself.
Prevalence (%) is expressed as # of cases of condition divided by total n of subjects in either PGDM or GDM group.
Calculated for vaginal deliveries only; Total n for PGDM = 10,620, GDM = 82,294.
A subgroup analysis was then performed to compare PGDM vs. GDM risks in the teenage population (age 15–19 years). Teenagers with PGDM compared to those with GDM were found to have higher adjusted rates of thyroid dysfunction (OR 5.32, 95% CI 2.71–10.45), preterm delivery (OR 2.21, 95% CI 1.72–2.85), fetal CNS malformations (OR 7.35, 95% CI 1.63–33.13), intrauterine fetal demise (OR 10.28, 95% CI 4.35–24.29), preeclampsia (OR 1.41, 95% CI 1.03–1.93), severe preeclampsia (OR 3.57, 95% CI 2.26–5.62), and chronic hypertension (OR 3.93, 95% CI 1.87–8.24), and cesarean delivery (OR 1.39, 95% CI 1.15–1.68). PGDM teenagers had lower rates of post-term delivery (OR 0.27, 95% CI 0.15–0.48), postpartum hemorrhage (OR 0.53, 0.30–0.94).
4. Discussion
Our results demonstrate that both PGDM and GDM differ in several aspects — age distribution, race/ethnicity, and associations with clinical morbidities. Both diseases have increased over time, highlighting the need to investigate possible population-based clinical and demographic interactions.
Our study, to our knowledge, is the first to investigate the increased morbidity of PGDM compared to GDM using a large population. El Mallah et al. described that GDM and PGDM were similar in maternal, fetal, and neonatal complications (El Mallah, Narchi, Kulaylat, & Shaban, 1997); however, the study was under-powered due to a small sample size of 71 subjects. Ray et al. demonstrated different results between PGDM and GDM morbidity, showing increased risks of cesarean delivery, shoulder dystocia, and gestational hypertension (Ray, Vermeulen, Shapiro, & Kenshole, 2001). However, with a still relatively small sample size of 126 PGDM and 498 GDM subjects, the study was not powered to address rare outcomes (such as intrauterine fetal demise). The pathogenesis leading to adverse outcome in PGDM and GDM is believed to stem from abnormal glucose levels affecting both mother and fetus. The Hyperglycemia and Adverse Pregnancy Outcomes study by Landon demonstrated a linear relationship between maternal glucose levels and adverse pregnancy outcome (Metzger et al., 2008; Landon et al., 2011). Thus, it would be logical that PGDM, which is more likely to have elevated glucose levels in early pregnancy, would have increased adverse pregnancy outcome as well. Our results confirm this expectation — most adverse pregnancy outcomes are increased in all stages of pregnancy.
There is a well-established relationship between increasing age and increased prevalence of both GDM and Type 2 DM (in non-pregnant adults) (Cowie et al., 2006; McFarland & Case, 1985). This appears to be congruent with our results, as we found a much higher PGDM prevalence with older pregnant subjects. The same was found for GDM. In fact, as shown in Fig. 3, the prevalences of GDM and PGDM were each increased more than 10-fold when comparing subjects age 45+ to those aged 15–19 years old.
Both GDM and PGDM incidence increased during the study time period (2001–2007). Other studies have shown similar findings (Davenport, Campbell, & Mottola, 2010; Ferrara, 2007; Lawrence et al., 2008). One possible rationale for the increase in diabetic disease in pregnancy is that the average age of pregnant women is increasing. National Vital Statistics reports as well as a systematic review by Carolan, et. al both demonstrated that the average maternal age of patients undergoing their first pregnancies has risen significantly (Carolan & Frankowska, 2011; Martin et al., 2010).
Our findings were consistent with previously published studies showing that the races/ethnicities at highest risk for GDM are Hispanics, Asians, and Native Americans (Berkowitz, Lapinski, Wein, & Lee, 1992; Caughey, Cheng, Stotland, Washington, & Escobar, 2010;Ferrara, 2007). The races at highest risk of PGDM in our study were Native Americans, Hispanics, and blacks, while Asians and Caucasians had the lowest risk. These findings differ slightly from those found in the literature. The Center for Disease Control’s 2011 National Diabetes Fact Sheet, identified using current U.S. population data that Asians, Hispanics and blacks have the highest risks of DM (Centers for Disease Control and Prevention, 2011). A large prospective study using Nurses’ Health Study data found that races/ethnicities at the highest risk of developing adult-onset type 2 DM were Asians, Hispanics, and blacks. The population age was comprised of an older population, with a mean age at identification of 66 years (Shai et al., 2006). Our population was different since it was comprised of younger, reproductive age, pregnant females. Subjects who present with PGDM in pregnancy are more likely to have a mixture of type 1 DM (which has an earlier age at onset) in addition to type 2 DM. McElduff et al. looked at a group of 180 PGDM pregnancies and found that slightly more (55%) were type 2 as opposed to type 1 DM (McElduff et al., 2005). This may explain the difference in our findings from those of the older adult-onset type 2 DM subjects in the aforementioned studies.
We also demonstrated that Asians had the highest prevalence of GDM but one of the lowest prevalences of PGDM (see Fig. 4). A higher GDM prevalence in Asians has been demonstrated in the literature previously. Some publications have also shown evidence of worse outcome in Asian PGDM subjects compared to their GDM counterparts (Ferrara, 2007; Jayathilaka, Dahanayake, Abewardhana, Ranaweera, & Rishard, 2012; Shefali, Kavitha, Deepa, & Mohan, 2006) Our findings suggest that races with the highest predilection for diabetes during pregnancy may not necessarily have the highest racial predilection for type 2 or type 1 DM. To our knowledge, this is the first time that such racial/ethnic comparisons have been made between PGDM and GDM in a single population.
There are several limitations to a study of this large retrospective database nature. It has been suggested that in large population studies, the actual strength of association may be overestimated by the calculated odds ratio (Davies, Crombie, & Tavakoli, 1998). Thus, ORs especially in the lower range (e.g. OR 1 to 2) should be interpreted with caution, as they may not be as clinically significant. However, our subgroup analysis of teenage deliveries with significantly smaller comparison group sizes of 545 (PGDM) and 2495 (GDM) still demonstrates some very strong associations with PGDM morbidity. This may help confirm the validity of some of the results of our study.
We also acknowledge that in studies such as ours based on ICD-9-CM coding, underreporting of conditions and data quality issues may be present. Yasmeen et al. performed a validation study using OSHPD data from 1992 to 1993 and found that coding was found to be reasonably accurate in conditions such as gestational diabetes, preeclampsia, chorioamnionitis, and preterm labor. Gestational diabetes coding validity was found to have a sensitivity of 78% and positive predictive value of 94%(Yasmeen, Romano, Schembri, Keyzer, & Gilbert, 2006 Apr). A study looking at hospital morbidity data by Taylor et al. found in a sample of 1000 maternal and neonatal charts that there was an extremely high accuracy in ICD-10 coding for gestational diabetes, with a sensitivity and specificity of 95.5% and 98.8% respectively (Taylor, Travis, Pym, Olive, & Henderson-Smart, 2005). This provides some assurance that case coding should still be relatively reflective of the true prevalence in our study population.
The California OSHPD protects patient record confidentiality by unique combinations of a select set of demographic masking certain variables — this resulted in a large portion of our original cohort being eliminated simply due to missing age, race/ethnicity data. Finally, we acknowledge that certain covariates such as level of glycemic control, presence of obesity, type of diabetes, parity, and certain socioeconomic aspects could not be obtained using the data available and thus could not be used in our logistic regression models. We were unable to ascertain screening method, which may alter GDM prevalence depending on the diagnostic testing used (e.g. potentially a higher rate of diagnosis using testing recommended by the International Association of the Diabetes and Pregnancy Study Group) (Visser & de Valk, 2013). Neonatal outcomes and breakdown of PGDM type (e.g. type 1 and type 2) were not possible to assess as well.
The strength of our study lies in its sample size. The dataset, having been derived from statewide discharge data, is comprehensive of the entire diverse California population, including deliveries of all risk stratifications. A study of this sample size also allows sufficient power for precise assessment of rare outcomes. Our prevalence of GDM and PGDM studies is overall quite congruent with other results cited in the literature. Finally, the strength of association is adjusted using logistic regression analysis.
In conclusion, we have shown in a large California cohort that both GDM and PGDM appear to be rising, and that PGDM appears to have certain demographic associations as well as increased clinical morbidity compared to GDM. We believe that our results serve as an assessment of several key differences between PGDM and GDM in a large population based study.
Footnotes
The authors report no conflict of interest.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jdiacomp.2013.08.009.
References
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S88–S90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Lapinski RH, Wein R, Lee D. Race/ethnicity and other risk factors for gestational diabetes. American Journal of Epidemiology. 1992;135(9):965–973. doi: 10.1093/oxfordjournals.aje.a116408. [DOI] [PubMed] [Google Scholar]
- Carolan M, Frankowska D. Advanced maternal age and adverse perinatal outcome: A review of the evidence. Midwifery. 2011;27(6):793–801. doi: 10.1016/j.midw.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Caughey AB, Cheng YW, Stotland NE, Washington AE, Escobar GJ. Maternal and paternal race/ethnicity are both associated with gestational diabetes. American Journal of Obstetrics and Gynecology. 2010;202(6):616e1–616e5. doi: 10.1016/j.ajog.2010.01.082. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States. Atlanta, GA US: Department of Health and Human Services CfDCaP; 2011. [Google Scholar]
- Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- Davenport MH, Campbell MK, Mottola MF. Increased incidence of glucose disorders during pregnancy is not explained by pre-pregnancy obesity in London, Canada. BMC Pregnancy and Childbirth. 2010;10:85. doi: 10.1186/1471-2393-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. 1998;316(7136):989–991. doi: 10.1136/bmj.316.7136.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl. 1):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mallah KO, Narchi H, Kulaylat NA, Shaban MS. Gestational and pre-gestational diabetes: Comparison of maternal and fetal characteristics and outcome. International Journal Of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics. 1997;58(2):203–209. doi: 10.1016/s0020-7292(97)00084-2. [DOI] [PubMed] [Google Scholar]
- Ferrara A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care. 2007;30(Suppl 2):S141–S146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- ICD-9-CM: International classification of diseases, 9th revision; clinical modification, 6th edition/ Practice Management Information Corporation (PMIC) Los Angeles: 2006. 2005. Edition Hospital edition. [Google Scholar]
- Jayathilaka K, Dahanayake S, Abewardhana R, Ranaweera A, Rishard M. Diabetes in pregnancy among Sri Lankan women: Gestational or pre-gestational? Sri Lanka Journal of Diabetes Endocrinology and Metabolism. 2012;1(1):8–13. [Google Scholar]
- Landon MB, Mele L, Spong CY, Carpenter MW, Ramin SM, Casey B, et al. The relationship between maternal glycemia and perinatal outcome. Obstetrics and Gynecology. 2011;117(2 Pt 1):218–224. doi: 10.1097/aog.0b013e318203ebe0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31(5):899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- Macintosh MC, Fleming KM, Bailey JA, Doyle P, Modder J, Acolet D, et al. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: Population based study. BMJ. 2006;333(7560):177. doi: 10.1136/bmj.38856.692986.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Osterman MJ, et al. Births: Preliminary data for 2010. National vital statistics reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System, 2011. 2010;60(2) [Google Scholar]
- McElduff A, Ross GP, Lagstrom JA, Champion B, Flack JR, Lau SM, et al. Pregestational diabetes and pregnancy: An Australian experience. Diabetes Care. 2005;28(5):1260–1261. doi: 10.2337/diacare.28.5.1260. [DOI] [PubMed] [Google Scholar]
- McFarland KF, Case CA. The relationship of maternal age on gestational diabetes. Diabetes Care. 1985;8(6):598–600. doi: 10.2337/diacare.8.6.598. [DOI] [PubMed] [Google Scholar]
- Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. The New England Journal of Medicine. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: A large, population-based study. Diabetes Care. 2009;32(11):2005–2009. doi: 10.2337/dc09-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JG, Vermeulen MJ, Shapiro JL, Kenshole AB. Maternal and neonatal outcomes in pregestational and gestational diabetes mellitus, and the influence of maternal obesity and weight gain: The DEPOSIT study. Diabetes Endocrine Pregnancy Outcome Study in Toronto. QJM: Monthly Journal of the Association of Physicians. 2001;94(7):347–356. doi: 10.1093/qjmed/94.7.347. [DOI] [PubMed] [Google Scholar]
- Shai I, Jiang R, Manson JE, Stampfer MJ, Willett WC, Colditz GA, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: A 20-year follow-up study. Diabetes Care. 2006;29(7):1585–1590. doi: 10.2337/dc06-0057. [DOI] [PubMed] [Google Scholar]
- Shefali AK, Kavitha M, Deepa R, Mohan V. Pregnancy outcomes in pregestational and gestational diabetic women in comparison to non-diabetic women —A prospective study in Asian Indian mothers (CURES-35) The Journal of the Association of Physicians of India. 2006;54:613–618. [PubMed] [Google Scholar]
- Taylor LK, Travis S, Pym M, Olive E, Henderson-Smart DJ. How useful are hospital morbidity data for monitoring conditions occurring in the perinatal period? The Australian & New Zealand Journal of Obstetrics & Gynaecology. 2005;45(1):36–41. doi: 10.1111/j.1479-828X.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- Vambergue A, Valat AS, Dufour P, Cazaubiel M, Fontaine P, Puech F. Pathophysiology of gestational diabetes. Journal de Gynécologie, Obstétrique et Biologie de la Reproduction. 2002;31(6 Suppl):4S3–4S10. [PubMed] [Google Scholar]
- Visser GH, de Valk HW. Is the evidence strong enough to change the diagnostic criteria for gestational diabetes now? American Journal of Obstetrics and Gynecology. 2013;208(4):260–264. doi: 10.1016/j.ajog.2012.10.881. [DOI] [PubMed] [Google Scholar]
- Wier LM, Witt E, Burgess J, Elixhauser A. Hospitalizations related to diabetes in pregnancy, 2008: Statistical brief #102. Rockville (MD): Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. 2006 [PubMed] [Google Scholar]
- Women's health fact sheet. No 334. Vol. 2009. World Health Organization; 2009. Nov, [Google Scholar]
- Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. American Journal of Obstetrics and Gynecology. 2006 Apr;194(4):992–1001. doi: 10.1016/j.ajog.2005.08.058. [DOI] [PubMed] [Google Scholar]