Abstract
Purpose
The purpose of this trial was to evaluate the effect of a Web-based, self-report assessment and educational intervention on symptom distress during cancer therapy.
Patients and Methods
A total of 752 ambulatory adult participants were randomly assigned to symptom/quality-of-life (SxQOL) screening at four time points (control) versus screening, targeted education, communication coaching, and the opportunity to track/graph SxQOL over time (intervention). A summary of the participant-reported data was delivered to clinicians at each time point in both groups. All participants used the assessment before a new therapeutic regimen, at 3 to 6 weeks and 6 to 8 weeks later, completing the final assessment at the end of therapy. Change in Symptom Distress Scale–15 (SDS-15) score from pretreatment to end of study was compared using analysis of covariance and regression analysis adjusting for selected variables.
Results
We detected a significant difference between study groups in mean SDS-15 score change from baseline to end of study: 1.27 (standard deviation [SD], 6.7) in the control group (higher distress) versus −0.04 (SD, 5.8) in the intervention group (lower distress). SDS-15 score was reduced by an estimated 1.21 (95% CI, 0.23 to 2.20; P = .02) in the intervention group. Baseline SDS-15 score (P < .001) and clinical service (P = .01) were predictive. Multivariable analyses suggested an interaction between age and study group (P = .06); in subset analysis, the benefit of intervention was strongest in those age > 50 years (P = .002).
Conclusion
Web-based self-care support and communication coaching added to SxQOL screening reduced symptom distress in a multicenter sample of participants with various diagnoses during and after active cancer treatment. Participants age > 50 years, in particular, may have benefited from the intervention.
INTRODUCTION
Including self-reported cancer symptoms and quality-of-life (SxQOL) concerns in cancer care has increased over the last three decades for several reasons: focus on patient-reported outcomes (PROs) in clinical trials for product development, the need to adjust therapeutic doses based on toxicities, the number of patients with complex multimodal therapies, the emphasis on patient-centered care, and the shift from inpatient to ambulatory and home administration of therapy. Simultaneously, resource constraints have reduced face-to-face patient/clinician time. Web-based SxQOL assessment and support of patients during active cancer treatment offer the potential to efficiently and conveniently collect PROs for both research and clinical use as well as to improve patient outcomes. In addition to our own prior randomized trial,1 pilot studies2 and randomized trials in non-US settings3–5 have shown SxQOL clinical screening to be feasible and clinically beneficial with regard to communication and patient outcomes.
In the first Electronic Self-Report Assessment–Cancer (ESRA-C) randomized clinical trial,1 we demonstrated the feasibility, acceptability, and efficacy of computerized SxQOL screening at a large comprehensive cancer center in Seattle, Washington, increasing the frequency of patient/clinician communication about problematic issues as measured in audiorecorded clinic visits. We did not collect SxQOL outcome data past the visit targeted for intervention and could not evaluate impact of the screening plus clinician summary on later SxQOL outcomes. Moreover, we found that even when clinicians received summaries of patient-reported SXQOL, the most frequently addressed issues were those either regulated by certification bodies (eg, pain) or likely to be affected by supportive care medications previously ordered by the clinician (eg, nausea with antiemetics). These issues were discussed whether or not the patient had problems, whereas other issues reported as problematic by the patient (eg, insomnia) often were left unaddressed. We designed a new trial in which the clinician summary intervention would be delivered for all participants, and the new intervention would support patients directly through self-care strategies and communication of priority issues. The new intervention offered tailored education, communication coaching, and SxQOL tracking and was accessible from home at times convenient to patient users.
PATIENTS AND METHODS
Study Design and Participants
ESRA-C II was a prospective, randomized clinical trial designed to determine the effect of a patient education and coaching intervention added to a self-report assessment with clinician summary on SxQOL outcomes throughout a new therapeutic regimen for patients with various cancer diagnoses. The quality health outcomes model6 informed our trial design. The authors posited that system and provider variables could influence the outcome of practically every intervention delivered. Thus, we enhanced the usual care system with self-report and a clinician summary and measured many potentially influential aspects (eg, use of intervention at home or clinic) and face-to-face communication patterns between patients and providers.7
The study was conducted in ambulatory care of two comprehensive cancer centers between April 2009 and June 2011 with approval by the institutional review boards of the Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium and the Dana-Farber Cancer Institute. Eligible patients had a diagnosis of cancer, were ambulatory and age ≥ 18 years, were starting a new therapeutic regimen, and spoke/read English (Fig 1). Patients were recruited from the transplantation service in one cancer center (Seattle, WA) and from medical and radiation oncology in the other (Boston, MA). Baseline ESRA-C assessments were offered to all patients meeting eligibility criteria, accessible from a home computer or touchscreen notebook in clinic waiting areas. Patients were queried on screen for interest in a study about the computer program. Research staff obtained written informed consent, which included use of the baseline questionnaire (T1) as research data. The initial response rate was lower than expected; after 14 months, we changed to in-person recruitment.
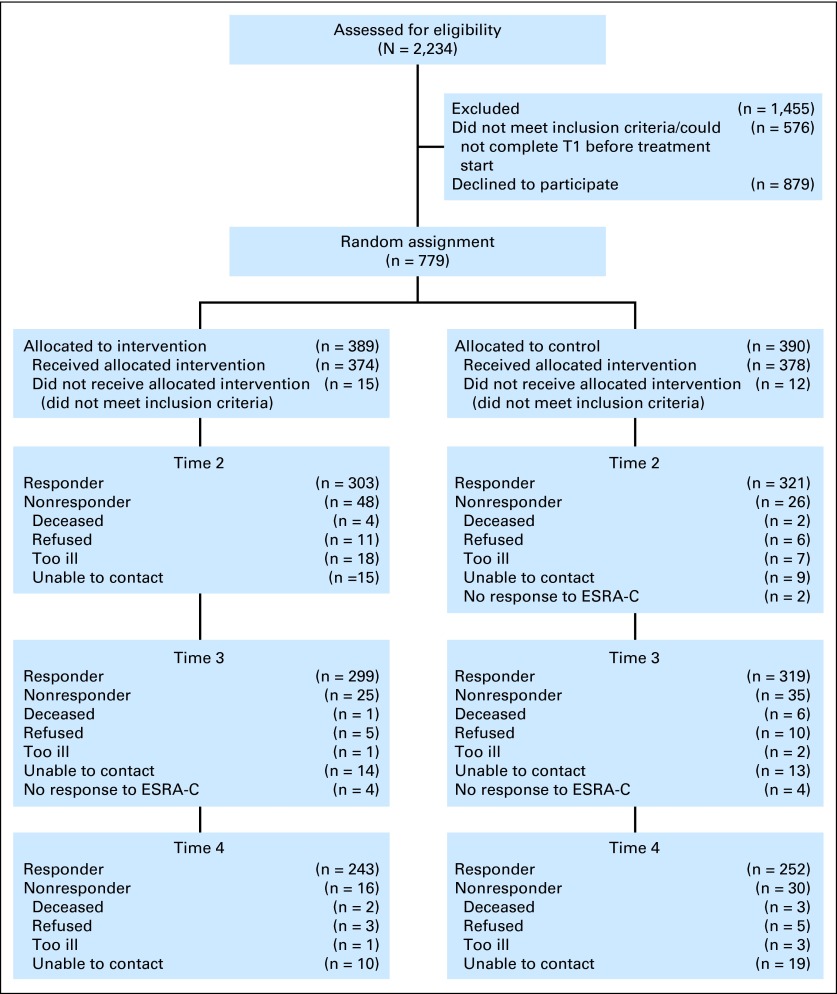
Fig 1.
CONSORT diagram; study exclusion and inclusion, random assignment, and follow-up information. Electronic Self-Report Assessment–Cancer (ESRA-C).
Participants self-identified as home or clinic users, and within these strata, the computer application randomly allocated them at a ratio of one to one in blocks of four to the intervention or control group. Participants in the intervention group were given a brief tutorial on using the self-administered intervention; those without home computers could use the intervention in clinic at any time. Home users received a 1-week follow-up telephone call to answer questions and remind them of the available intervention features.
Participants completed a second assessment (T2) approximately 3 to 6 weeks after starting treatment when symptoms were likely to be prevalent. A third assessment (T3) was scheduled 2 weeks later to assess participants' response to T2 problem SxQOL issues. The final assessment (T4) was planned 2 to 4 weeks after treatment ended. During the study, the decision was made to administer a T4 assessment to those participants who would continue to receive treatment indefinitely (eg, palliative treatment, maintenance therapy) at the next restaging visit.
Intervention and Control Conditions
Intervention-group participants used the new intervention to self-assess SxQOL not only when prompted at each study time point but also ad lib between visits. After each self-report, the program delivered three messages regarding SxQOL issues reported at a predetermined threshold (Appendix Table A1, online only): first, why and how often this issue typically occurs; second, what can be done; and third, how to talk to your clinical team about the issue. Participants were coached to verbalize specifics tailored for each SxQOL issue to providers: first, how often and when the issue occurs; second, intensity; third, alleviating or aggravating factors; and fourth, request help. Additional features alerted patients to call providers right away when levels of symptom distress, depression, and/or pain were severe or when any suicidal ideation was reported in between clinic visits. An illustration of the intervention components is provided in Figure 2.
Fig 2.
Illustration of the Electronic Self-Report Assessment–Cancer II patient-centered intervention. SQI, self-reported cancer symptom and quality-of-life issue.
Participants could visualize graphed responses over time for each of 24 SxQOL issues, whether at threshold levels or not, and annotate the results using a journal feature. All intervention participants could explore self-care strategies and coaching for any SxQOL issue at any time.
Control-group participants completed the same SxQOL assessments, but only at each study time point; summary reports were delivered to clinicians as in the intervention group. Research staff verbally notified the provider of any severe levels of depression and/or pain reported at the time of the clinic visit. Both groups were provided the same patient education as was typically available in each clinic.
Measures
The ESRA-C questionnaires screen for cancer-related SxQOL issues: symptom distress, quality of life, depression, peripheral neuropathy, skin changes, and pain. The assessment has been described previously.1 The primary outcome for the study was symptom distress, a construct that addresses multiple symptoms common across cancer diagnoses. For this trial, we used the original 13-item Symptom Distress Scale (SDS)8 plus two items (impact on sexual activity and interest, fever/chills) to form the SDS-15. Each item had a 5-point response option from 1 (no or minimal distress) to 5 (maximal distress). Item scores were summed for a total, unweighted scale score ranging from 15 to 75. Internal consistency of the SDS-15 was calculated as 0.83 at baseline and 0.86 at the end of study.
Statistical Analyses
Our primary end point was change in SDS-15 total score from baseline to the end-of-study time point (T4); if a patient had finished treatment before T3, T4 was not administered, and the T3 score was used. With a target of 315 patients in each arm (630 patients total), the study was designed to detect an effect size of 0.26 with 90% power, at the two-sided significance level of .05 with a t test. Given the attrition rate of 11% in the previous study,1 the target enrollment sample originally was 702. Because the observed attrition rate during this trial was approximately 20%, the study was amended to a maximum sample size of 796.
Baseline patient characteristics were summarized with descriptive statistics. Attrition was assumed missing at random and validated by checking baseline characteristics for participants with and without complete data.
Change in SDS-15 total score from baseline to end of study was calculated for each patient and compared between study groups using an analysis of covariance (ANCOVA) approach, linear regression adjusting for baseline SDS-15 score. All randomly assigned eligible patients with outcome data were included according to the intent-to-treat principle. As a process check, we calculated intervention access frequencies in server logs. Nonparametric smoothing techniques were used to explore the impact of baseline SDS-15 score and age on score change. Covariates previously identified as influencing symptom distress (age, clinical service, working status, and baseline SDS-15 score) were assessed with univariable analysis and then adjusted in multivariable analysis to improve the precision for estimating the intervention effect. Emotional functioning (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 [EORTC QLQ-C30]) and depression (Patient Health Questionnaire–Depression [PHQ9]) scores at baseline were also explored as covariates, but because the two variables were highly correlated with SDS-15 at baseline (data not shown), they were removed from the analysis. Possible two-way interactions were checked in the multivariable model. Type III P values were used to assess overall significance in all models. Because an age and study group interaction was suggested from the smoothing graph and multivariable regression model (P = .06), subset analyses were conducted within two age groups.
Participants lost to attrition before T4 were excluded from the analysis according to protocol. However, 37 were still undergoing therapy and had SDS-15 scores available at T3. Thus, we added these 37 to the analysis sample for a secondary sensitivity analysis (n = 618) using the last available SDS-15 score (T3) to test the impact of attrition on outcome. All analyses were conducted using SAS (version 9.2; SAS Institute, Cary, NC) and R software (http://www.r-project.org); all statistical tests were two sided at a significance level of .05.
RESULTS
We enrolled and randomly assigned 779 participants (Fig 1). Twenty-seven enrolled participants were found to be ineligible after random assignment. Table 1 lists demographic and clinical characteristics of the eligible study sample (n = 752). There were no significant differences in age, sex, race/ethnicity, disease stage, or attrition among participants recruited on screen or by personal contact (data not shown). The study groups were well balanced except for age; participants were younger in the intervention group compared with the control group (P = .04). A large majority (86%) of patients enrolled as home users. Completion rates for end-of-study outcomes were similar for both arms (control, 77.2%; intervention, 77.3%).
Table 1.
Demographic and Baseline Clinical Characteristics of Eligible Participants (N = 752)
| Characteristic | Control (n = 378) |
Treatment (n = 374) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years* | ||||
| Median | 59 | 56 | ||
| Range | 19-88 | 22-86 | ||
| Age group, years | ||||
| < 50 | 90 | 24 | 126 | 34 |
| ≥ 50 | 288 | 76 | 248 | 66 |
| Sex | ||||
| Male | 204 | 54 | 185 | 50 |
| Female | 174 | 46 | 189 | 50 |
| Ethnicity/race | ||||
| Minority | 27 | 7 | 33 | 9 |
| Missing | 43 | 11 | 37 | 10 |
| Working status | ||||
| Working† | 222 | 59 | 222 | 60 |
| Not working | 113 | 30 | 123 | 33 |
| Missing | 43 | 11 | 29 | 8 |
| Clinical service | ||||
| Medical oncology | 210 | 56 | 211 | 56 |
| Radiation oncology | 117 | 31 | 125 | 33 |
| Hematopoietic stem-cell transplantation | 51 | 14 | 38 | 10 |
| Cancer diagnosis | ||||
| Breast | 97 | 26 | 109 | 30 |
| Colorectal | 31 | 8 | 34 | 9 |
| Pancreatic | 10 | 3 | 4 | 1 |
| Esophageal | 7 | 2 | 11 | 3 |
| Gastric | 5 | 1 | 3 | 1 |
| GI, other‡ | 13 | 3 | 16 | 4 |
| Head and neck | 26 | 7 | 24 | 6 |
| Prostate | 60 | 16 | 62 | 17 |
| Bladder | 13 | 3 | 10 | 3 |
| Renal cell | 4 | 1 | 9 | 2 |
| Testicular | 11 | 3 | 9 | 2 |
| Sarcoma | 15 | 4 | 19 | 5 |
| Leukemia | 22 | 6 | 14 | 4 |
| Myeloma | 17 | 4 | 13 | 4 |
| Non-Hodgkin lymphoma | 21 | 6 | 23 | 6 |
| Hodgkin lymphoma | 11 | 3 | 6 | 2 |
| Other§ | 10 | 3 | 7 | 2 |
| Unknown primary | 5 | 1 | 1 | 0.3 |
| Group stage (solid tumors) | ||||
| 0 (DCIS) | 9 | 3 | 3 | 0.6 |
| I | 58 | 19 | 52 | 16 |
| II | 71 | 23 | 86 | 27 |
| III | 51 | 17 | 66 | 21 |
| IV | 111 | 36 | 101 | 32 |
| Missing | 7 | 2 | 10 | 3 |
Abbreviation: DCIS, ductal carcinoma in situ.
P = .04.
Working includes: full time, part time, working at home, on medical leave, or any one of these categories plus retired.
Adenocarcinoma (appendix, small bowel, ampullary gland, duodenum, gallbladder), Bismuth IV Klatskin tumor, carcinoid tumor (small bowel), cholangiocarcinoma, GI stromal tumor, mucinous neoplasm (appendix), and pancreatic neuroendocrine.
Carcinoid tumor (unknown primary origin, bronchial), Merkel cell carcinoma, retroperitoneal germ cell tumor, and Wilms tumor.
A total of 581 eligible participants (17% attrition rate) who completed the study per protocol comprised the target analytic sample, including those who answered at T4 and those who completed cancer treatment before T3. Participants missing full SDS-15 scores at baseline (n = 44) or end of study (n = 22) were removed from the main analysis, for a final sample of 523. Participants with missing data were older (P = .0002) and from minority backgrounds (P = .06). Table 2 summarizes the available sample sizes and SDS-15 scores for each time point.
Table 2.
Means and SDs of SDS-15 Scores and Sample Sizes at Each Time Point (n = 581)
| Group | T1 (baseline) |
T2 |
T3/4 (end of study) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | |
| Control (n = 292) | 268 | 24.1 | 6.8 | 269 | 26.6 | 7.5 | 282 | 25.4 | 7.9 |
| Treatment (n = 289) | 269 | 24.3 | 6.7 | 264 | 26.6 | 7.7 | 277 | 24.2 | 6.7 |
Abbreviations: SD, standard deviation; SDS-15, Symptom Distress Scale–15.
Participants randomly assigned to the intervention arm had lower symptom distress; mean change in SDS-15 score was 1.27 (standard deviation [SD], 6.7) in the control group (higher distress) and −0.04 (SD, 5.8) in the intervention group (lower distress). In our primary ANCOVA analysis, SDS-15 score was reduced by an estimated 1.21 (95% CI, 0.23 to 2.20; P = .02) in the intervention group versus the control group. For the 289 intervention participants, ESRA-C server logs revealed a median access rate of four (range, two to four) at study time points and of one (range, zero to eight) at voluntary times. One outlier (66 voluntary access sessions) was removed from the calculation.
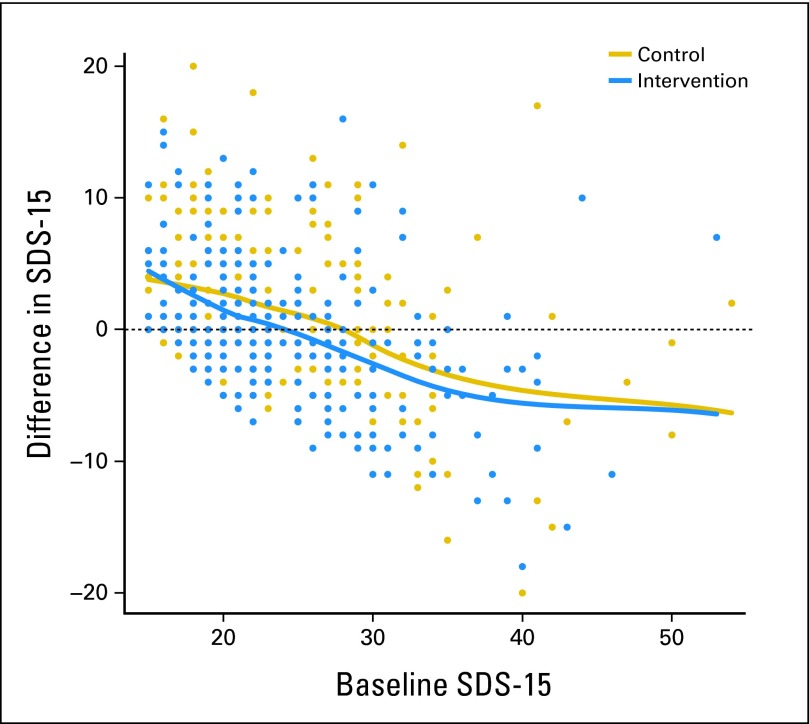
Study group and age were significantly associated with SDS-15 score change in univariable analyses (Appendix Table A2, online only). In multivariable analysis of SDS-15 score change, the intervention effect remained significant (95% CI, −2.07 to −0.03; P = .04; Appendix Table A2). In addition, clinical service and baseline SDS-15 score were significantly predictive. Score changes indicating lower symptom distress were evident for the treatment group and participants treated with radiation therapies. Visual inspection of results from the smoothing techniques (Fig 3) suggested that the higher the baseline symptom distress (SDS-15 score), the greater the reduction in SDS-15 score by end of study, although those with very low baseline values had increased symptom distress by end of study.
Fig 3.
Effect of baseline Symptom Distress Scale–15 (SDS-15) score on SDS-15 score change between baseline and end of study.
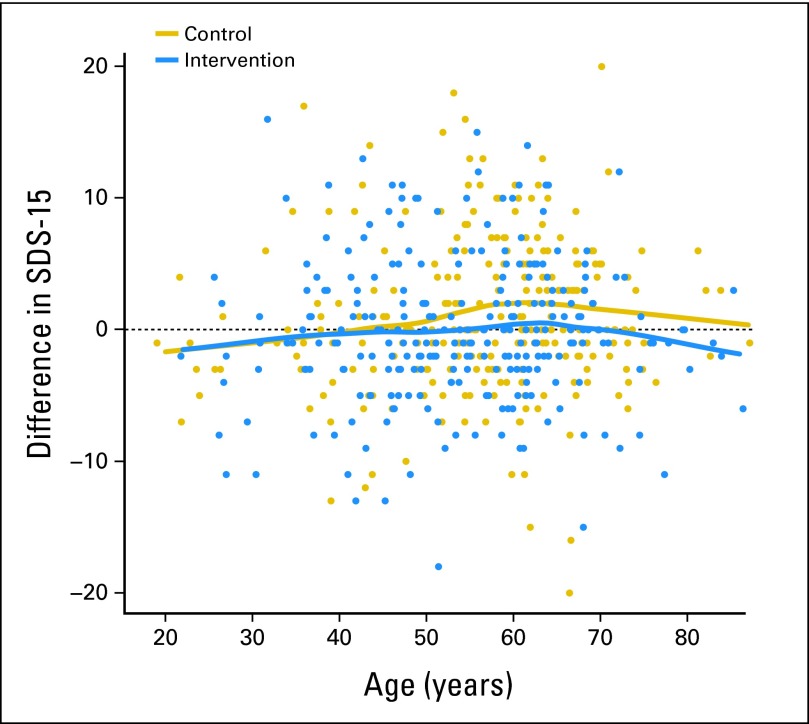
The only interaction suggested by multivariable analysis was between age and study group (P = .06); this was explored with smoothing techniques. The results shown in Figure 4 suggested there was minimal difference in SDS-15 score change between control and intervention groups for younger participants (age < 50 years), whereas the difference was evident among older participants (age ≥ 50 years). For the group of older participants, SDS-15 score seemed to increase from baseline to end of study in the control group, whereas it remained stable in the intervention group (Fig 4).
Fig 4.
Effect of age on Symptom Distress Scale–15 (SDS-15) score change between baseline and end of study.
We performed a subset multivariable regression analysis, using the ≥ 50 years of age mark suggested by the smoothing curve, and a statistically significant intervention effect (Table 3) was identified among participants age ≥ 50 years; a reduction of an average 1.93 points in SDS-15 score change was seen in the intervention group compared with the control group (95% CI, −3.16 to −0.70; P = .002). There was no significant intervention effect detected among participants age < 50 years (P = .40). Clinical service was significantly associated with SDS-15 score change only among older participants (P = .01), and working status was significant only in the younger group (P = .04).
Table 3.
Multivariable Regression Analysis of SDS-15 Score Change by Age (n = 523)
| Variable | Age < 50 Years (n = 162) |
Age ≥ 50 Years (n = 361) |
||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Estimate | 95% CI | P | |
| Study group (intervention v control) | 0.87 | −0.94 to 2.67 | .4 | −1.93 | −3.16 to −0.70 | .002 |
| Age* | 0.05 | −0.07 to 0.17 | .4 | −0.01 | −0.10 to 0.077 | .8 |
| Service | .3 | .01 | ||||
| HSCT v radiation oncology | 0.44 | −2.88 to 3.75 | .8 | −0.21 | −2.72 to 2.30 | .9 |
| Medical oncology v radiation oncology | 1.57 | −0.51 to 3.65 | .1 | 1.85 | 0.55 to 3.14 | .005 |
| Work status (not working v other) | 2.26 | 0.08 to 4.43 | .04 | 0.87 | −0.55 to 2.28 | .2 |
| SDS-15 baseline | −0.27 | −0.40 to −0.14 | < .001 | −0.43 | −0.53 to −0.34 | < .001 |
Abbreviations: HSCT, hematopoietic stem-cell transplantation; SDS-15, Symptom Distress Scale–15.
Continuous variable.
Sensitivity analyses revealed a difference by treatment group in SDS-15 score change between baseline and end of study (estimate, 1.21; 95% CI, 0.23 to 2.20; P = .02), similar to the difference in the primary analysis. The intervention effect remained significantly (P = .04) associated with SDS-15 score change in multivariable analysis, and it significantly interacted with age (P = .05; Appendix Table A3, online only); the intervention effect was significant (P = .001) among older but not younger participants (P = .20; Appendix Table A4, online only).
DISCUSSION
Use of the ESRA-C patient-centered intervention resulted in lower symptom distress when compared with symptom distress under control conditions over the course of ambulatory cancer treatment in a sample of adult patients of various cancer diagnoses and stages at two comprehensive cancer centers in the United States. ESRA-C access frequencies in the intervention-group participants indicated interest in remote access between clinic visits. These findings extend the potential efficacy of the ESRA-C program, with the addition of the patient-centered intervention (ESRA-C II) to the clinician-centered intervention of the ESRA-C I trial (control condition in ESRA-C II).
Our organizing framework, the health outcomes model,6 has been confirmed in part with our results. Patient variables, age in particular, influenced the effect of the intervention on outcomes. In an earlier analysis, we found that patient self-appraisal of change in quality of life was influential with regard to quality-of-life outcomes.9
Other randomized controlled trial results are similar; however, these trials involved primarily samples outside of the United States and were limited to single institutions or selected diagnoses.3–5 The one US randomized trial10 was a nurse-led, telephone-based intervention based on pain and depression algorithms delivered to patients with cancer from 16 urban and rural care sites in Indiana. The intervention resulted in significantly lower pain and depression in patients who had reported moderate to severe pain and/or depression at enrollment.
Although the statistically significant difference in change between study groups was small, it was realized over the likely positive impact of the summary clinician report for both groups. What is the meaning of an SDS-15 score change difference of 1.21 for the entire sample or of 1.93 (adjusted model) in those age > 50 years? A difference of 1 to 2 points could reflect the difference between intensity or distress from one symptom or a 1-point change on two separate items. Reducing symptom distress, even at a small magnitude, with an intervention that engages patients in self-care and adds no time to a clinic visit is patient-centered care in action.11
Ruland et al4 in Norway reported a small significant difference in global symptom distress measure by the Memorial Symptom Assessment Scale–Short Form12 when testing a Web-based symptom reporting and support system in those with breast or prostate cancer. As with our findings, variability in stage and diagnosis likely had an impact on effect size. There are many descriptive studies using the SDS, but few randomized studies published in which SDS score was the primary outcome and was significantly different between groups.
There are several plausible explanations for finding the stronger intervention effect in older participants. Middle-age and older adults may have needed more support to access reliable and focused self-care information on the Internet than younger participants. Furthermore, older participants may have benefited from communication coaching, whereas the younger participants in both groups may have been more inclined to articulate SxQOL and ask more questions, as has been found in other communication research.13,14
Generalizing our results outside of a comprehensive cancer center is questionable; the results are likely to be associated more with the demographics of patients who seek care at such a center than the setting itself. A majority of our participants had and used Internet access at home or work. Patients who refused participation may have lacked familiarity with technology. Although only a small percent of the sample had missing data on the SDS, older and minority individuals were more likely to skip items.
Racial and ethnic diversity rates in our mainly New England sample were lower than the Massachusetts population, in which approximately 19% report minority race or ethnicity.15 Our study results cannot be generalized to non–English speaking patients. Our interpretation of the effect of clinical service is limited by that the overlap of that variable with accrual site. Differences we attributed to service may have resulted from other clinical or patient population characteristics of the accruing sites.
Screening for psychosocial problems in patients with cancer has been included in the American College of Surgeons and Commission on Cancer 2012 Cancer Program standards.16 The ESRA-C system addresses such screening needs, common cancer-specific physical symptoms and adverse effects, and self-care needs of patients. Combining screening, communication coaching, and automated, tailored self-care information adds value to the required screening and does so with minimal staff involvement. ESRA-C may facilitate integrated organizational processes that link identified needs to coordinated, patient-centered treatment and tracking of clinical outcomes.17 Because ESRA-C provides patients with the opportunity to self-identify priority SxQOL issues among items scored at the moderate to high distress level, two important types of information can be used clinically: the current level of symptomatology and the patient's self-appraisal of the problem. In the future, ESRA-C can be adapted for the specific needs of a clinic patient population and revised for deployment and use on a variety of remote devices.
Future analyses and studies could examine the mechanisms by which communication coaching reduces symptom distress and the individual characteristics predisposing patients to benefit from the intervention, as well as dosing effects. Other outcomes relevant to symptomatology should be evaluated, such as emergency room visits and clinic resource use.
In conclusion, adding self-care support and educational messages to the ESRA-C assessment program reduced symptom distress in a multicenter sample of participants with various diagnoses during and after active cancer treatment. Participants age > 50 years, in particular, may have benefited from the intervention.
Supplementary Material
Appendix
Table A1.
SxQOL Issues Assessed During ESRA-C Study
| SxQOL Issue | Instrument Used for Assessment | Score Used for Intervention |
|---|---|---|
| Nausea frequency | SDS (original 13-item scale)* | Each item was scored 1 (lowest) to 5 (highest) in severity; scores ≥ 3 were color coded as problematic |
| Nausea intensity | ||
| Appetite | ||
| Insomnia | ||
| Pain frequency | ||
| Pain intensity | ||
| Fatigue | ||
| Bowel troubles | ||
| Concentration | ||
| Appearance | ||
| Breathing | ||
| Fear and worry | ||
| Cough | ||
| Impact on sexuality | Additional items in SDS-15 | |
| Fever and chills | ||
| Pain intensity | Numeric 0-10 rating scale | Scores ≥ 5 were color coded as problematic |
| Depression | PRIME-MD PHQ9† | Scores of 10 (moderate depression) or higher were color coded as problematic |
| Physical functioning | EORTC QLQ-C30 (version 3)‡ | Each scale (two to five items) was scored 0 (worst) to 100 (best); scores < 50 were color coded as problematic |
| Emotional functioning | ||
| Social functioning | ||
| Cognitive functioning | ||
| Role functioning (work and leisure) | ||
| Sensory neuropathy | EORTC QLQ-CIPN20§ | Each scale (three to eight items) was scored 0 (best) to 100 (worst); scores ≥ 50 were color coded as problematic |
| Motor neuropathy | ||
| Autonomic neuropathy | ||
| Skin problems | Adapted‖ | One item rated severity of skin problems from 1 (not present) to 6 (very severe); scores of 3 (mild) or higher were color coded as problematic |
Abbreviations: EORTC, European Organisation for Research and Treatment of Cancer; ESRA-C, Electronic Self-Report Assessment–Cancer; PHQ9, Patient Health Questionnaire–Depression; PRIME-MD, Primary Care Evaluation of Mental Disorders; QLQ-C30, Quality of Life Questionnaire–Core 30; QLQ-CIPN20, Quality of Life Questionnaire–Chemotherapy-Induced Peripheral Neuropathy; SDS, Symptom Distress Scale; SxQOL, cancer symptoms and quality of life.
McCorkle R: Semin Oncol Nurs 3:248-256, 1987.
Kroenke K, et al: J Gen Intern Med 16:606-613, 2001.
Aaronson NK, et al: J Natl Cancer Inst 85:365-376, 1993.
Postma TJ, et al: Eur J Cancer 41:1135-1139, 2005.
Ryan JL, et al: Br J Cancer 97:14-21, 2007.
Table A2.
Univariable and Multivariable Regression Analyses of SDS-15 Score Change (n = 523)
| Variable | Univariable Analysis |
Multivariable Analysis |
||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Estimate | 95% CI | P | |
| Study group (intervention v control) | −1.30 | −2.36 to −0.24 | .016 | −1.05 | −2.07 to −0.03 | .04 |
| Age | 0.04 | 0.0003 to 0.086 | .05 | 0.03 | −0.01 to 0.08 | .12 |
| Service | .12 | .01 | ||||
| HSCT v radiation oncology | −0.71 | −2.84 to 1.42 | .51 | 0.30 | −1.71 to 2.30 | .77 |
| Medical oncology v radiation oncology | 0.94 | −0.19 to 2.07 | .10 | 1.60 | 0.49 to 2.71 | .005 |
| Work status (not working v other) | 0.37 | −0.83 to 1.58 | .54 | 1.03 | −0.13 to 2.19 | .08 |
| SDS-15 baseline | −0.35 | −0.42 to −0.27 | < .001 | −0.37 | −0.45 to −0.30 | < .001 |
Abbreviations: HSCT, hematopoietic stem-cell transplantation; SDS-15, Symptom Distress Scale–15.
Table A3.
Univariable and Multivariable Regression Analyses of SDS-15 Score Change in Sensitivity Analysis (n = 555)
| Variable | Univariable Analysis |
Multivariable Analysis |
||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Estimate | 95% CI | P | |
| Study group (intervention v control) | −1.14 | −2.19 to −0.08 | .04 | −1.04 | −2.05 to −0.03 | .04 |
| Age | 0.03 | −0.01 to 0.07 | .13 | 0.02 | −0.02 to 0.07 | .28 |
| Service | .01 | |||||
| HSCT v radiation oncology | −1.25 | −3.30 to 0.80 | .23 | −0.07 | −2.00 to 1.86 | .94 |
| Medical oncology v radiation oncology | 1.24 | 0.11 to 2.37 | .03 | 1.79 | 0.68 to 2.90 | .002 |
| Work status (not working v other) | 0.43 | −0.75 to 1.62 | .48 | 1.19 | 0.048 to 2.34 | .04 |
| SDS-15 baseline | −0.33 | −0.41 to −0.26 | < .001 | −0.37 | −0.44 to −0.30 | < .001 |
Abbreviations: HSCT, hematopoietic stem-cell transplantation; SDS-15, Symptom Distress Scale–15.
Table A4.
Multivariable Regression Analysis of SDS-15 Score Change by Age in Sensitivity Analysis (n = 555)
| Variable | Age < 50 Years (n = 177) |
Age ≥ 50 Years (n = 378) |
||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Estimate | 95% CI | P | |
| Study group (intervention v control) | 1.14 | −0.60 to 2.88 | .20 | −2.01 | −3.25 to −0.78 | .001 |
| Age | 0.02 | −0.09 to 0.14 | .68 | −0.03 | −0.12 to 0.057 | .48 |
| Service | .07 | .01 | ||||
| HSCT v radiation oncology | −0.58 | −3.78 to 2.61 | .72 | −0.06 | −2.48 to 2.35 | .96 |
| Medical oncology v radiation oncology | 1.91 | −0.14 to 3.95 | .07 | 1.91 | 0.61 to 3.22 | .004 |
| Work status (not working v other) | 2.29 | 0.18 to 4.40 | .03 | 1.13 | −0.28 to 2.54 | .12 |
| SDS-15 baseline | −0.26 | −0.38 to −0.14 | < .001 | −0.44 | −0.53 to −0.35 | < .001 |
Abbreviations: HSCT, hematopoietic stem-cell transplantation; SDS-15, Symptom Distress Scale–15.
Footnotes
Supported by Grant No. R01 NR008726 (2008-2010) from the National Institute of Nursing Research, National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00852852.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Donna L. Berry, Jesse R. Fann, Seth Wolpin, William B. Lober, Nigel E. Bush, Upendra Parvathaneni, Anthony L. Back, Rosemary Ford
Administrative support: Donna L. Berry, Barbara Halpenny, Rosemary Ford
Provision of study materials or patients: Donna L. Berry, Ann H. Partridge, Rosemary Ford
Collection and assembly of data: Donna L. Berry, Barbara Halpenny, Ann H. Partridge, Seth Wolpin, Dagmar Amtmann
Data analysis and interpretation: Donna L. Berry, Fangxin Hong, Barbara Halpenny, Ann H. Partridge, Jesse R. Fann, Seth Wolpin
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Berry DL, Blumenstein BA, Halpenny B, et al. Enhancing patient-provider communication with the electronic self-report assessment for cancer: A randomized trial. J Clin Oncol. 2011;29:1029–1035. doi: 10.1200/JCO.2010.30.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abernethy AP, Herndon JE, 2nd, Wheeler JL, et al. Feasibility and acceptability to patients of a longitudinal system for evaluating cancer-related symptoms and quality of life: Pilot study of an e/Tablet data-collection system in academic oncology. J Pain Symptom Manage. 2009;37:1027–1038. doi: 10.1016/j.jpainsymman.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. J Clin Oncol. 2004;22:714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 4.Ruland CM, Andersen T, Jeneson A, et al. Effects of an internet support system to assist cancer patients in reducing symptom distress: A randomized controlled trial. Cancer Nurs. 2013;36:6–17. doi: 10.1097/NCC.0b013e31824d90d4. [DOI] [PubMed] [Google Scholar]
- 5.Carlson LE, Groff SL, Maciejewski O, et al. Screening for distress in lung and breast cancer outpatients: A randomized controlled trial. J Clin Oncol. 2010;28:4884–4891. doi: 10.1200/JCO.2009.27.3698. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell PH, Ferketich S, Jennings BM. Quality health outcomes model: American Academy of Nursing expert panel on quality health care. Image J Nurs Sch. 1998;30:43–46. doi: 10.1111/j.1547-5069.1998.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 7.Berry DL, Hong F, Halpenny B, et al. The electronic self report assessment and intervention for cancer: Understanding the results of a randomized trial. Presented at the Oncology Nursing Society Connections Conference; November 16-18, 2012; Phoenix, AZ. (abstr E577) [Google Scholar]
- 8.McCorkle R, Cooley M, Shea J. A user's manual for the Symptom Distress Scale. http://fhsson.mcmaster.ca/apn/images/stories/pdfs/Symptom_Distress_Scale_user_manual.pdf.
- 9.Hong F, Bosco JL, Bush N, et al. Patient self-appraisal of change and minimal clinically important difference on the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 before and during cancer therapy. BMC Cancer. 2013;13:165. doi: 10.1186/1471-2407-13-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroenke K, Theobald D, Wu J, et al. Effect of telecare management on pain and depression in patients with cancer: A randomized trial. JAMA. 2010;304:163–171. doi: 10.1001/jama.2010.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen JD, Berry DL. Multi-media support for informed/shared decision-making before and after a cancer diagnosis. Semin Oncol Nurs. 2011;27:192–202. doi: 10.1016/j.soncn.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Chang VT, Hwang SS, Feuerman M, et al. The Memorial Symptom Assessment Scale Short Form (MSAS-SF) Cancer. 2000;89:1162–1171. doi: 10.1002/1097-0142(20000901)89:5<1162::aid-cncr26>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Eggly S, Harper FW, Penner LA, et al. Variation in question asking during cancer clinical interactions: A potential source of disparities in access to information. Patient Educ Couns. 2011;82:63–68. doi: 10.1016/j.pec.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krueger JL, Hermansen-Kobulnicky CJ. Patient perspective of medication information desired and barriers to asking pharmacists questions. J Am Pharm Assoc (2003) 2011;51:510–519. doi: 10.1331/JAPhA.2011.10069. [DOI] [PubMed] [Google Scholar]
- 15.Cáceres I, Orejuela-Hood M, West J. Racial and ethnic health disparities by EOHHS regions in Massachusetts. http://www.mass.gov/eohhs/docs/dph/research-epi/disparity-report.pdf.
- 16.American College of Surgeons, Commission on Cancer. Cancer program standards 2012: Ensuring patient-centered care. http://www.facs.org/cancer/coc/programstandards2012.pdf.
- 17.Fann JR, Ell K, Sharpe M. Integrating psychosocial care into cancer services. J Clin Oncol. 2012;30:1178–1186. doi: 10.1200/JCO.2011.39.7398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.