Abstract
Objectives:
The objectives of this study were to establish a bisphosphonate-related osteonecrosis of the jaw (BRONJ) rat model and to analyse the effects of teriparatide (TP) on this model.
Methods:
Sprague-Dawley rats were divided into three groups: I—zoledronic acid (ZA, n = 10); II—ZA and teriparatide (ZA + TP, n = 10); III—control (n = 10). Osteonecrosis was induced by administering zoledronic acid to groups ZA and ZA + TP. A week after the injections, rats underwent extraction of the first left mandibular molar. Following a four week period, TP was administered to the ZA + TP group for 28 days. Upon killing, extraction sockets were examined clinically, radiologically and histopathologically.
Results:
Clinical examination revealed necrotic bone exposure in none of the animals. MicroCT (µCT) examination showed that bone mineral density of the newly formed bone in the extraction socket was lower in the ZA group than in the ZA + TP group (p < 0.05). Histopathological examination revealed that only the ZA and ZA + TP groups developed osteonecrosis, and the osteonecrotic bone area in the ZA group was larger than that in the ZA + TP group (p < 0.05). Tartrate-resistant acid phosphatase (TRAcP) enzyme histochemistry revealed that the number of detached and large osteoclasts were higher in the ZA group than in other groups, whereas the number of apoptotic osteoclasts in both ZA and ZA + TP groups were higher than in the control group (p < 0.05).
Conclusions:
Our data indicate that bisphosphonate-related osteonecrosis of the jaw model used in the present study is an attractive model to investigate treatment modalities and that TP might be an effective treatment in BRONJ.
Keywords: bisphosphonate-associated osteonecrosis of the jaw, zoledronic acid, teriparatide, Microcomputed tomography, tartrate-resistant acid phosphatase
Introduction
The most important clinical effect of bisphosphonates (BP) is the inhibition of osteoclast-mediated bone resorption.1 However, in combination with a bone defect (e.g. tooth extraction), the drug appears to induce the development of osteonecrosis of the jaw (BRONJ).2 The occurrence of osteonecrosis is related to the type and dose of BP, not to the severity or type of the underlying disease.3 Patients receiving intravenous zoledronic acid seem to be at particular risk, and intraoral surgical interventions should be avoided in this group. One of the hallmarks of the BRONJ is the presence of exposed osteonecrotic bone and impaired wound healing in the oral cavity,1 but in some patients, this does not occur.4 Histopathological examination can be carried out only on bone sequestrae or surgical debridement material surrounding the osteonecrotic area. Therefore, there are still debates on the definition and staging of this entity, as well as treatment strategies. Case studies have shown that teriparatide (TP) increases bone mass and diameter and restores the microstructure of the bone, and it has an anabolic effect on bone.5 Recent studies also showed a healing effect in refractory BRONJ patients.6,7
Owing to the limitations on the study of BRONJ in humans, there is a need for simple and reliable animal models, which enable the collection of more detailed information. Previous animal studies have been performed predominantly on the development of BRONJ lesions but not on treatment methods.3,8,9 Since tooth extraction increases the risk of BRONJ, it is commonly used in BRONJ models. Biasotto et al3 chose a rat model in which they extracted maxillary molars, and concluded that the main advantage of this model was to be able to use large numbers of animals and eliminate the pathogenetic role of intracortical remodelling suppression in BRONJ. Histomorphometry is traditionally used to measure bone formation and is considered the gold standard.10 The disadvantage of this method is the long time required for all processing steps.10,11 MicroCT provides a fast and non-destructive technique to characterize and measure the three-dimensional geometric and density properties of a bone specimen.12 It was shown that both analyses are reliable but are used preferably in combination.11
The objective of this study was to demonstrate the healing effects of TP in an animal model of BRONJ, using microCT and histology. BRONJ was induced in Sprague-Dawley rats by intraperitoneal administration of zoledronic acid (ZA), which is the most potent BP, and a mandibular tooth extraction. An 8 week period after tooth extraction was considered to be sufficient for osteonecrosis to develop.
Materials and methods
All animal and surgical procedures were performed in accordance with the guidelines following approval of the Ethics in Animal Research Committee of the Yeditepe University (protocol number: 260/2012, YÜDHEK, Istanbul, Turkey). Healthy, female Sprague-Dawley rats, aged between 10 weeks and 12 weeks (YÜDETAM Laboratories, Istanbul, Turkey), and weighing between 164 g and 196 g, were included. Animals were acclimatized prior to the study, and housed with three to four animals per cage. The cages were provided with filtered air at a temperature of 21 °C and 40–60% relative humidity. A 12 h light–dark cycle was maintained. The animals were fed with a standard rat diet and had access to water ad libitum.
The rats were randomly divided into three groups: I—ZA (n = 10) group, II—ZA and teriparatide (ZA + TP, n = 10) group and III—a control (n = 10) group. To induce BRONJ lesions in the ZA and ZA + TP groups, rats were injected intraperitoneally with 0.2 mg kg−1 ZA in a 0.1 mg ml−1 sterile saline solution, three times a week for 6 weeks, whereas the rats in the control group were injected only with a similar volume of the vehiculum saline solution for the same time period. A week after the final injection, the rats were anesthetized with a single intramuscular injection of ketamine HCl (80–100 mg kg−1) and 2% xylazine HCl (10 mg kg−1), and the first left mandibular molar was extracted. Supplemental local anaesthesia was given to the rats, as needed, by local infiltration with a few drops of 2% lidocaine containing 1:100 000 epinephrine delivered into the mucobuccal fold. Sterile instruments and equipment were used during the extraction of molars, and teeth were removed by using a sharpened dental explorer. Extraction sockets were left to heal spontaneously. After the extractions, two rats died: one from the ZA group and the other from the control group. Following a 4 week period, the rats in the ZA + TP group were injected subcutaneously with 30 μg kg−1 of TP in a 0.1 mg ml−1 sterile saline solution, daily for 28 days. At the end of this period, all rats were sacrificed.
Clinical examination
Throughout the course of the study, the weight of the animals was measured four times: firstly at baseline, secondly prior to molar extraction, thirdly prior to TP injections and fourthly prior to sacrificing. On sacrificing, extraction sockets were examined clinically for the presence of mucosal ulceration, abscess and fistula formation and necrotic bone exposure. After clinical examination, the mandible of each animal was removed and placed into 10% formaldehyde.
Radiological evaluation
The mandibles were scanned using a high-resolution microCT system (µCT40; Scanco Medical AG, Brüttisellen, Switzerland) to measure their three-dimensional geometry, and the mineral density of the newly formed bone in the extraction sockets. Prior to scanning, a methacrylate monomer block was prepared in the shape of rectangular prism, and each mandible was glued upside down on the block, in a way that the occlusal plane became horizontal to the bottom. The spatial resolution of the scans was 18 µm and the beam energy 55 kVp. To minimize noise, an integration time of 1250 ms was used. The scanner measured the linear attenuation coefficient of the X-ray beam for each volume element (voxel) and computed the degree of mineralization of each voxel from this attenuation coefficient. The scanner was calibrated every week with a phantom containing hydroxyapatite (HA) densities of 0, 100, 200, 400 and 800 mg cm−3.13
Bone tissue was distinguished from bone marrow using a fixed threshold of 507.6 mg HA cm−3, which was calculated by averaging the thresholds determined visually in several slices. The width of the alveolar bone and the volumetric bone mineral density (vBMD) of extraction sockets were measured on axial sections. Alveolar bone width was measured from the widest part of the alveolar bone at the extraction socket. The density of the newly formed bone in the socket was measured as the density of a volume in the extraction socket of 20 sections and a 1 ×1 mm area. The root apices of the right mandibular molar were used as a reference for this volume.
In the ZA + TP group, one rat was excluded from the study because of extreme bone formation on the periosteal surface. Furthermore, one rat from the ZA group, one from the ZA + TP group and two from the control group were excluded from density measurement because of a widespread osteolytic area extending beyond the roots caused by an infection in the extraction socket.
Histopathological examination
After radiological examination, bone samples including extraction sockets were decalcified in ethylenediaminetetraacetic acid for 4 weeks,14 embedded in paraffin and sectioned as sections of 6 µm thickness. Haematoxylin and eosin stained sections were examined for the presence of necrotic bone tissue. Histomorphometric analysis of the osteonecrotic bone area of the groups on haematoxylin and eosin sections was performed on three sections of each rat.
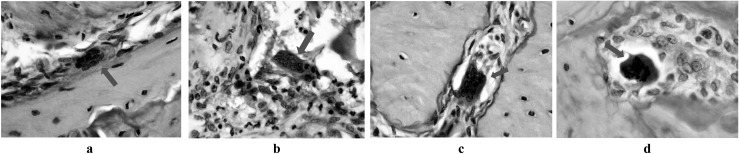
To stain osteoclasts, tartrate-resistant acid phosphatase (TRAcP) enzyme histochemical staining was performed. Prior to staining, the enzyme activity of tissue samples that decreased during paraffin embedding procedures was reactivated in alkaline (pH, 9.0) Tris-buffered saline solution. Sections were incubated for 2 h at 37 °C with a substrate solution composed of naphthol AS-BI phosphate dissolved in dimethylformamide, acetate buffer in polyvinyl alcohol mixed with MgCl2 6H2O and KNa-tartrate 4H2O and pararosanilin mixed with NaNO2. On TRAcP staining, sections were counterstained with haematoxylin and eosin staining. The number of osteoclasts attached to the bone surface, the bone surface area occupied with osteoclasts, the number of detached osteoclasts and the number of large and apoptotic osteoclasts were counted on TRAcP-stained sections (Figure 1a–d). The presence of other cells between the bone surface and the osteoclast was the criteria for the definition of detached osteoclast15 (Figure 1b). A large osteoclast was defined as an osteoclast with eight or more nuclei in the plane of sectioning16 (Figure 1c). A light microscope (Olympus BX60; Olympus Corporation, Tokyo, Japan) was used for the histopathological examinations.
Figure 1.
Micrographs of (a) an osteoclast attached to the bone surface (arrow), (b) a detached osteoclast (arrow), (c) a giant osteoclast (arrow), (d) an apoptotic osteoclast (arrow), taken from the extraction socket (tartrate-resistant acid phosphatase staining, ×1000)
Statistical analysis
Statistical tests were performed using SPSS® (SPSS Inc., Chicago, IL) for Windows 21.0. Descriptive analysis (mean and standard deviation) was used. Data were initially subjected to the Kolmogorov–Smirnov normality test. Then, comparisons were performed among groups using the one-way ANOVA test or Welch's test followed by either Tukey or Dunnett's test as post-hoc test. For the data that did not pass the normality test, the Kruskal–Wallis test was performed followed by the Mann–Whitney U test as post-hoc test. Statistical significance was set at p < 0.05 with 95% confidence intervals.
Results
Clinical examination revealed mucosal ulceration, abscesses, fistula formation or necrotic bone exposure in none of the animals. The weight of the animals prior to sacrificing was higher for the control group than for the ZA group (p < 0.05) (Table 1).
Table 1.
Weight measurements of the rats in zoledronic acid (ZA), ZA + teriparatide (TP) and control groups at baseline, prior to molar extraction, prior to teriparatide injections and prior to sacrificing
| Weight measurements (g) | n | Mean ± SD | F | p-value | |
|---|---|---|---|---|---|
| Baseline | |||||
| ZA | 9 | 185.22 ± 9.23 | 1.345 | 0.279 | |
| ZA + TP | 10 | 178.50 ± 8.66 | |||
| Control | 9 | 181.78 ± 8.89 | |||
| Overall | 28 | 181.71 ± 9.03 | |||
| Prior to molar extraction (Week 7) | |||||
| ZA | 9 | 219.11 ± 12.29 | 2.481 | 0.104 | |
| ZA + TP | 10 | 214.20 ± 11.96 | |||
| Control | 9 | 225.11 ± 8.31 | |||
| Overall | 28 | 219.29 ± 11.23 | |||
| Prior to teriparatide injections (Week 11) | |||||
| ZA | 9 | 219.44 ± 21.01 | 2.452 | 0.107 | |
| ZA + TP | 10 | 222.60 ± 17.45 | |||
| Control | 9 | 236.22 ± 11.64 | |||
| Overall | 28 | 226.96 ± 18.05 | |||
| Prior to sacrificing (Week 15) | |||||
| ZA | 9 | 224.78 ± 15.36 | 3.627 | 0.041a | |
| ZA + TP | 10 | 230.70 ± 18.28a | |||
| Control | 9 | 243.33 ± 9.31a | |||
| Overall | 28 | 232.86 ± 16.34 |
SD, standard deviation.
Comparisons were performed among groups using one-way ANOVA.
The Tukey test was used as post hoc to define significantly different groups. Significance was set at p < 0.05.
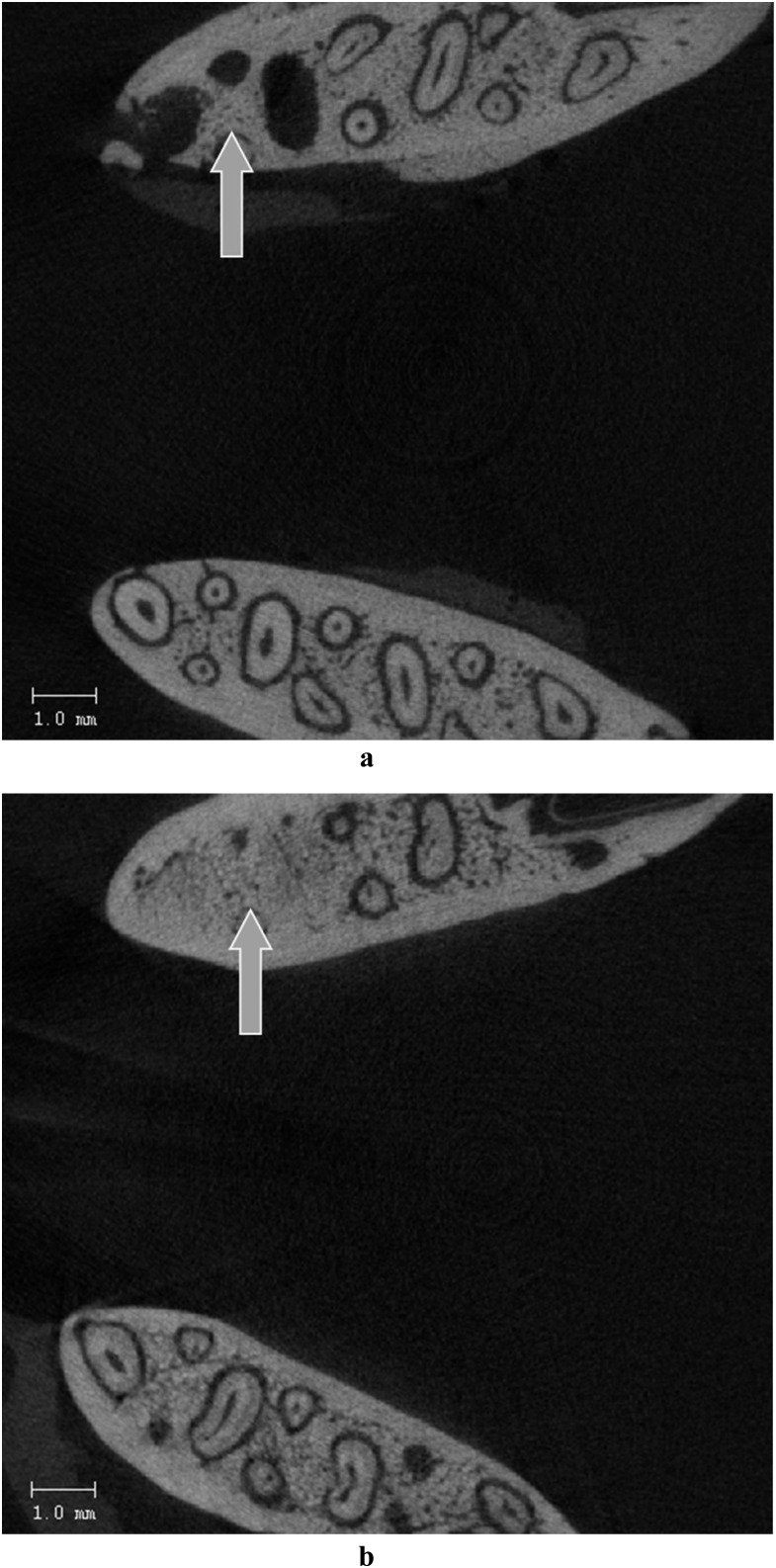
Radiological examination performed with microCT showed that alveolar bone width of the ZA and ZA + TP groups was higher than in the control group (p < 0.05) (Table 2). vBMD of the newly formed bone in the extraction sockets of ZA group was lower than the vBMD of ZA + TP (p < 0.05) (Table 2) (Figure 2a,b).
Table 2.
MicroCT measurements of alveolar bone width and volumetric bone mineral density (vBMD) of the bone formed in the extraction sockets of the rats in the zoledronic acid (ZA), ZA + teriparatide (TP) and control groups
| Radiological measurements | n | Mean ± SD | p-value |
|---|---|---|---|
| Alveolar bone width (mm) | |||
| ZA | 9 | 3.16 ± 0.35a | 0.0032a |
| ZA + TP | 9 | 3.31 ± 0.32a | |
| Control | 9 | 2.79 ± 0.20a | |
| vBMD (mg HA cm−3) | |||
| ZA | 8 | 791.38 ± 22.79a | 0.0247a |
| ZA + TP | 8 | 835.53 ± 35.33a | |
| Control | 7 | 823.94 ± 31.83 |
SD, standard deviation.
Comparisons were performed among groups using one-way ANOVA.
The Tukey test was used as post hoc to define significantly different groups. Significance was set at p < 0.05.
Figure 2.
MicroCT image of a rat mandible of the (a) zoledronic acid group, which indicates the impaired wound healing in the extraction socket (arrow, axial view) and (b) control group, which indicates the bone formation in the extraction socket (arrow, axial view)
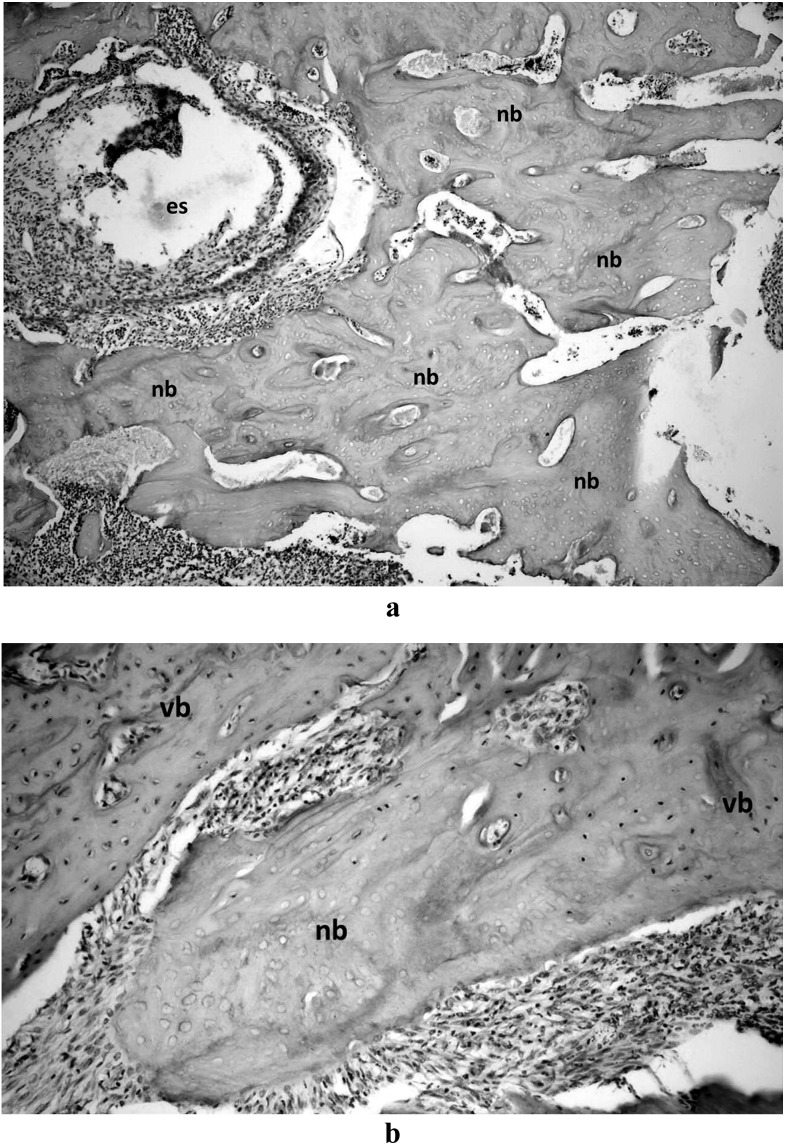
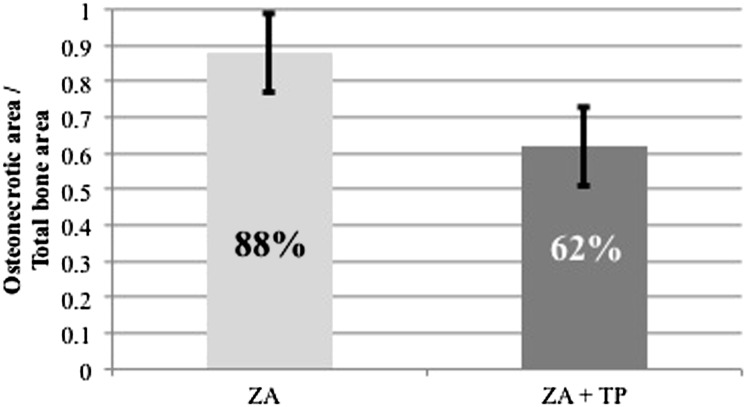
Histopathological examination revealed that no osteonecrosis was found in the control group, whereas all animals in the ZA and ZA + TP groups developed osteonecrosis (Figure 3a,b). Histomorphometric measurements showed that the mean percentage of necrotic bone area in the ZA group was higher than in the ZA + TP group (88% and 62%, respectively; p < 0.05) (Figure 4).
Figure 3.
Micrograph of the haematoxylin and eosin stained section showing (a) failure in healing of the bone in the extraction socket of a rat of the zoledronic acid (ZA) group (×100), (b) a small necrotic portion of the alveolar bone surrounding the extraction socket of a rat of the ZA + teriparatide group (×200). es, extraction socket; nb, necrotic bone; vb, vital bone
Figure 4.
Quantification of the ratio of necrotic bone area to the total bone area in the zoledronic acid (ZA) and ZA + teriparatide (TP) groups
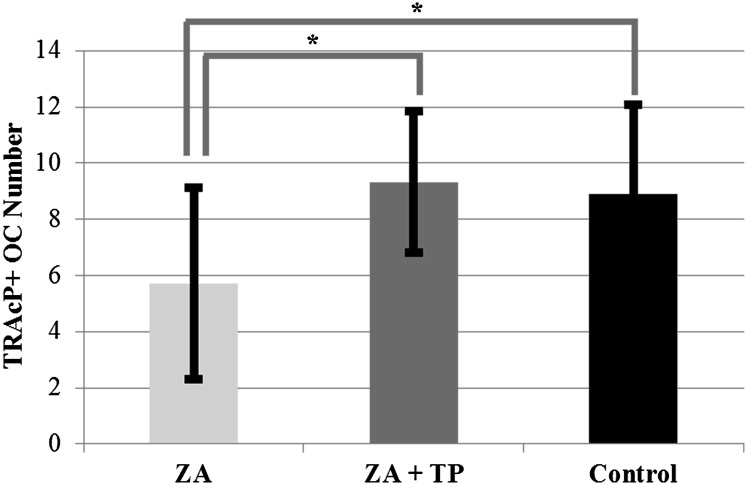
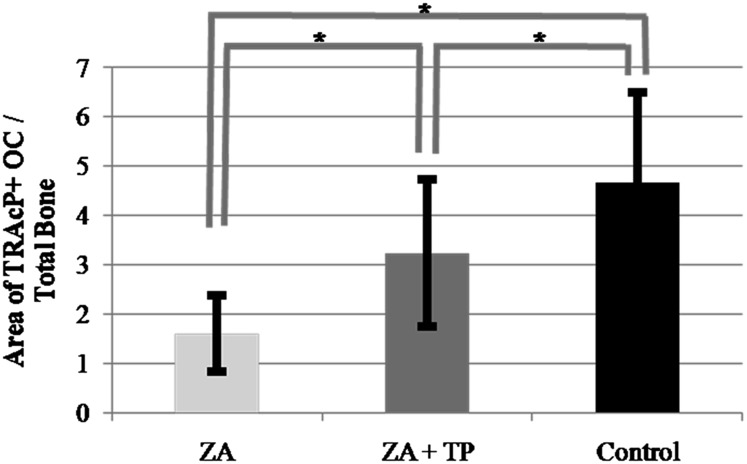
TRAcP enzyme histochemistry revealed that in the ZA + TP and control group, the number of osteoclasts attached to the bone surface and the bone surface area occupied with osteoclasts were higher than in the ZA group (9.3 ± 2.5, 8.9 ± 3.2, 5.7 ± 3.4, p < 0.05; 3.2 ± 1.5, 4.7 ± 1.8, 1.6 ± 0.8, p < 0.05, respectively) (Figures 5 and 6). The number of TRAcP-positive detached and large osteoclasts were higher in the ZA group than in the ZA + TP and control group (p < 0.05), whereas the number of TRAcP-positive apoptotic osteoclasts in both ZA and ZA + TP groups were higher than in the control group (p < 0.05) (Table 3).
Figure 5.
Number of tartrate-resistant acid phosphatase positive (TRAcP+) osteoclasts (OCs) attached to the bone of the rats of the zoledronic acid (ZA), ZA + teriparatide (TP) and control groups (*p < 0.05)
Figure 6.
Bone surface area occupied by tartrate-resistant acid phosphatase positive attached osteoclasts of the rats of the zoledronic acid (ZA), ZA + teriparatide (TP) and control groups (*p < 0.05)
Table 3.
Mean numbers of tartrate-resistant acid phosphatase–positive, detached, giant and apoptotic osteoclasts of zoledronic acid (ZA), ZA + teriparatide (TP) and control groups
| n | Mean ± SD | p-value | |
|---|---|---|---|
| Detached OCs | |||
| ZA | 9 | 2.06 ± 1.434a | <0.01a |
| ZA + TP | 9 | 0.78 ± 0.943a | |
| Control | 9 | 0.83 ± 2.036a | |
| Giant OCs | |||
| ZA | 9 | 1.44 ± 1.294a | <0.01a |
| ZA + TP | 9 | 0.44 ± 0.616a | |
| Control | 9 | 0.11 ± 0.323a | |
| Apoptotic OCs | |||
| ZA | 9 | 2.72 ± 1.274a | <0.01a |
| ZA + TP | 9 | 2.11 ± 1.568a | |
| Control | 9 | 0.22 ± 0.548a |
OC, osteoclast; SD, standard deviation.
Comparisons were performed using Mann–Whitney U test.
The Kruskal Wallis test was used as post hoc to define significantly different groups. Significance was set at p < 0.01.
Discussion
Since clinical findings do not always reflect the extension and severity of BRONJ lesions beneath the oral mucosa, the usage of radiological methods become inevitable.17 Recent studies reported that CT is one of the most useful imaging modalities in BRONJ cases; however, the radiological signs are not specific for BRONJ.18–21 Although radiological interpretation is crucial for the definition of BRONJ lesions, in cases that necrotic bone is present but not yet exposed into the oral cavity, radiographic images may also appear normal.22 Unfortunately, there is only a limited number of publications about the diagnostic importance of radiological evaluation in these lesions.
Despite insignificant appearances on radiological images, periosteal new bone formation is frequently seen in BRONJ patients.22 It was shown that, especially in the mandible, with progression of the severity of the BRONJ lesion, formation of new periostal bone increases.20,22,23 In a periodontal disease induced BRONJ rat model, Aghaloo et al24 reported increased periosteal new bone formation and wider alveolar bone on microCT images. Intermittent parathyroid hormone administration was also shown to induce an increase in alveolar bone formation in rats.25 In the present study, alveolar bone width of the ZA group was found to be greater than that of the control group. This finding might be the result of the inductive effect of BRONJ lesion on periosteal new bone formation. Also, the ZA + TP group had the largest alveolar bone width at the extraction site. We assume that this results from the additive inductive effect of BRONJ and TP on periosteal bone formation.
The use of TP on refractory BRONJ lesions was first defined by Harper and Fung,6 who observed soft-tissue healing in a patient with a 3 month TP administration. In a review paper on BRONJ treatment with the use of TP, Subramanian et al26 suggested that healing results in more than the mere elimination of necrotic bone, and the necrotic tissue might be used as an osteoconductive scaffold for bone regeneration. Additionally, in a case study, Ohbayashi et al27 demonstrated bone regeneration 6 months after TP therapy in a refractory BRONJ patient. Unfortunately, in the literature, there is no detailed information on the radiological examination of TP effect on tooth extraction and BRONJ. In the present study, it was shown by quantitative analyses with microCT that the vBMD of the newly formed bone in the non-treated group was lower than in the treated group (Table 2). As with the radiological evaluation, histomorphometry analysis also revealed that the mean percentage of osteonecrotic area was significantly higher in the ZA group than in the ZA + TP and control groups (Figure 4).
TP regulates bone resorption by increasing osteoclastic activity.28 Ma et al29 showed that TP reverses the inhibitory effects of antiresorptive drugs such as BPs in vivo. The BPs suppress osteoclastic activity by inducing apoptosis of these cells and cause them to detach from the bone surface.30 Bertoldo et al2 reported that during resorption of bone following tooth extraction, BPs within the bone are taken up by osteoclasts and this results in apoptosis. Kuroshima et al31 showed that in mice injected with ZA for 13 months, there was an increase in the number of detached osteoclasts. Weinstein et al,16 in a study on postmenopausal women who received oral BPs, reported an increased number of giant, detached and apoptotic osteoclasts. Hokugo et al,8 who induced BRONJ lesions with ZA and maxillary molar extraction in a rat model, found that the number of apoptotic osteoclasts in the ZA group was higher than in the control group. In the present study, consistent with other studies, the ZA group had a statistically significantly higher number of apoptotic, detached and giant osteoclasts in the ZA group.
Although weight gain is one of the side effects of ZA, weight loss can be seen in BRONJ patients.32,33 For ZA-induced BRONJ in rats, no information is available on weight differences. In this study, the weight measurements prior to sacrificing showed that the rats of the control group weighed significantly more than the rats of the ZA group. Although not significant, the weight of the TP group prior to sacrifice was found to be higher than that of the ZA group. Better nourishment with the diminution in the osteonecrotic area might have given rise to an increase in the weight of rats in the TP group.
According to the recommendations of American Association of Oral and Maxillofacial Surgeons, necrotic bone exposure for more than 8 weeks is one of the hallmarks of BRONJ.1,34 Khosla et al34 suggested that in patients using BPs, the presence of symptoms like pain, swelling, paresthesia and suppuration, unless they are associated with necrotic bone exposure, is not enough to diagnose BRONJ. On the other hand, it is postulated that 30% of BRONJ cases presents itself without necrotic bone exposure. These patients are evaluated as Stage 0 according to the recommendations of American Association of Oral and Maxillofacial Surgeons and are left undiagnosed and untreated. Since, there are still disagreements on BRONJ definition and staging systems, some researchers suggested their own staging systems.35,36 Also, it is not known how the 8 week period determined for humans should be adapted to rats. In the present study, all extraction sockets were covered with mucosa, and there were no signs of bone exposure 8 weeks after tooth extractions. We suggest that all signs and symptoms in clinical examination, including weight alterations, should be taken into consideration while establishing the diagnosis of BRONJ.
Increased rates of osteosarcoma were reported in rats with the use of TP.5 Although development of osteosarcoma was not reported in humans, there is no long-term information, thus TP treatment for more than 2 years is not recommended.37 In the previously reported case reports, TP was injected for 1–6 months in BRONJ treatment and is considered to be a short-term treatment.37 TP, which is indicated in osteoporosis prevention and treatment, is also indicated in osteoporotic BRONJ patients. Although TP is contraindicated in patients who have malignant bone diseases and developed BRONJ,28 studies on the suitability of short-term TP therapy in these patients are needed.
In our study, radiographical evaluation and histopathological examination proved to be helpful in the diagnosis of BRONJ. Therefore, we suggest that in addition to radiological examination, if possible, histopathological examination should also be performed. However, in patients who are under BP treatment, bone biopsies are contraindicated and radiological examination remains the only tool. Additionally, one of the findings of this study was that the mandible becomes wider at the site of extraction. Owing to the inactivation of the osteoclasts, bone can no longer be remodelled properly. When the bone tissue has deteriorated because of osteonecrosis, it seems that the only way to prevent failure is to produce new bone. Although more research is required, our findings suggest that tomography may be a reliable method to detect osteonecrosis.
In conclusion, systemic administration of TP has shown to be effective in diminution of the osteonecrotic area, and the findings of this study support the view that TP might be an effective treatment modality for BRONJ patients. Standardized three-dimensional radiographic assessment of bone mineral density seems to provide a helpful parameter for monitoring BRONJ status.
References
- 1.Advisory Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 2007; 65: 369-376. 10.1016/j.joms.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 2.Bertoldo F, Santini D, Lo Cascio V. Bisphosphonates and osteomyelitis of the jaw: a pathogenic puzzle. Nat Clin Pract Oncol 2007; 4: 711-721. 10.1038/ncponc1000 [DOI] [PubMed] [Google Scholar]
- 3.Biasotto M, Chiandussi S, Zacchigna S, Moimas S, Dore F, Pozzato G, et al. A novel animal model to study nonspontaneous bisphosphonates osteonecrosis of jaw. J Oral Pathol Med 2010; 35: 390-396. 10.1111/j.1600-0714.2009.00878.x [DOI] [PubMed] [Google Scholar]
- 4.Junquera L, Gallego L. Nonexposed bisphosphonate-related osteonecrosis of the jaws: another clinical variant? J Oral Maxillofac Surg 2008; 66: 1516-1517. 10.1016/j.joms.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 5.Tashjian AH Jr, Chabner BA. Commentary on clinical safety of recombinant human parathyroid hormone 1-34 in the treatment of osteoporosis in men and postmenopausal women. J Bone Miner Res 2002; 17: 1151-1161. 10.1359/jbmr.2002.17.7.1151 [DOI] [PubMed] [Google Scholar]
- 6.Harper RP, Fung E. Resolution of bisphosphonate-associated osteonecrosis of the mandible: possible application for intermittent low-dose parathyroid hormone (rhPTH[1-34]). J Oral Maxillofac Surg 2007; 65: 573-580. 10.1016/j.joms.2006.10.076 [DOI] [PubMed] [Google Scholar]
- 7.Subramanian G, Quek SY. Teriparatide's role in the management of bisphosphonate-associated osteonecrosis of the jaw. Osteoporos Int 2012; 23: 2727-2728; author reply 2729–2730. 10.1007/s00198-012-1951-8 [DOI] [PubMed] [Google Scholar]
- 8.Hokugo A, Christensen R, Chung EM, Sung EC, Felsenfeld AL, Sayre JW, et al. Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J Bone Miner Res 2010; 25: 1337-1349. 10.1002/jbmr.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasconcelos AC, Berti-Couto SA, Azambuja AA, Salum FG, Figueiredo MA, da Silva VD, et al. Comparison of effects of clodronate and zoledronic acid on the repair of maxilla surgical wounds—histomorphometric, receptor activator of nuclear factor-kB ligand, osteoprotegerin, von Willebrand factor, and caspase-3 evaluation. J Oral Pathol Med 2012; 41: 702-712. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Heredia MA, Bongio M, Cuijpers VM, van Dijk NW, van den Beucken JJ, Wolke JG, et al. Bone formation analysis: effect of quantification procedures on the study outcome. Tissue Eng Part C Methods 2012; 18: 369. 10.1089/ten.TEC.2011.0353 [DOI] [PubMed] [Google Scholar]
- 11.Gielkens PF, Schortinghuis J, de Jong JR, Huysmans MC, Leeuwen MB, Raghoebar GM, et al. A comparison of micro-CT, microradiography and histomorphometry in bone research. Arch Oral Biol 2008; 53: 558. 10.1016/j.archoralbio.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 12.Jones AC, Milthorpe B, Averdunk H, Limaye A, Senden TJ, Sakellariou A, et al. Analysis of 3D bone ingrowth into polymer scaffolds via micro-computed tomography imaging. Biomaterials 2004; 25: 4947. 10.1016/j.biomaterials.2004.01.047 [DOI] [PubMed] [Google Scholar]
- 13.van Ruijven LJ, Mulder L, van Eijden TM. Variations in mineralization affect the stress and strain distributions in cortical and trabecular bone. J Biomech 2007; 40: 1211-1218. 10.1016/j.jbiomech.2006.06.004 [DOI] [PubMed] [Google Scholar]
- 14.Warshawsky H, Moore G. A technique for the fixation and decalcification of rat incisors for electron microscopy. J Histochem Cytochem 1967; 15: 542-549. [DOI] [PubMed] [Google Scholar]
- 15.Hata K, Kukita T, Akamine A, Kukita A, Kurisu K. Trypsinized osteoclast-like multinucleated cells formed in rat bone marrow cultures efficiently form resorption lacunae on dentine. Bone 1992; 13: 139-146. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med 2009; 360: 53-62. 10.1056/NEJMoa0802633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilde F, Heufelder M, Winter K, Hendricks J, Frerich B, Schramm A, et al. The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 111: 153-163. 10.1016/j.tripleo.2010.04.015 [DOI] [PubMed] [Google Scholar]
- 18.Chiandussi S, Biasotto M, Cavalli F, Cova MA, Di Learda R. Clinical and diagnostic imaging of bisphosphonate-associated osteonecrosis of the jaws. Dentomaxillofac Radiol 2006; 35: 236-243. 10.1259/dmfr/27458726 [DOI] [PubMed] [Google Scholar]
- 19.Bisdas S, Chambron Pinho N, Smolarz A, Sader R, Vogl TJ, Mack MG. Biphosphonate-induced osteonecrosis of the jaws: CT and MRI spectrum of findings in 32 patients. Clin Radiol 2008; 63: 71-77. [DOI] [PubMed] [Google Scholar]
- 20.Olutayo J, Olubanwo Agbaje J, Jacobs R, Verhaeghe V, Vande Velde F, Vinckier F. Bisphosphonate-related osteonecrosis of the jaw bone: radiological pattern and the potential role of CBCT in early diagnosis. J Oral Maxillofac Res 2010; 1: e3 [cited 29 April 2012]. Available from: http://www.ejomr.org/JOMR/archives/2010/2/e3/e3ht.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elad S, Gomori MJ, Ben-Ami N, Friedlander-Barenboim S, Regev E, Lazarovici TS, et al. Bisphosphonate-related osteonecrosis of the jaw: clinical correlations with computerized tomography presentation. Clin Oral Investig 2010; 14: 43-50. 10.1007/s00784-009-0311-3 [DOI] [PubMed] [Google Scholar]
- 22.Wilde F, Heufelder M, Lorenz K, Liese S, Liese J, Helmrich J, et al. Prevalence of cone beam computed tomography imaging findings according to the clinical stage of bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114: 804-811. 10.1016/j.oooo.2012.08.458 [DOI] [PubMed] [Google Scholar]
- 23.Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD. Histopathological features of bisphosphonate-associated osteonecrosis: findings in patients treated with partial mandibulectomies. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114: 785-791. 10.1016/j.oooo.2012.08.457 [DOI] [PubMed] [Google Scholar]
- 24.Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, et al. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res 2011; 26: 1871-1882. 10.1002/jbmr.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller SC, Hunziker J, Mecham M, Wronski TJ. Intermittent parathyroid hormone administration stimulates bone formation in the mandibles of aged ovariectomized rats. J Dent Res 1997; 76: 1471-1476. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian G, Cohen HV, Quek SY. A model for the pathogenesis of bisphosphonate-associated osteonecrosis of the jaw and teriparatide's potential role in its resolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 112: 744-753. 10.1016/j.tripleo.2011.04.020 [DOI] [PubMed] [Google Scholar]
- 27.Ohbayashi Y, Miyake M, Sawai F, Minami Y, Iwasaki A, Matsui Y. Adjunct teriparatide therapy with monitoring of bone turnover markers and bone scintigraphy for bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 115: e31-e37. 10.1016/j.oooo.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 28.Pleiner-Duxneuner J, Zwettler E, Paschalis E, Roschger P, Nell-Duxneuner V, Klaushofer K. Treatment of osteoporosis with parathyroid hormone and teriparatide. Calcif Tissue Int 2009; 84: 159-170. 10.1007/s00223-009-9218-x [DOI] [PubMed] [Google Scholar]
- 29.Ma YL, Bryant HU, Zeng Q, Schmidt A, Hoover J, Cole HW, et al. New bone formation with teriparatide [human parathyroid hormone-(1-34)] is not retarded by long-term pretreatment with alendronate, estrogen, or raloxifene in ovariectomized rats. Endocrinology 2003; 144: 2008-2015. [DOI] [PubMed] [Google Scholar]
- 30.Rogers MJ. From molds and macrophages to mevalonate: a decade of progress in understanding the molecular mode of action of bisphosphonates. Calcif Tissue Int 2004; 75: 451-461. 10.1007/s00223-004-0024-1 [DOI] [PubMed] [Google Scholar]
- 31.Kuroshima S, Go VA, Yamashita J. Increased numbers of nonattached osteoclasts after long-term zoledronic acid therapy in mice. Endocrinology 2012; 153: 17-28. 10.1210/en.2011-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng A, Mavrokokki A, Carter G, Stein B, Fazzalari NL, Wilson DF, et al. The dental implications of bisphosphonates and bone disease. Aust Dent J 2005; 50: 4-13. [DOI] [PubMed] [Google Scholar]
- 33.Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg 2007; 65: 415-423. 10.1016/j.joms.2006.10.061 [DOI] [PubMed] [Google Scholar]
- 34.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007; 22: 1479-1491. 10.1359/jbmr.0707onj [DOI] [PubMed] [Google Scholar]
- 35.McMahon RE, Bouquot JE, Glueck CJ, Griep JA, Adams WR, Spolnik KJ, et al. Staging bisphosphonate-related osteonecrosis of the jaw should include early stages of disease. J Oral Maxillofac Surg 2007; 65: 1899-1900. 10.1016/j.joms.2007.04.021 [DOI] [PubMed] [Google Scholar]
- 36.Patel S, Choyee S, Uyanne J, Nguyen AL, Lee P, Sedghizadeh PP, et al. Non-exposed bisphosphonate-related osteonecrosis of the jaw: a critical assessment of current definition, staging, and treatment guidelines. Oral Dis 2012; 18: 625-632. 10.1111/j.1601-0825.2012.01911.x [DOI] [PubMed] [Google Scholar]
- 37.Kwon YD, Lee DW, Choi BJ, Lee JW, Kim DY. Short-term teriparatide therapy as an adjunctive modality for bisphosphonate-related osteonecrosis of the jaws. Osteoporos Int 2012; 23: 2721-2725. 10.1007/s00198-011-1882-9 [DOI] [PubMed] [Google Scholar]