Abstract
In the 40 years since the founding of the National Institute on Alcohol Abuse and Alcoholism (NIAAA), researchers have gained a better understanding of the brain circuits and brain chemical (i.e., neurotransmitter) systems involved in the development and maintenance of alcoholism and other drug dependence. This understanding has led to the identification of numerous potential targets for pharmacotherapy of addiction. For example, insight into the roles of signaling molecules called endogenous opioids and the neurotransmitter glutamate were fundamental for developing two medications—naltrexone and acamprosate—now used in the treatment of alcoholism. However, the processes of dependence development (e.g., reinforcement, sensitization, and withdrawal) are highly complex and involve a plethora of contributing influences, which also may differ from patient to patient. Therefore, existing pharmacotherapies still are effective only for some but not all alcoholic patients. Accordingly, researchers are continuing to explore the processes involved in addiction development to identify new targets for treatment and develop new medications that can address different aspects of the dependence syndrome, thereby increasing the likelihood of successful treatment. NIAAA continues to play a pivotal role in funding and conducting this research in order to provide effective treatment options to millions of alcohol-dependent patients.
Keywords: Alcohol and other drug dependence, alcoholism, brain, brain circuit, brain chemistry, neurotransmitters, treatment, pharmacotherapy, medications
Alcoholism, like addiction to other drugs, is a chronic, relapsing disorder characterized by compulsive alcohol use that is thought to include three stages (Koob and Le Moal 1997). These stages have heuristic value as a construct because they are components of the addiction process in general (i.e., have face validity), and they have value in predicting not only the neurobiological bases of addiction but also medications for the treatment of addiction (i.e., have construct validity) (Koob et al. 2009). The three stages include the following:
The binge–intoxication stage, during which a downregulation of positive reward pathways occurs—that is, increasing drug levels are needed to trigger the brain reward system. During this stage, alcohol and other drug (AOD) use is motivated primarily by positive rewarding experiences.
The withdrawal–negative-affect stage, during which the drug user transitions to AOD addiction and experiences negative consequences (i.e., withdrawal symptoms) when AOD use is discontinued. At this stage, AOD use begins to be motivated primarily by the desire to avoid negative experiences (i.e., by negative reinforcement).
The preoccupation–anticipation (“craving”) stage, which is characterized by exaggerated motivation for drug use (i.e., craving).
All three stages are associated with specific changes in the structure and function of various brain-signaling molecule (i.e., neurotransmitter) systems and in the circuits connecting various brain regions to relay information related to a specific function. As a result of these neurochemical and neurocircuitry changes, the person eventually loses behavioral control over drug seeking and drug taking. Because neurotransmitters and the circuits they act on are pivotal players in the development of alcohol dependence and other addictions, they also are prime targets for pharmacotherapies for these disorders. Accordingly, over the past 40 years, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) has strongly supported research into the neurochemical mechanisms underlying the development of various aspects of addiction, such as positive and negative reinforcement, development of tolerance, sensitization to alcohol’s effects, and development of withdrawal symptoms after cessation of drinking. Other research has focused on the brain circuits that are altered by repeated exposure to alcohol and on the development of animal and human laboratory models that reflect various aspects of addiction. This comprehensive approach led to the development of the drug naltrexone (Revia®, Depade®), which acts at one type of neurotransmitter receptor affected by alcohol (i.e., opioid receptors). Naltrexone was approved for the treatment of alcohol dependence based on two NIAAA-supported clinical trials (O’Malley et al. 1992; Volpicelli et al. 1992). NIAAA continues supporting the identification of new targets for pharmacotherapy interventions, development of candidate compounds, and testing of these compounds in validated preclinical models and clinical studies. This article reviews some of the neurobiological targets currently studied for the development of new treatment approaches for alcoholism and other drug addictions and their proposed actions during the various stages of AOD addiction.
Neurobiological Targets in the Treatment of Addiction
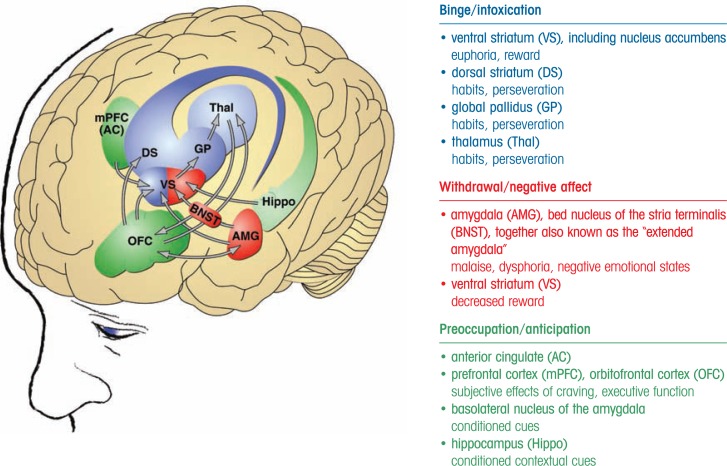
For researchers to select appropriate targets for novel pharmacotherapeutic approaches, they must first gain an understanding of the neurobiology of addiction—that is, of the brain circuits and signaling systems involved in the various stages of addiction. Several neurobiological circuits have been identified, each of which are central to one or more of the three stages of addiction (Koob and Le Moal 2006) and which may be promising targets for potential pharmacotherapy (see the figure):
The mesocorticolimbic dopamine system and its terminal areas in the ventral striatum, which includes signaling by the neurotransmitter dopamine and signaling molecules called opioid peptides, is involved in mediating the positive reinforcing drug effects associated with the binge–intoxication stage of the addiction cycle (Nestler 2005).
The body’s stress response system—known as the hypothalamic–pituitary–adrenal (HPA) stress response—and the brain’s stress response become activated during the withdrawal–negative-affect stage (Koob 2008). At the same time, neuronal systems implicated in the positive reinforcing effects of AODs are disrupted (e.g., dopamine activity decreases). Both body and brain stress responses involve a molecule called corticotropin-releasing factor (CRF); in the HPA response, CRF acts in the hypothalamus or pituitory, whereas for the brain’s stress response CRF acts outside the hypothalamus on the pituitary (i.e., extra-hypothalamic) in a region called the amydala. With repeated cycles of drinking and withdrawal, the HPA response becomes weaker (i.e., blunted), whereas the extrahypothalamic CRF stress system becomes more sensitive (Koob 2008).
The preoccupation–anticipation stage is thought to involve brain circuits using signal transmission via the neurotransmitter glutamate from the prefrontal cortex, basolateral amygdala, insula, and hippocampus to the extended amygdala and nucleus accumbens (NAc) (Kalivas and McFarland 2003). Furthermore, the compulsive drug-seeking behavior that occurs during this stage engages additional circuits (i.e., dorsal striatal– dorsal pallidal–thalamic–cortical circuits) (Vanderschuren and Everitt 2005), combined with decreased activity in reward circuits (Koob and Le Moal 2005,Koob and Le Moal 2008).
Figure.
Neurocircuitry schematic illustrating the combination of neuroadaptations in the brain circuitry for the three stages of the addiction cycle that drive drug-seeking behavior in the addicted state. Note the activation of the ventral striatum/dorsal striatum in the binge intoxication stage. During the withdrawal–negative-affect stage, the dopamine systems are compromised and brain stress systems such as CRF are activated to reset further the salience of drugs and drug-related stimuli in the context of an aversive dysphoric state. During the preoccupation–anticipation stage, contextual cues via the hippocampus and stimuli cues via the basolateral amygdala converge with frontal cortex activity to drive drug seeking. Other components in the frontal cortex are compromised, producing deficits in executive function.
SOURCE: Koob et al. 2008.
The delineation of these circuits and the signaling molecules involved has allowed, and will continue to allow, researchers to identify appropriate targets for additional therapeutic interventions, develop medications that act on these targets, and test these medications in validated animal models and, if there is evidence of effectiveness, in human clinical trials.
New Targets for Medication Development
Three key sources can provide information for the development of new medications: research into basic neurobiological mechanisms underlying the different stages of the addiction cycle, the effects of currently approved medications on animal models of the different stages of the addiction cycle, and clinical studies of medications approved for other indications that overlap with specific components of addiction. Information from these sources has led to investigations of a variety of neurotransmitter systems affecting different aspects of the dependence syndrome. The emphasis of this discussion is on neuronal circuits and neurotransmitter systems involved in the withdrawal–negative-affect stage of addiction, because the fear of experiencing withdrawal symptoms (which are caused by the brain’s adaptation to the continued presence of alcohol) is one motivation for alcoholics to continue drinking (Koob and Le Moal 2008), and, even more importantly, negative emotional states during protracted abstinence provide a strong motivation for relapse. These neurotransmitter systems involved include the dopamine, γ-aminobutyric acid (GABA), CRF, and glutamate systems, all of which target circuits that can restore deregulated reward systems involved during the withdrawal–negative-affect stage. Moreover, dopamine can affect the binge–intoxication stage and glutamate acts during the preoccupation–anticipation stage. Some of these neurotransmitter systems (i.e., GABA, glutamate, and CRF) already are targets of medications used to treat addiction, but all of them still have the potential to yield new medications (see table). (Koob et al. 2009).
Table.
Neurotransmitter Systems in the Brain Involved in Different Stages of the Addiction Cycle and Existing and Potential Pharmacotherapies Targeting Them in the Treatment of Alcohol Dependence
| Neurotransmitter System | Existing and Potential Pharmacotherapies |
|---|---|
| Dopamine system | Dopamine receptor partial agonists
Dopamine receptor antagonists
|
| γ-Aminobutyic acid (GABA) system | GABA receptor modulators
|
| Brain stress system | CRF-related targets
Non–CRF-related targets
|
| Glutamate system | Glutamate receptor agonists and antagonists
|
Agents Acting on the Dopamine System
The mesolimbic dopamine system projects from the ventral tegmental area (VTA) to basal forebrain sites, the NAc, and the central nucleus of the amygdala; it has a key role in motivation and mediates the reinforcing actions of many drugs of abuse, including alcohol. Moreover, normal functioning of this system is disrupted during acute withdrawal from all major drugs of abuse (Weiss and Koob 2001). For example, dopamine levels in the NAc decrease substantially in animals undergoing withdrawal from alcohol (Weiss et al. 1996). Moreover, dopamine-releasing neurons in the VTA show decreased activity during withdrawal from most major drugs of abuse (Melis et al. 2005). Therefore, it appears plausible that medications altering dopamine activity could be effective in the treatment of AOD addiction.
Researchers have studied the effects of dopamine partial agonists1—agents that block the activities of the dopamine receptors (i.e., act as dopamine antagonists) when normal dopamine activity is high but enhance receptor activity (i.e., act as agonists) when dopamine activity is low. Such an approach is thought to generate less severe or fewer side effects than full agonists or antagonists (Pulvirenti and Koob 2002). The validity of this approach has been demonstrated by findings that partial agonists of one of the dopamine receptors (i.e., the D2 receptor) decreased the reinforcing effects of orally self-administered alcohol in nondependent2 rats (Bono et al. 1996). Studies using animals that self-administered psychostimulants (e.g., amphetamine) found that D2 partial agonists can reverse some of the effects of withdrawal (e.g., Orsini et al. 2001), but the approach using partial agonists has yet to be explored in animal models of compulsive drinking.
Other agents targeting the dopamine system also have shown effectiveness in preventing some aspects of alcohol dependence. For example, antagonists of the dopamine D3 receptor have blocked cue-induced reinstatement of cocaine and alcohol self-administration (Heidbreder et al. 2005), and other investigators are studying the effects of D1 receptor antagonists on various aspects of drug dependence. Together, the results obtained to date suggest that altered dopamine signaling contributes to craving for and relapse to AOD use and that therefore dopamine partial agonists may be effective in treating certain aspects of addiction (Spanagel and Kiefer 2008).
Agents Acting on the GABA System
GABA also acts at several different receptors (i.e., GABAA and GABAB receptors) that have been studied as targets for medications to treat alcoholism and other addictions. For example, GABAA receptor antagonists and inverse agonists3 decrease alcohol self-administration in animals (Hyytia and Koob 1995; Rassnick et al. 1993). However, these agents cause excessive excitability of certain brain cells, limiting their usefulness. In contrast, GABA receptor agonists or agents that modulate receptor activity can block drug-seeking behavior by acting on a variety of brain systems. For example, in nondependent rats, GABA receptor modulators that increased GABAergic activity reduced the animal’s self-administration of alcohol (Colombo et al. 2003). GABA receptor agonists also block alcohol withdrawal in animals (Frye et al. 1983) and decrease drinking and certain components of craving in alcoholics (Addolorato et al. 2002a,b). The GABAB agonist baclofen even has been reported to reduce alcohol craving and intake in a preliminary double-blind, placebo-controlled trial (Addolorato et al. 2002a). However, these agents have substantial sedative effects at therapeutic doses, limiting their usefulness. To circumvent this, researchers are studying GABA receptor modulators that act more indirectly.
One of these is gabapentin (Neurontin®; Pfizer), a molecule designed to have a similar structure as GABA that mainly interacts with voltage-gated N-type calcium channels (Sills 2006). It originally was developed as an anticonvulsant drug, but because it increases GABA concentrations in the brain (Taylor et al. 1998), it also has been evaluated for alcohol dependence. In animal studies, gabapentin had strikingly different effects in nondependent and alcohol-dependent rats (Roberto et al. 2008). In nondependent rats, it facilitated GABAergic transmission in the central nucleus of the amygdala but did not affect alcohol intake. In dependent rats, however, gabapentin decreased both GABAergic transmission and alcohol intake. These findings suggest that during the development of alcohol dependence, neuroadaptive changes occur in the GABA system, including a reduction in sensitivity and/or number of the GABAB receptors (Roberto et al. 2008). Moreover, in human laboratory studies, gabapentin decreased craving and reversed or ameliorated some of the consequences of protracted abstinence (Mason et al. 2009). These findings provide a prime example for how analyses of neurobiological processes associated with dependence can lead to testing of novel agents (or agents that were developed for a different purpose) in animal models and, subsequently, humans, potentially resulting in new treatment approaches.
Agents Acting on the Brain Stress System
Agents Acting on the CRF System
Alcohol is a powerful activator of stress systems involving both the HPA axis and extrahypothalamic CRF systems in the extended amygdala; the latter also become hyperactive during withdrawal, leading to increased CRF levels in certain brain regions (i.e., the central nucleus of the amygdala and the bed nucleus of the stria terminalis) (Funk et al. 2006; Merlo-Pich et al. 1995; Olive et al. 2002). In animal models, acute withdrawal and protracted abstinence from alcohol and all other major drugs of abuse produce anxiety-like responses that are mediated by CRF and can be reversed by CRF receptor antagonists (Knapp et al. 2004; Overstreet et al. 2004). The effects of these antagonists also appear to be specific to alcohol-dependent animals. Thus, an antagonist that acts at different CRF receptors had no effect on alcohol self-administration in nondependent rats but eliminated excessive drinking in dependent rats during acute withdrawal and protracted abstinence (Valdez et al. 2002). Similarly, injections of other CRF antagonists blocked increased alcohol intake during acute withdrawal and protracted abstinence in alcohol-dependent rats but not in nondependent rats (Funk et al. 2007; Gehlert et al. 2007). These data suggest that extrahypothalamic CRF is an important mediator in the increased self-administration associated with alcohol dependence and therefore a promising target for pharmacotherapy. However, no human laboratory studies or clinical trials have yet been initiated to investigate the effects of CRF antagonists on alcohol dependence.
Agents Acting on Non-CRF Brain Stress Targets
Other neurotransmitter systems and neuromodulators within the stress systems of the extended amygdala also may be deregulated during the development of AOD dependence. Thus, deregulation of noradrenaline, dynorphin, vasopressin, orexin, and substance P all appear to play a role in alcohol dependence (Koob 2008). Accordingly, these signaling systems also may be appropriate targets for pharmacotherapy. For example, administration of an antagonist acting at one of the noradrenergic receptors decreases self-administration in alcohol-dependent rats (Walker et al. 2008). Noradrenaline also interacts with CRF during the brain stress activation associated with withdrawal from AODs (Koob 2008).
Dynorphins are a class of endogenous opioids that interact with the κ-opioid receptor and are thought to mediate negative emotional states. Accordingly, κ-opioid receptor agonists produce depression and dysphoria in humans (Pfeiffer et al. 1986). Furthermore, κ-opioid receptors have been linked to reinstatement of drug-seeking behavior because in alcohol-dependent rats, a κ-opioid receptor antagonist selectively blocked the increase in alcohol self-administration associated with withdrawal (Walker and Koob 2008). Therefore, the dynorphin system may provide another avenue to treating alcohol dependence. This approach already has been tested successfully in models of addiction to other drugs and clearly warrants study in models of alcohol dependence.
Finally, some neuromodulatory systems that counteract CRF in the brain stress response are being investigated as potential targets for pharmacotherapy of alcohol dependence. These include, for example, signaling systems using molecules called neuropeptide Y, nociceptin, and substance P. Agonists of the neuropeptide Y and nociceptin systems have been shown to reduce excessive drinking associated with alcohol dependence (Ciccocioppo et al. 2000; Heilig et al. 1994). Conversely, approaches to reduce the activity of the receptor system for substance P reduced voluntary drinking in high-alcohol–consuming mice and reduced craving and other physiological responses in recently detoxified alcoholic patients (George et al. 2008).
Taken together, all of these findings suggest that agents which interfere with various CRF-mediated and non-CRF–mediated responses of the brain stress system to alcohol might have potential as new treatment approaches for alcoholism and should be investigated further. Continued support with funding from NIAAA and other sources will be essential for the translation of laboratory findings in animals and humans into treatments for large numbers of alcoholic patients. Particularly critical at this juncture is the continued cross-validation of animal and human laboratory models that are predicative of treatment efficacy.
Agents Acting on the Glutamate System
The neurotransmitter glutamate interacts with several receptors, including the N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), kainate, and metabotropic glutamate receptors. The glutamate system acts at several sites in the neurobiology of alcohol dependence, many of which provide potential targets for medications development. For example, at low doses alcohol acts as an NMDA receptor antagonist, and this reduction of glutamatergic activity may mediate the acute rewarding effects of alcohol (Hoffman et al. 1989). Conversely, the glutamatergic system shows excessive activity during acute and prolonged abstinence from alcohol. Analyses of these effects of alcohol consumption and withdrawal on the glutamate system have led to the development of the medication acamprosate, which is a partial agonist of the NMDA receptor and an antagonist of metabotropic glutamate receptors (de Witte et al. 2005; Spanagel and Kiefer 2008).
AMPA or kainate receptors also appear to mediate some of glutamate’s effects in addiction processes, as indicated by the results of clinical trials with the anticonvulsant medication topiramate. This agent acts as an antagonist at AMPA and kainate receptors but not NMDA receptors. In animal models of alcoholism, topiramate decreased alcohol consumption and preference but failed to block place preference to alcohol in mice (Gabriel and Cunningham 2005; Gremel et al. 2006). Topiramate did block stress-induced increases in alcohol consumption and preference (Farook et al. 2009) and conditioned abstinence behavior in mice (Farook et al. 2007). These and other findings suggest that topiramate may act on brain stress systems to reduce the motivation for alcohol seeking in dependence. In clinical trials involving alcohol-dependent patients, topiramate reduced drinking behavior and improved the participants’ quality of life (Johnson et al. 2007; Olmsted and Kockler 2008). However, this treatment also produced significant adverse effects on memory and concentration.
Because direct glutamatergic antagonists can induce significant side effects, medications that modulate the system may be more promising candidates for the treatment of addiction, including alcoholism. Such agents may not only be able to dampen the hyperexcitability of glutamatergic systems during protracted abstinence from alcohol but also may decrease drug- and cue-induced reinstatement of alcohol self-administration. For example, both an antagonist of metabotropic glutamate receptor 5 (mGluR5) and agonists of mGluR2 and mGluR3, all of which decrease glutamate function, blocked cue-induced reinstatement of alcohol self-administration (Schroeder et al. 2008; Zhao et al. 2006).
Conclusions and Future Directions
The U.S. National Institutes of Health, and particularly NIAAA, has supported much of the research into the mechanisms underlying the development of alcohol dependence, thereby laying the foundation for the development of effective pharmacotherapies for this debilitating disorder. This NIAAA-funded research has led to tremendous breakthroughs in elucidating the basic neurobiology of addiction and in developing and validating behavioral and pharmacological treatments of alcoholism. For example, preclinical and clinical proof-of-concept in the development of naltrexone for the treatment of alcoholism would not have been possible without NIAAA funding.
However, none of the existing medications are effective in all patients, and additional agents need to be identified and developed that allow for effective treatment of additional patient subgroups. As described here, analyses of the neurobiology underlying both the withdrawal–negative-affect stage and the preoccupation–anticipation (craving) stage of the addiction cycle already have revealed numerous potential new targets for pharmacotherapy development. In fact, agents that act on neurotransmitter systems associated with both of these stages would be optimal.
Thus, there is substantial potential for the development of future pharmacotherapies for treatment of addiction. Moreover, further analyses of currently available medications in validated animal models can yield additional information on the neuronal circuits and neuropharmacological mechanisms involved in the development and maintenance of addiction, thereby providing a means to identify additional targets and to develop and evaluate future medications.
Animal models can be defined as experimental paradigms developed for the purpose of studying a given phenomenon found in humans, and animal models remain key elements for exploring the neurobiological bases of psychiatric disorders and providing targets for medications development. However, animal models for a complete syndrome of a psychiatric disorder are unlikely to be possible either conceptually or practically. Thus, although there are no complete animal models of psychiatric disorders, animal models do exist for individual elements of each syndrome. One approach to the development of animals models of heuristic value is that animal models are most likely to have construct validity when the model mimics only the specific signs or symptoms associated with a pathological condition (Geyer and Markou 1995). Using this conceptual approach, new procedures in animal models and human laboratory models in alcoholism are being refined in the context of the three stages of the addiction cycle outlined above. New data provide compelling evidence for a process termed the “Rosetta Stone” approach, in which existing pharmacotherapies are used to validate and improve animal models and human laboratory models, resulting in improved translation to human clinical therapeutics (Koob et al. 2009). Using this approach, animal models in the substance use disorders domains have excellent face and construct validity and have led to major insights into their neurobiological mechanisms of action. Such information not only provides a rich substrate for targets for pharmcotherapeutic approaches but also provides an excellent heuristic basis for validating the efficacy of behavioral approaches to treatment of alcoholism.
Other efforts could focus on molecular changes that occur in response to alcohol exposure at the level of signal transduction within or between cells or in the genes encoding the involved molecules. Such basic research can provide insights into how the neuronal circuits described in this article become deregulated during the different stages of the addiction cycle, thereby providing new potential targets for medication development. Moreover, such effects may identify targets for genetic pharmacology in which treatments could be individualized for patients based on specific genetic polymorphisms carried by the individual. To date, no medication targets have been identified using analyses of such molecular changes but eventually, molecular studies may become key to understanding the vulnerability to addiction as well as help identify possible targets for pharmacotherapeutic approaches.
Footnotes
To exert its effects, dopamine (like any other neurotransmitter) is released by a signal-emitting nerve cell (i.e., neuron) and interacts with docking molecules (i.e., receptors) on the surface of the signal-receiving neuron. This interaction induces a chain of reactions in the signal-receiving cell, resulting either in the generation or suppression of a new nerve signal, depending on the neurotransmitter and cells involved. Agents that bind to a neurotransmitter receptor and induce the same response as the normal neurotransmitter are called agonists; agents that bind to the receptor, thereby blocking the normal neurotransmitter or an agonist from binding and preventing receptor activation, are called antagonists.
The term nondependent refers to animals with limited access to alcohol that do not show physical or motivational symptoms of withdrawal when alcohol is removed. Conversely, dependent rats had, with sufficient exposure to alcohol, to show physical or motivational symptoms of withdrawal when alcohol is removed.
Inverse agonists bind to the same receptor as a normal neurotransmitter or agonist but induce the opposite effect (rather than just preventing neurotransmitter or agonist actions by blocking their interaction with the receptor).
Financial Disclosure
The author declares that he has no competing financial interests.
References
- Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake: A preliminary double-blind randomized controlled study. Alcohol and Alcoholism. 37:504–508. doi: 10.1093/alcalc/37.5.504. 2002a. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Capristo E, et al. Rapid suppression of alcohol withdrawal syndrome by baclofen. American Journal of Medicine. 112:226–229. doi: 10.1016/s0002-9343(01)01088-9. 2002b. [DOI] [PubMed] [Google Scholar]
- Bono G, Balducci C, Richelmi P, et al. Dopamine partial receptor agonists reduce ethanol intake in the rat. European Journal of Pharmacology. 1996;296:233–238. doi: 10.1016/0014-2999(95)00592-7. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Panocka I, Massi M. Nociceptin/orphanin FQ and drugs of abuse. Peptides. 2000;21:1071–1080. doi: 10.1016/s0196-9781(00)00245-x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Vacca G, Serra S, et al. Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology. 2003;167:221–224. doi: 10.1007/s00213-003-1397-y. [DOI] [PubMed] [Google Scholar]
- de Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: Elucidating the mechanism of action. CNS Drugs. 2005;19:517–537. doi: 10.2165/00023210-200519060-00004. [DOI] [PubMed] [Google Scholar]
- Farook JM, Lewis B, Littleton JM, Barron S. Topiramate attenuates the stress-induced increase in alcohol consumption and preference in male C57BL/6J mice. Physiology & Behavior. 2009;96:189–193. doi: 10.1016/j.physbeh.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Farook JM, Morrell DJ, Lewis B, et al. Topiramate (Topamax) reduces conditioned abstinence behaviours and handling-induced convulsions (HIC) after chronic administration of alcohol in Swiss-Webster mice. Alcohol and Alcoholism. 2007;42:296–300. doi: 10.1093/alcalc/agm047. [DOI] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Differential sensitivity of ethanol withdrawal signs in the rat to gamma-aminobutyric acid (GABA) mimetics: Blockade of audiogenic seizures but not forelimb tremors. Journal of Pharmacology and Experimental Therapeutics. 1983;226:720–725. [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. Journal of Neuroscience. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, et al. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biological Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel KI, Cunningham CL. Effects of topiramate on ethanol and saccharin consumption and preferences in C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2005;29:75–80. doi: 10.1097/01.alc.0000150014.79657.64. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, et al. 3-(4-Chloro-2-mor-pholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b] pyridazine: A novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. Journal of Neuroscience. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 787–798. [Google Scholar]
- George DT, Gilman J, Hersh J, et al. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Gabriel KI, Cunningham CL. Topiramate does not affect the acquisition or expression of ethanol conditioned place preference in DBA/2J or C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2006;30:783–790. doi: 10.1111/j.1530-0277.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: Role in emotional integration. Trends in Neurosciences. 1994;17:80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Moses F, Tabakoff B. N-methyl-d-aspartate receptors and ethanol: Inhibition of calcium flux and cyclic GMP production. Journal of Neurochemistry. 1989;52:1937–1940. doi: 10.1111/j.1471-4159.1989.tb07280.x. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. European Journal of Pharmacology. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: A randomized controlled trial. JAMA: Journal of the American Medical Association. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature Neuroscience. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. London: Elsevier; 2006. [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Lloyd GK, Mason BJ. Development of pharmacotherapies for drug addiction: A Rosetta Stone approach. Nature Reviews Drug Discovery. 2009;8:500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Williams LD, Drobes DJ. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: Effects of gabapentin. Addiction Biology. 2009;14:73–83. doi: 10.1111/j.1369-1600.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: Hypodopaminergic state. International Review of Neurobiology. 2005;63:101–154. doi: 10.1016/S0074-7742(05)63005-X. [DOI] [PubMed] [Google Scholar]
- Merlo-Pich E, Lorang M, Yeganeh M, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by micro-dialysis. Journal of Neuroscience. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature Neuroscience. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, et al. Naltrexone and coping skills therapy for alcohol dependence: A controlled study. Archives of General Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacology, Biochemistry, and Behavior. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted CL, Kockler DR. Topiramate for alcohol dependence. Annals of Pharmacotherapy. 2008;42:1475–1480. doi: 10.1345/aph.1L157. [DOI] [PubMed] [Google Scholar]
- Orsini C, Koob GF, Pulvirenti L. Dopamine partial agonist reverses amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:789–792. doi: 10.1016/S0893-133X(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacology, Biochemistry, and Behavior. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Koob GF. Being partial to psychostimulant addiction therapy. Trends in Pharmacological Sciences. 2002;23:151–153. doi: 10.1016/s0165-6147(00)01991-x. [DOI] [PubMed] [Google Scholar]
- Rassnick S, D’Amico E, Riley E, Koob GF. GABA antagonist and benzodiazepine partial inverse agonist reduce motivated responding for ethanol. Alcoholism: Clinical and Experimental Research. 1993;17:124–130. doi: 10.1111/j.1530-0277.1993.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, O’Dell LE, et al. Cellular and behavioral interactions of gabapentin with alcohol dependence. Journal of Neuroscience. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, et al. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: Blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills GJ. The mechanisms of action of gabapentin and pregabalin. Current Opinions in Pharmacology. 2006;6:108–113. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Kiefer F. Drugs for relapse prevention of alcoholism: Ten years of progress. Trends in Pharmacological Sciences. 2008;29:109–115. doi: 10.1016/j.tips.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Gee NS, Su TZ, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Research. 1998;29:233–249. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, et al. Increased ethanol self-administration and anxiety-like behavior during acute withdrawal and protracted abstinence: Regulation by corticotropin-releasing factor. Alcoholism: Clinical and Experimental Research. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Behavioral and neural mechanisms of compulsive drug seeking. European Journal of Pharmacology. 2005;526:77–88. doi: 10.1016/j.ejphar.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Archives of General Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of κ-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. α1-Noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Koob GF. Drug addiction: Functional neurotoxicity of the brain reward systems. Neurotoxicity Research. 2001;3:145–156. doi: 10.1007/BF03033235. [DOI] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, et al. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. Journal of Neuroscience. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]