Abstract
Alcohol abuse suppresses multiple arms of the immune response, leading to an increased risk of infections. The course and resolution of both bacterial and viral infections is severely impaired in alcohol-abusing patients, resulting in greater patient morbidity and mortality. Multiple mechanisms have been identified underlying the immunosuppressive effects of alcohol. These mechanisms involve structural host defense mechanisms in the gastrointestinal and respiratory tract as well as all of the principal components of the innate and adaptive immune systems, which are compromised both through alcohol’s direct effects and through alcohol-related dysregulation of other components. Analyses of alcohol’s diverse effects on various components of the immune system provide insight into the factors that lead to a greater risk of infection in the alcohol-abusing population. Some of these mechanisms are directly related to the pathology found in people with infections such as HIV/AIDS, tuberculosis, hepatitis, and pneumonia who continue to use and abuse alcohol.
Keywords: Alcohol abuse, alcohol and other drug effects and consequences, immune system, immune response, immunosuppressive effect, infection, bacterial infection, viral infection, communicable disease, host defense mechanisms
Both acute and chronic alcohol abuse can induce significant defects in the body’s defense against microorganisms (i.e., pathogens) by interfering with multiple aspects of the immune response. The resulting increased risk and severity of infections in chronic alcoholics has been recognized as early as 1785, by Benjamin Rush, the first Surgeon General of the United States. The impact of alcohol abuse on risk and severity of infection has been demonstrated particularly well for infections of the respiratory tract, especially bacterial pneumonia and tuberculosis (Zhang et al. 2008). Alcohol consumption also is associated with a higher prevalence of hepatitis C infection (Prakash et al. 2002) and increases the risk of infection with the human immunodeficiency virus (HIV), particularly in binge drinkers (Baliunas et al. 2009). In addition to increasing the risk of infections, alcohol abuse has been reported to contribute to the morbidity and mortality resulting from these infections in alcohol-abusing patients. This is particularly relevant in chronic infections, such as HIV and hepatitis C. After providing a brief overview of the human immune system and its various components, this article summarizes alcohol’s diverse effects on these components.
Overview of the Human Immune System
The body constantly is exposed to pathogens that penetrate either our external surface (i.e., the skin), through wounds or burns, or the internal surfaces (i.e., epithelia) lining the respiratory and gastrointestinal (GI) tracts. The body responds to such an infectious challenge with a two-level response. The first line of defense is called the innate immunity;1 it exists from birth, before the body is even exposed to a pathogen. It is an immediate and rapid response that is activated by any pathogen it encounters (i.e., is nonspecific); in addition, it plays a key role in the activation of the second level of the immune response, termed the adaptive or acquired immunity. This part of the immune response is specific to one particular pathogen and also creates an “immune memory” that allows the body to respond even faster and more effectively if a second infection with the same pathogen occurs. Both innate and adaptive immunity rely on a multitude of different cells and molecules. Thus, both types of immunity are mediated partly by the actions of specific immune cells (i.e., include a cell-mediated response) and partly by the actions of molecules secreted by various immune cells (i.e., include a humoral response).
The Innate Immune Response
The innate immune response comprises five main elements:
The physical barrier formed by epithelial cells in the skin, gut mucosa, and airways that prevents the entry of pathogens into the body;
A chemical shield to prevent microbial growth and invasion that is provided by antimicrobial peptides, reactive oxygen species, and the pH and lipid composition of the internal and external surfaces;
A pathogen recognition system that identifies invading pathogens (e.g., through molecules called Toll-like receptors);
An inducible response to invading pathogens that includes cell-mediated and humoral components; and
The coordinated recruitment of other cells that amplify the response.
Virtually all of these components are affected by alcohol; however, the discussion in the following sections will focus on the first and fourth of these elements.
The cell-mediated arm of the innate immunity is orchestrated primarily by granulocytes, monocytes/macrophages, dendritic cells, and natural killer (NK) cells. Granulocytes are white blood cells (i.e., leukocytes) that derive their name from the large granules that are visible when the cells are stained for microscopic analysis. They further are characterized by oddly shaped nuclei with multiple lobes and therefore also are called polymorphonuclear leukocytes (PMNs). They represent approximately 60 percent of all circulating leukocytes. The most abundant type of PMNs is called neutrophils. These cells act as phagocytes—that is, they engulf pathogens and ingest them in a process called phagocytosis. In addition, they can excrete toxic substances from their granules that can kill pathogens. PMNs produce a host of bacteria-killing (i.e., bactericidal) molecules (e.g., myeloperoxidase, defensins, azurophil-derived bactericidal factors, bactericidal permeability-increasing protein, cationic proteins, gelatinase, and lactoferrin). In addition, PMNs participate in the regulation of the local defense response by releasing signaling molecules called cytokines and chemokines (e.g., tumor necrosis factor [TNF]-α; interleukin [IL]-1β, IL-6, and IL-8; and macrophage inflammatory protein [MIP]-2). These molecules help recruit and activate additional PMNs as well as macrophages to the site of an injury or infection.
Monocytes and macrophages are leukocytes with a single-lobed nucleus that also act as phagocytes and which therefore also are called mononuclear phagocytes. Monocytes are an immature form of these cells that circulate in the blood until they are alerted to the presence of a pathogen in a particular tissue. Once they are at the site of infection, they swell in size and develop into the mature defensive cells—the macrophages—that enter the tissues. After eliminating pathogens by phagocytosis, the monocytes exhibit pathogen-derived proteins and other molecules (i.e., antigens) on their surfaces. This is important for activating the cells of the adaptive immune response. Finally, monocytes and macrophages also produce certain cytokines that help regulate immune system activity.
Dendritic cells also are mononuclear phagocytes derived from monocytes. Their main role is to capture, ingest, and process antigens in order to present them on their surface to cells of the adaptive immune response (i.e., to the T-lymphocytes). Thus, dendritic cells play a crucial role in linking innate and adaptive immune responses. Lastly, NK cells are abundant in the liver (Gao et al. 2009) and recognize cells that have low levels of a protein called class I major histocompatibility complex (MHC) on their surface. This reduced class I MHC expression can result from infection with certain types of viruses. NK cells eliminate cells with low class I MHC expression as well as cancer cells.
The most important components of the humoral arm of the innate immune response include the following molecules:
Cytokines and chemokines. Cytokines are proteins made and released by one cell that affect the behavior of other cells (e.g., activate other cells) and cell–cell interactions. Thus, cytokines released by immune cells control immune processes by regulating the production of new immune cells from precursor cells, activating lymphocytes and phagocytes, coordinating the cell-mediated and humoral immune responses, mediating the process of inflammation, and killing cells directly. Important cytokines are TNF-αand the ILs. Chemokines are similar to cytokines; however, their main function is to attract additional cells (e.g., monocytes and neutrophils) to the site of an infection.
Interferons (IFNs) are proteins that are involved in the immune response to viral infection. Thus, they participate in inducing a state of resistance to viral replication and upregulate the cell-mediated immune response to viral infection.
The complement system comprises a large number of distinct plasma proteins that react with one another to cover the surface of a pathogen so that it can be recognized and ingested by phagocytes. This process, which is called opsonization, induces a series of inflammatory responses that help combat the infection. The complement system can be activated through three different biochemical pathways.
Acute-phase proteins are, as the name implies, produced early during an inflammatory response to infection. They participate in the opsonization of pathogens and of monocytes that have ingested pathogens as well as in the activation of the complement cascade. Important acute-phase proteins are C-reactive protein, mannan-binding lectin, and pulmonary surfactants A and D.
The innate immune response orchestrated by all these components provides the first line of defense against invading pathogens and plays a key role in the activation and orientation of adaptive immunity, as well as in the maintenance of tissue integrity and repair. Only if a pathogen can evade the different components of this response (i.e., structural barriers as well as cell-mediated and humoral responses) does the infection become established and an adaptive immune response ensues.
The Adaptive Immune Response
The innate immune response to a pathogen is followed by an adaptive immune response that is activated only after the body is exposed to the pathogen for the first time and which is specific to that one pathogen. This activation of the adaptive immune response depends on the display of antigens from the invading pathogen (or any other foreign molecule) on the surface of antigen-presenting cells (e.g., monocytes or dendritic cells) in a way that can be recognized by the cells mediating the adaptive immune response—that is, the T-lymphocytes (or T-cells) and the B-lymphocytes (or B-cells).
T-cells are responsible for the cell-mediated arm of the adaptive immune response. After their formation in the bone marrow and maturation in the thymus, they remain in an inactive (naïve) state until they encounter a specific antigen. This encounter activates the T-cells, which then further differentiate into different subtypes. Two important subtypes of T-cells are the following:
Helper T-cells produce cytokines to stimulate the activity of other immune cells. According to the cytokines they produce, they are categorized into three subsets: (1) Th1 helper cells that produce IFN-γ and mediate immunity against intracellular pathogens; (2) Th2 helper cells that produce IL-4, IL-5, and IL-13 and promote humoral immunity and allergic responses; and (3) Th17 helper cells that produce IL-17, IL-21, and IL-22 and are implicated in host defense and autoimmunity. Helper T-cells (as well as a few other immune cells, such as macrophages) are characterized by the presence of a molecule called CD4 on their surface; this molecule serves as the receptor to which HIV can bind when it infects the cells. Accordingly, CD4-carrying (i.e., CD4+) helper T-cells are the major target of HIV infection. Their depletion leads to the development of the acquired immunodeficiency syndrome (AIDS) and the development of numerous opportunistic infections, including pneumonia caused by infection with the fungus Pneumocystis or infections with the yeast Candida albicans, the tuberculosis pathogen Mycobacterium tuberculosis, and several other pathogens that usually cause no harm in people with a healthy immune system (Phair 1990).
Cytotoxic T-cells recognize antigens on the surface of virus-infected or transplanted cells and destroy these cells; each cytotoxic T-cell recognizes only one specific antigen. Cytotoxic T-cells are characterized by the presence of a molecule called CD8 on their surface.
B-cells are responsible for the humoral arm of the adaptive immune response. They produce immune molecules called antibodies or immunoglobulins that they can either display on their surface or secrete. The antibodies can recognize and interact with antigens, and each B-cell produces antibodies that recognize only one specific antigen. The antigen– antibody interaction leads to the activation of the B-cell. The activated B-cell then begins to multiply and mature fully in a series of developmental processes that are accompanied by changes in the class of immunoglobulin that the cell produces (i.e., immunoglobulin class switching).2 In most cases, the resulting daughter cells develop into plasma cells, which secrete many copies of the antibody into the blood or fluid between cells. These antibodies then will bind to any matching antigen molecules they encounter in the blood or on other cells, thereby marking them for destruction. Some B-cells, however, become memory cells that will remain dormant in the body for years and can be activated rapidly if a second infection with the same pathogen occurs. The activities of T-cells and B-cells are intricately intertwined through the actions of various cytokines to orchestrate an effective immune response to any pathogen the organism may encounter.
Both the innate and the adaptive immune response are critical for effective host defense to infectious challenges. Multiple aspects of both arms of the immunity response are significantly affected by alcohol abuse, as described in the following sections.
Alcohol and the Innate Immune Response
Alcohol and Structural Host Defense Mechanisms
The first line of host defense involves both structural (i.e., epithelial) cells and immune cells (i.e., macrophages and dendritic cells) at mucosal surfaces. The epithelial cells function as a physical barrier as well as regulators of the innate and adaptive immunity. Particularly important are the epithelial immune barriers of the reproductive, GI, and respiratory tracts. Several lines of evidence suggest that alcohol abuse significantly disrupts the GI and respiratory tract immune barriers.
Effects on the GI Tract
The GI tract is the organ exposed to the highest concentration of alcohol during acute or chronic ingestion. Therefore, it has been studied extensively with respect to the pathologic effects of alcohol, particularly as they impact the ability of the intestinal barrier to allow passage of certain substances into the blood (i.e., intestinal permeability). Collective evidence from animal and human studies indicates that chronic alcohol abuse results in excessive intestinal permeability, which may underlie several of the health consequences of excessive alcohol consumption (Keshavarzian et al. 1999; Rao et al. 2004). For example, alterations in cell structures called tight junctions in the epithelial cells lining the intestine contribute to the pathophysiology of alcohol-induced intestinal permeability (Rao 2009). These tight junctions are areas where two epithelial cells are closely associated with each other. They serve to hold the cells together and to prevent the direct passage of water and other molecules from the intestine into the blood stream. Thus, if the tight junctions are damaged (e.g., by alcohol’s actions), material from the intestine can “leak” into the blood, as has been shown by increased levels of bacterial molecules called lipopolysaccharides (LPSs) in the blood of alcoholic patients (Hanck et al. 1998). Alcohol interferes with tight-junction functioning through several mechanisms. For example, alcohol (or its metabolite acetaldehyde) impairs trafficking of epithelial tight-junction proteins, such as zona occludens (ZO)-1 and occludin (Atkinson and Rao 2001). Moreover, alcohol-induced epigenetic effects may modulate the production of tight-junction protein. Thus, studies found that alcoholics with liver disease exhibited dramatically increased expression of a small, regulatory molecule called microRNA (miR) 212 in colon tissue samples (Tang et al. 2008). miR-212 can bind to the messenger RNA (mRNA) from which the ZO-1 protein is produced; this binding prevents ZO-1 production, thereby contributing to alcohol-induced increased permeability of the intestinal epithelium and to the “leaky” alcoholic gut.
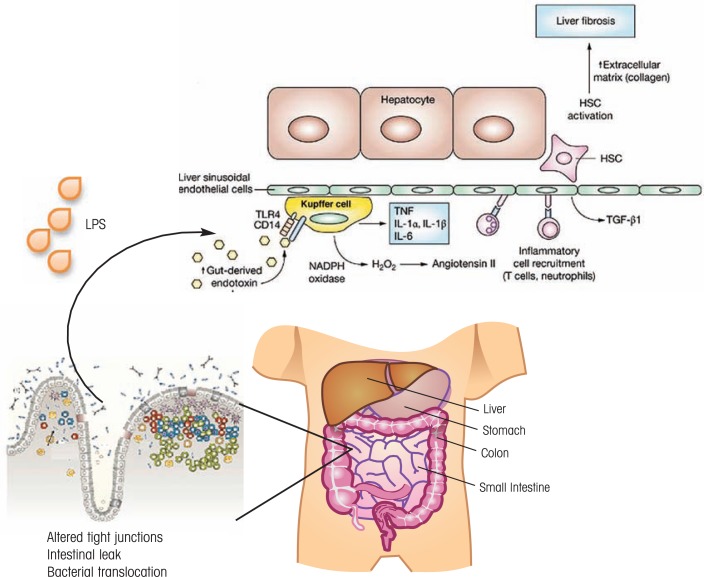
The consequences of impaired gut structural integrity are significant (see figure 1). Increased intestinal leakage allows bacteria-derived products, such as LPSs, to enter the blood stream supplying the liver (i.e., the portal circulation) and, in the liver, to activate a variety of cells, including endothelial cells, liver macrophages (i.e., Kupffer cells), stellate cells, and the main liver cells (i.e., hepatocytes). This results in a chronic inflammatory environment conducive to liver injury.
Figure 1.
Alcohol’s effects on the structural host defense of the gastrointestinal (GI) tract. Alcohol-induced changes in tight junctions cause increased intestinal leaks that lead to translocation of bacteria-derived products such as lipopolysaccharide (LPS). These molecules enter the circulation to the liver where they activate endothelial and stellate cells as well as hepatocytes, resulting in a chronic inflammatory environment aggravating organ injury. This also may contribute to HIV disease pathophysiology.
NOTE: CD14 = cluster of differentiation 14; HSC = hepatic stellate cell; IL = interleukin; NADPH = nicotinamide adenine dinucleotide phosphate; TGF= tissue growth factor; TNF = tumor necrosis factor; TLR4 = toll-like receptor 4.
In addition to contributing to the pathogenesis of alcoholic liver disease (Rao 2009), other observations suggest that enhanced endothelial permeability is detrimental to HIV disease course in alcohol-abusing patients. In the simian immunodeficiency virus (SIV)/rhesus macaque model of HIV infection, chronic alcohol feeding increased the number of virus particles in the blood (i.e., plasma viral load) and hastened the progression to AIDS (Bagby et al. 2006; Poonia et al. 2006). Both HIV and SIV infection themselves cause extensive intestinal disease and enhanced intestinal permeability during advanced disease stages; moreover, evidence from HIV-infected humans and SIV-infected primates shows a compelling association between the entry of microbial antigen into the circulation and the progression of retroviral disease (Brenchley et al. 2006). It is hypothesized that the HIV-related leakage results in chronic activation of the immune system. Because HIV infects primarily immune cells (i.e., CD4+ T-cells and macrophages), this activation leads to the generation of more target cells for the virus; eventually, however, the body’s capacity to replenish CD4+ T-cells is exhausted, which results in disease progression to AIDS. The enhanced gut permeability resulting from alcohol abuse is likely to exacerbate the gut leak associated with HIV/SIV infection, thereby further accelerating disease progression.
Effects on the Respiratory System
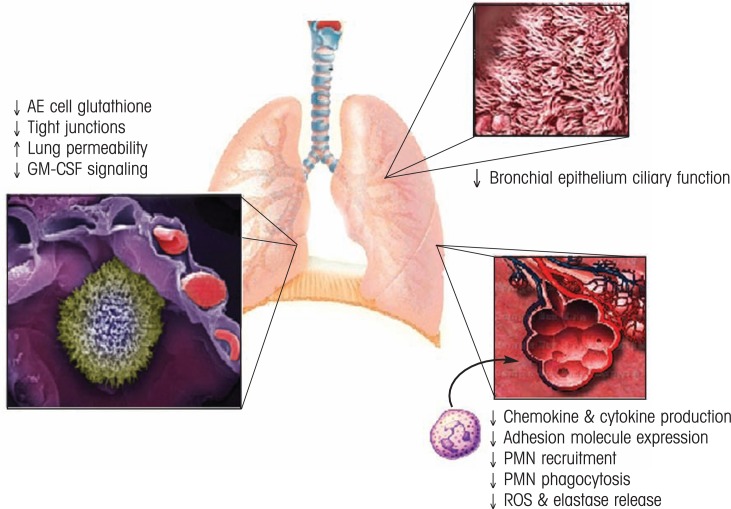
Mucosal organ “leakiness” resulting from chronic alcohol exposure also contributes, through a variety of mechanisms, to the pathophysiology of acute respiratory distress syndrome (ARDS) or acute lung injury, a serious complication frequently associated with sepsis and trauma in alcohol-abusing patients (Moss et al. 1996) (see figure 2). Chronic alcohol abuse decreases the levels of the antioxidant glutathione in the lung, leading to oxidative injury that predisposes to ARDS (Holguin et al. 1998). Alcohol abuse also affects the tight junctions between the epithelial cells in the small airsacs (i.e., alveoli) where the exchange of oxygen and carbon dioxide occurs in the lung. Moreover, chronic alcohol abuse interferes with the actions of a signaling molecule called granulocyte/macrophage colony–stimulating factor (GM–CSF), which is secreted by various cells (including epithelial cells) and stimulates the production of granulocytes and monocytes. GM–CSF signaling by alveolar epithelial type II (AE2) cells is important for protecting the body against lung infections because it induces macrophage maturation and promotes epithelial barrier maintenance (Joshi and Guidot 2007). Finally, the ciliated epithelium of the airways (i.e., bronchi), which also is a critical structural component of innate lung immunity, has been reported to be impaired by alcohol (Elliott et al. 2007). This increases the risk of airborne bacteria entering the lungs, contributing to the increased risk of infection associated with alcohol abuse.
Figure 2.
Alcohol abuse decreases host defense against bacterial infections. In the lung, alcohol decreases barrier integrity, antioxidant capacity, chemokine and cytokine production and release in response to infection, and recruitment and activation of polymorphonuclear cells. Together, these defects in host response are associated with increased risk, morbidity, and mortality from infections in the alcohol-abusing host.
NOTE: AE = alveolar epithelial; GM–CSF = granulocyte/macrophage-colony stimulating factor; ROS = reactive oxygen species; PMN = polymorphonuclear.
Alcohol and Cell-Mediated Host Defense Mechanisms
The innate cellular response, which is mediated primarily by monocytes/macrophages and neutrophils, involves the recognition, phagocytosis, and destruction of pathogens—processes essential to subsequent adaptive responses. Acute and chronic alcohol abuse can interfere with the actions of these cells at various levels.
Alcohol’s Effects on PMNs
Alcohol abuse results in profound defects in PMN function. For example, alcohol suppresses tissue recruitment of PMNs during infection and inflammation, which can lead to increased susceptibility to bacterial infections (particularly pneumonia), decreased removal of invading bacteria (i.e., bacterial clearance), and increased mortality from pneumonia (Zhang et al. 2002). Thus, alcohol interferes with various processes necessary to deliver neutrophils to the site of an infection, such as expression of a molecule called CD18 on PMNs in response to inflammatory stimuli and PMN “hyperadherence” to endothelial cells following appropriate stimulation (MacGregor et al. 1988). In addition, alcohol significantly inhibits PMN phagocytic activity as well as the production or activity of several molecules (e.g., superoxide or elastase) that are involved in the PMNs’ bactericidal activity (Stoltz et al. 1999), so that overall bactericidal activity ultimately is reduced.
Alcohol abuse also profoundly affects the production of new granulocytes (i.e., granulopoiesis), particularly in response to infection (Zhang et al. 2009). In fact, alcohol abusers with severe bacterial infection often present with abnormally low granulocyte levels (i.e., granulocytopenia), which in preclinical and clinical studies was associated with increased mortality (Perlino and Rimland 1985). Moreover, alcohol intoxication can inhibit cell division and the differentiation of precursor cells (i.e., hematopoietic stem cells) into granulocytes, which is a critical step in granulopoiesis triggered by infection (Zhang et al. 2009). These observations suggest that alcohol-mediated effects on PMNs range from the initial stages of primitive hematopoietic precursor commitment to impaired recruitment to and function within infected tissues.
Effects on Mononuclear Phagocytes
Mononuclear phagocytes include monocytes in the blood, macrophages that reside in the tissues, and dendritic cells. Studies found that alcohol abuse impairs the phagocytic function of these cells. This effect is particularly important in the setting of tuberculosis, because in healthy people more than 90 percent of the inhaled tuberculosis pathogens (i.e., mycobacteria) are ingested and destroyed by alveolar macrophages. This initial defense is critical for clearing the infection and preventing the mycobacteria from further proliferating. Chronic alcohol abuse also affects monocytes in the blood: Although the number of these cells increases, their functioning is impaired at various levels. Thus, there are significant reductions in monocyte phagocytosis (Mørland et al. 1988), adherence to other cells (which is essential for their recruitment to the tissues), production of reactive oxygen species, and intracellular microbe killing (Bermudez and Young 1991), as well as alterations in the expression of various proteins (i.e., receptors) on the monocytes’ surface.
Alcohol also induces enhanced expression of a molecule called CCR5 on the surface of macrophages, which is particularly important in patients with concurrent HIV infection. This molecule normally serves as a chemokine receptor. In HIV-infected patients, however, it also acts as a coreceptor (together with CD4) for HIV, allowing certain HIV or SIV strains to infect macrophages (Wang et al. 2002). Accordingly, alcohol-induced enhanced expression of CCR5 leads to enhanced infectivity of these HIV strains in the macrophages. Similar effects have been observed in chronic alcohol-fed rhesus macaques that show an increase in the percentage of CCR5-expressing monocytes (Marcondes et al. 2008). This increase correlates with an increase in the SIV viral “set point”3 in the circulation (Bagby et al. 2003, 2006), which in turn is associated with more rapid SIV disease progression.
Chronic alcohol ingestion also decreases the number of dendritic cells (Laso et al. 2007; Siggins et al. 2009), interferes with their differentiation, and impairs their functions, such as their ability to stimulate other cells (Szabo et al. 2004), absorb and ingest particles from outside the cell, and express co-stimulatory receptors (Lau et al. 2009). This alcohol-mediated dendritic cell dysfunction prevents the organism from generating virus-specific adaptive immune responses involving CD4+ and CD8+ lymphocytes, which may contribute to the acquisition and persistence of hepatitis C infection (Siu et al. 2009).
Effects on NK Cells
NK cells are quantitatively and qualitatively altered by alcohol abuse, particularly in patients with advanced liver cirrhosis (Cook et al. 1997; Zhang et al. 2008). For example, alcohol interferes with the expression of several NK cell proteins (e.g., proteins called perforin and granzymes A and B), and this inhibition leads to a decrease in the NK cells’ ability to destroy their target cells. This impairment in NK cell activity may play a role in alcohol-associated tumor development and viral infection (Pan et al. 2006). Moreover, chronic alcohol feeding enhances liver fibrosis in response to treatment with a chemical called carbon tetrachloride (CCl4). This enhanced fibrotic response is associated with reduced NK cell cytotoxicity and reduced expression of IFN-γ, a cytokine know to inhibit liver fibrosis (Jeong et al. 2008). Finally, alcohol activates a subgroup of NK cells called NKT cells that also express CD3, and the activation of these cells has been associated with enhanced liver injury (Minagawa et al. 2004) and hepatocyte apoptosis (Jaruga et al. 2004).
Alcohol and the Innate Humoral Response to Infections
The induced innate humoral response plays a critical role in clearing or containing infection while an adaptive response develops. It is characterized by the release of mediators of inflammatory reactions, such as cytokines and chemokines, as well as activation of the complement cascade. In addition, viral infections induce the production of various IFNs and acute-phase proteins. Many of these components are affected by acute or chronic alcohol exposure.
Effects on Cytokines and Chemokines
The effects of alcohol on cytokine and chemokine production differ according to duration of alcohol exposure or administration. Acute alcohol exposure generally suppresses cytokine (Pruett et al. 2004) and chemokine responses. Conversely, chronic alcohol exposure frequently is associated with enhanced expression of inflammation-promoting (i.e., proinflammatory) cytokines (Mandrekar et al. 2009), particularly TNF (Nagy 2004). These effects appear to be independent of the type of alcoholic beverage consumed (Romeo et al. 2007). The enhanced expression of pro-inflammatory cytokines induced by chronic alcohol exposure or consumption clearly leads to inflammation-mediated tissue injury. Conversely, the suppression of proinflammatory cytokines and increased expression of anti-inflammatory cytokines resulting from acute alcohol exposure have been associated with impaired host defense against infection.
Acute alcohol reduces the production of proinflammatory cytokines such as TNF-α and IL-1β in macrophages of the spleen and the lungs (Nelson et al. 1989) as well as in human blood monocytes.4 In addition, both acute and chronic alcohol consumption enhance expression of anti-inflammatory cytokines (Mandrekar et al. 2009). For example, chronic alcoholic patients undergoing cardiac and gastric surgery had higher levels of the anti-inflammatory cytokine IL-10, as well as a lower ratio between the proinflammatory IL-6 and the anti-inflammatory IL-10. These changes were associated with a marked increase in infection rates after the surgery (Sander et al. 2002). Preclinical studies have confirmed that injuries obtained during alcohol intoxication result in increased morbidity and mortality (Greiffenstein and Molina 2008), because the body’s ability to elicit an appropriate response to a subsequent inflammatory or infectious challenge (e.g., infection with the bacterium Klebsiella pneumoniae) (Zambell et al. 2004) is impaired. Similarly, excess alcohol at the time of burn injury (Choudhry and Chaudry 2006) or prior to a surgical intervention (Spies et al. 2008) is associated with impaired host defense response to infections.
In addition to these changes in cytokine function, investigators also have shown a contribution of barrier dysfunction to the postinjury increase in infections in intoxicated people (Choudhry et al. 2004). Thus, alcohol intoxication can suppress chemokine production and impair the expression of proteins that allow neutrophils to adhere to other cells at the site of infection, which also contributes to increased susceptibility to infection. For example, in a model of lung infection, acute alcohol intoxication suppressed the production of certain chemokines (i.e., CINC and MIP-2) during infection and inflammation, thereby markedly impairing the recruitment of additional neutrophils to the site of infection (Boé et al. 2003). This defective neutrophil recruitment could be partially restored by localized chemokine administration (Quinton et al. 2005).
Effects on IFNs
Various studies in isolated human spleen and blood mononuclear cells (Wagner et al. 1992), alcohol-ingesting rodents (Starkenburg et al. 2001), and nonalcoholic humans (Szabo et al. 2001) have demonstrated that acute alcohol exposure can suppress IFN secretion, which contributes to the risk and severity of infections. For example, the lungs of alcohol-fed rodents infected with Klebsiella pneumoniae showed a decreased and delayed production of IFN-γmRNA and protein, which was associated with reduced bacterial clearance from the lungs and reduced survival of the animals (Zisman et al. 1998).
Effects on Acute-Phase Proteins
Alcohol feeding suppresses the production and secretion of certain acute-phase proteins (i.e., type II cell surfactant). This effect may contribute to lung injury in response to inflammation (Holguin et al. 1998).
Effects on Complement
Few studies have investigated the effects of alcohol abuse on complement activation and its relationship with the incidence and severity of infection; instead, the focus of studies on alcohol-induced alterations in complement has been on liver injury (Pritchard et al. 2008). However, alcoholic patients frequently have abnormally low levels of complement in the blood. In addition, animal studies have indicated that acute alcohol intoxication can decrease complement activation in response to tissue injury resulting from disruptions in blood supply (i.e., ischemic injury). In contrast, chronic alcohol intake can activate the complement response (Roychowdhury et al. 2009), both by inducing the biochemical pathways that lead to activation of the complement cascade and by suppressing processes to terminate or regulate the cascade (Bykov et al. 2007).
Alcohol and the Adaptive Immune Response
Acute and chronic alcohol exposure can interfere with various aspects of the adaptive immune response, including the antigen presentation required to activate T- and B-cells, the activity of CD4+ and CD8+ T-cells, and the activity of B-cells.
Effects on Antigen Presentation
To elicit a response from the cell-mediated arm of the adaptive immunity, antigens need to be presented to the CD4+ and CD8+ T-cells. Studies in rodents found that chronic alcohol feeding can impair presentation of protein antigens in the spleen (Mikszta et al. 1995). Dendritic cells are among the most potent antigen-presenting cells. Acute alcohol intoxication impairs the antigen-presenting ability of these cells (Mandrekar et al. 2004). In addition, alcohol markedly affects the differentiation of dendritic cells in blood and tissues (Ness et al. 2008). The alcohol-induced defects in dendritic cell function include reduced levels of CD80 and CD86 on the cells’ surface (which are necessary to induce activation of T-cells) as well as reduced production of IL-12, which is critical for stimulating naïve CD4+ T-cells to become IFN-γ–producing Th1 cells.
Effects on CD4+ (Helper) T-Cells
Numerous studies have demonstrated alcohol-related impairment of T-cell responses to various challenges. For example, in rats that were administered the bacterium Klebsiella pneumoniae directly into the lungs, alcohol suppressed the IFN-γ response of Th1 cells; when the animals were genetically modified to express additional IFN-γ, however, their immune response was restored and they were able to clear the pathogen (Kolls et al. 1998). In other studies, chronic alcohol feeding impaired Th1 responses to a hepatitis C virus protein, a defect that was hypothesized to result from impaired secretion of IL-2 and GM–CSF by dendritic and T-cells (Geissler et al. 1997). This alcohol-induced defect in Th1 immunity correlates with suppression of IL-12 secretion by macrophages and dendritic cells (Waltenbaugh et al. 1998). Thus, it appears that alcohol inhibits Th1 immune responses and may predispose the organism to Th2 responses and that this shift is at least partly mediated by suppression of IL-12.
In addition to the Th1 response, alcohol appears to interfere with the Th17 response. For example, following an infectious challenge, acute alcohol can suppress alveolar macrophage expression of IL-23, which helps activate naïve T-cells to differentiate into Th17 cells (Happel et al. 2006). Similarly, as with the Th1 responses, alcohol inhibits the ability of dendritic cells to promote Th17 responses, thereby favoring Th2 responses (Heinz and Waltenbaugh 2007).
Effects on CD8+ (Cytotoxic) T-Cells
Chronic alcohol decreases the numbers of CD4+ and CD8+ T-cells in the thymus and spleen (Saad and Jerrels 1991). In addition, chronic alcoholics with cirrhosis have higher levels of unbound (i.e., soluble) CD8 protein in the blood, which could inhibit CD8+ T-cell activation. It is well documented that chronic alcoholics have more progressive hepatitis C infection as well as a diminished response to treatment, and this may be related to the alcohol-induced suppression of CD8+ T-cell function, which may complicate viral clearance (Jerrells 2002). Evidence supporting this hypothesis includes the observation that chronic alcohol also delays the clearance of another virus (i.e., cytomegalovirus) from the liver in mice, and that this delay is associated with defects in the normal IL-12 and IFN-γ responses. Moreover, chronic alcohol has been associated with increased activation of CD8+ T-cells (Cook et al. 2004), which could reflect homeostatic proliferation of T-cells and increased percentage of peripheral memory cells. However, the CD8+ T-cells that do infiltrate the liver in alcoholics with hepatitis C appear dysfunctional with respect to viral clearance.
Alcohol-mediated effects on CD8+ T-cell function also have been linked to impaired immunity in the lung in response to influenza infection (Meyerholz et al. 2008). Whether the increased viral load measured in SIV-infected chronic alcohol-fed macaques can be attributed to diminished CD8+ T-cell function remains to be established (Bagby et al. 2006; Kumar et al. 2005).
Effects on B-Cells
Several lines of evidence show that the number and function of B-cells are reduced by chronic alcohol. For example, chronic alcoholics exhibit loss of B-cells in the periphery and a reduced capacity to generate protective antibodies (Cook et al. 1996). In addition, chronic alcohol can decrease the number of B-cells that produce an antibody type called IgA5 in one of the layers of mucous membranes (i.e., the lamina propria), which is indicative of altered mucosal immunity (Lopez et al. 1994). Finally, alcohol inhibits the responsiveness of B-cells at certain developmental stages (i.e., blasts, which are the precursors to the antibody-secreting plasma cells) to various cytokines, particularly to IL-2 and IL-4. However, alcohol may have a dual effect on B-cell function because some studies have reported that B-cells also could be activated in alcohol-consuming people (Drew et al. 1984).
Alcohol’s effects on the number and function of B-cells may have several consequences, including the following:
Because B-cells also can function as antigen-presenting cells, an alcohol-induced reduction in the number of B-cells could inhibit antigen presentation.
As mentioned earlier, most activated B-cells differentiate into plasma cells; accordingly, alcohol-induced suppression of B-cell differentiation may explain why chronic alcoholics reportedly show a reduced antibody responses to hepatitis B vaccine (Mendenhall et al. 1988).
Chronic alcoholics have elevated levels of an immunoglobulin type called IgE, which is involved in allergic reactions; this elevation may be related to the previously mentioned shift in T-cell response from a Th1 response to a Th2 response (Dominguez-Santalla et al. 2001).
Despite these observations, which shed some light on alcohol’s effects on B-cells and their functions, some questions remain to be answered. For example, the acetaldehyde that is formed during alcohol metabolism can interact with other proteins in the cells, interfering with their function. Therefore, it is possible that acetaldehyde also interacts with antibodies and thereby may alter antibody responses; however, this remains to be established (Thiele et al. 2008). Similarly, more work is needed to determine whether alcohol inhibits specific aspects of B-cell differentiation, such as immunoglobulin class switching and cell survival.
Perspectives, Implications, and Future Research Directions
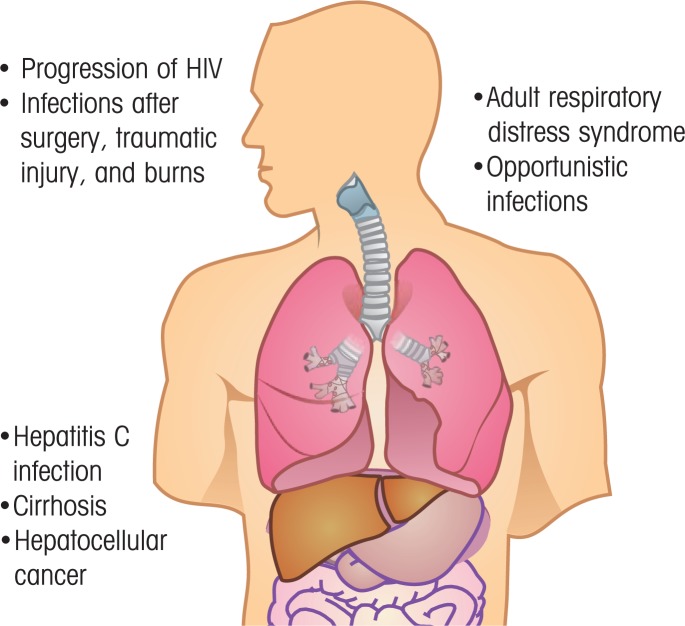
Alcohol has a broad range of effects on the structural, cellular, and humoral components of the immune system. This alcohol-induced dysregulation of the immune system renders the patient susceptible to a vast array of infectious pathogens, resulting in biomedical consequences such as increased risk of infections after surgery, traumatic injury, or burns; of liver disease, such as hepatitis C infection, fibrosis, and liver cancer; of ARDS and opportunistic infections in the lungs; and of accelerated progression of HIV disease (see figure 3).
Figure 3.
Biomedical consequences of alcohol-induced dysregulation of the immune system. These may include infections after surgery, traumatic injury, or burns; accelerated progression of HIV disease; adult respiratory distress syndrome and other opportunistic lung infections; and infection with hepatitis C virus, cirrhosis, or liver cancer (hepatocellular carcinoma).
Alcohol abuse is particularly prevalent in HIV-infected people, and its ability to interfere with antiviral treatment is now well recognized. In addition, current studies have identified interactions between alcohol and infectious diseases that not only increase risk of and susceptibility to infection but also contribute to comorbidities arising from continued alcohol abuse in infected individuals. The alcohol-induced alterations in the immune environment likely contribute to the pathogenesis and burden of disease in infected people, particularly in the case of HIV and hepatitis C infection (Marcondes et al. 2008). For example, patients who have a compromised immune system resulting from HIV infection and who chronically abuse alcohol are at increased risk for pneumonia. Similarly, chronic alcohol use by HIV-infected patients accelerates the disease course of HIV/AIDS (Shuper et al. 2010). Similar synergistic interactions exist for alcohol and hepatitis C infection. The prevalence of hepatitis C infection is 3- to 30-fold higher in alcoholics compared with the general population (Singal and Anand 2007), and these patients develop more severe fibrosis and have higher rates of cirrhosis and liver cancer compared with nondrinkers. Thus, alcohol abuse is associated not only with increased prevalence of HIV and hepatitis C infection, which can be attributed to behavioral and immune factors but also with more severe pathogenesis and accelerated disease progression that may at least in part result from decreased response rate to antiviral therapy (Siu et al. 2009). Given the substantial additional disease burden that alcohol imparts on people infected with HIV and viral hepatitis, a greater understanding of the precise mechanisms through which acute and chronic abuse alter the multiple facets of the host’s immune response to these infections is needed.
Acknowledgments
This work was funded by NIAAA grant AA09803–17.
Footnotes
For a definition of this and other technical terms, see the Glossary, pp. 161–164.
The different immunoglobulin classes are involved in different aspects of the immune response. However, all immunoglobulins produced by one B-cell and its daughter cells specifically recognize the same antigen.
The HIV (or SIV) set point is the stable viral load that is established in an HIV-infected person after the initial phase of the infection, when the person’s immune systems tries to fight the virus. The higher the viral load of the set point, the faster infection will progress to full-blown AIDS.
Expression of TNF-α and IL-1β requires the actions of a protein called nuclear factor (NF)- B. The activity of this protein is regulated by another molecule, inhibitor of NF- B (I B). Alcohol acts on this molecule (i.e., decreases phosphorylation of I B), thereby allowing I B to attach to NF- B, interfering with its activation of cytokine expression (Mandrekar et al. 1999). In addition, alcohol interferes with TNF expression by inhibiting the normal processing of newly produced TNF that is necessary for normal TNF functioning (Zhao et al. 2003).
IgA is an antibody that plays a critical role in immune responses in the mucous membranes. These membranes line the body cavities exposed to the external environment (e.g., the GI tract, respiratory tract, nostrils, mouth, or eyelids) and therefore are likely to come in contact with outside pathogens. IgA is the most common type of antibody produced in the body.
Financial Disclosure
The authors declare that they have no competing financial interests.
References
- Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. American Journal of Physiology Gastrointestinal and Liver Physiology. 2001;280(6):G1280–G1288. doi: 10.1152/ajpgi.2001.280.6.G1280. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Stoltz DA, Zhang P, et al. The effect of chronic binge ethanol consumption on the primary stage of SIV infection in rhesus macaques. Alcoholism: Clinical and Experimental Research. 2003;27(3):495–502. doi: 10.1097/01.ALC.0000057947.57330.BE. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Purcell JE, et al. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcoholism: Clinical and Experimental Research. 2006;30(10):1781–1790. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Baliunas D, Rehm J, Irving H, Shuper P. Alcohol consumption and risk of incident human immunodeficiency virus infection: A meta-analysis. International Journal of Public Health. 2009 Dec 1; doi: 10.1007/s00038-009-0095-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bermudez LE, Young LS. Ethanol augments intracellular survival of Mycobacterium avium complex and impairs macrophage responses to cytokines. Journal of Infectious Diseases. 1991;163(6):1286–1292. doi: 10.1093/infdis/163.6.1286. [DOI] [PubMed] [Google Scholar]
- Boé DM, Nelson S, Zhang P, et al. Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcoholism: Clinical and Experimental Research. 2003;27(11):1838–1845. doi: 10.1097/01.ALC.0000095634.82310.53. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Medicine. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Bykov I, Junnikkala S, Pekna M, et al. Effect of chronic ethanol consumption on the expression of complement components and acute-phase proteins in liver. Clinical Immunology. 2007;124(2):213–220. doi: 10.1016/j.clim.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Chaudry IH. Alcohol intoxication and post-burn complications. Frontiers in Bioscience. 2006;11:998–1005. doi: 10.2741/1857. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Rana SN, Kavanaugh MJ, et al. Impaired intestinal immunity and barrier function: A cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol. 2004;33(3):199–208. doi: 10.1016/j.alcohol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Cook RT, Li F, Vandersteen D, et al. Ethanol and natural killer cells. I. Activity and immunophenotype in alcoholic humans. Alcoholism: Clinical and Experimental Research. 1997;21(6):974–980. [PubMed] [Google Scholar]
- Cook RT, Waldschmidt TJ, Cook BL, et al. Loss of the CD5+ and CD45RAhi B cell subsets in alcoholics. Clinical and Experimental Immunology. 1996;103(2):304–310. doi: 10.1046/j.1365-2249.1996.d01-621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT, Zhu X, Coleman RA, et al. T-cell activation after chronic ethanol ingestion in mice. Alcohol. 2004;33(3):175–181. doi: 10.1016/j.alcohol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Domínguez-Santalla MJ, Vidal C, Viñuela J, et al. Increased serum IgE in alcoholics: Relationship with Th1/Th2 cytokine production by stimulated blood mononuclear cells. Alcoholism: Clinical and Experimental Research. 2001;25(8):1198–1205. doi: 10.1111/j.1530-0277.2001.tb02336.x. [DOI] [PubMed] [Google Scholar]
- Drew PA, Clifton PM, LaBrooy JT, Shearman DJ. Polyclonal B cell activation in alcoholic patients with no evidence of liver dysfunction. Clinical and Experimental Immunology. 1984;57(2):479–486. [PMC free article] [PubMed] [Google Scholar]
- Elliott MK, Sisson JH, Wyatt TA. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. American Journal of Respiratory Cell and Molecular Biology. 2007;36(4):452–459. doi: 10.1165/rcmb.2005-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: Immunobiology and emerging roles in liver diseases. Journal of Leukocyte Biology. 2009;86(3):513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler M, Gesien A, Wands JR. Inhibitory effects of chronic ethanol consumption on cellular immune responses to hepatitis C virus core protein are reversed by genetic immunizations augmented with cytokine-expressing plasmids. Journal of Immunology. 1997;159(10):5107–5113. [PubMed] [Google Scholar]
- Greiffenstein P, Molina PE. Alcohol-induced alterations on host defense after traumatic injury. Journal of Trauma. 2008;64(1):230–240. doi: 10.1097/TA.0b013e318158a4ad. [DOI] [PubMed] [Google Scholar]
- Hanck C, Rossol S, Böcker U, et al. Presence of plasma endotoxin is correlated with tumour necrosis factor receptor levels and disease activity in alcoholic cirrhosis. Alcohol and Alcoholism. 1998;33(6):606–608. doi: 10.1093/alcalc/33.6.606. [DOI] [PubMed] [Google Scholar]
- Happel KI, Odden AR, Zhang P, et al. Acute alcohol intoxication suppresses the interleukin 23 response to Klebsiella pneumoniae infection. Alcoholism: Clinical and Experimental Research. 2006;30(7):1200–1207. doi: 10.1111/j.1530-0277.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- Heinz R, Waltenbaugh C. Ethanol consumption modifies dendritic cell antigen presentation in mice. Alcoholism: Clinical and Experimental Research. 2007;31(10):1759–1771. doi: 10.1111/j.1530-0277.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. Journal of Clinical Investigation. 1998;101(4):761–768. doi: 10.1172/JCI1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaruga B, Hong F, Kim WH, et al. Chronic alcohol consumption accelerates liver injury in T cell-mediated hepatitis: Alcohol disregulation of NF-kappaB and STAT3 signaling pathways. American Journal of Physiology Gastrointestinal and Liver Physiology. 2004;287(2):G471–G479. doi: 10.1152/ajpgi.00018.2004. [DOI] [PubMed] [Google Scholar]
- Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134(1):248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells TR. Role of activated CD8+ T cells in the initiation and continuation of hepatic damage. Alcohol. 2002;27(1):47–52. doi: 10.1016/s0741-8329(02)00210-0. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Guidot DM. The alcoholic lung: Epidemiology, pathophysiology, and potential therapies. American Journal of Physiology Lung Cellular and Molecular Physiology. 2007;292(4):L813–L823. doi: 10.1152/ajplung.00348.2006. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Holmes EW, Patel M, et al. Leaky gut in alcoholic cirrhosis: A possible mechanism for alcohol-induced liver damage. American Journal of Gastroenterology. 1999;94(1):200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Lei D, Stoltz D, et al. Adenoviral-mediated interferon-gamma gene therapy augments pulmonary host defense of ethanol-treated rats. Alcoholism: Clinical and Experimental Research. 1998;22(1):157–162. [PubMed] [Google Scholar]
- Kumar R, Perez-Casanova AE, Tirado G, et al. Increased viral replication in simian immunodeficiency virus/simian-HIV-infected macaques with self-administering model of chronic alcohol consumption. Journal of the Acquired Immune Deficiency Syndrome. 2005;39(4):386–390. doi: 10.1097/01.qai.0000164517.01293.84. [DOI] [PubMed] [Google Scholar]
- Laso FJ, Vaquero JM, Almeida J, et al. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcoholism: Clinical and Experimental Research. 2007;31(5):846–854. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- Lau AH, Szabo G, Thomson AW. Antigen-presenting cells under the influence of alcohol. Trends in Immunology. 2009;30(1):13–22. doi: 10.1016/j.it.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Lopez MC, Huang DS, Borgs P, et al. Modification of lymphocyte subsets in the intestinal-associated immune system and thymus by chronic ethanol consumption. Alcoholism: Clinical and Experimental Research. 1994;18(1):8–11. doi: 10.1111/j.1530-0277.1994.tb00873.x. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Safford M, Shalit M. Effect of ethanol on functions required for the delivery of neutrophils to sites of inflammation. Journal of Infectious Diseases. 1988;157(4):682–689. doi: 10.1093/infdis/157.4.682. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Bala S, Catalano D, et al. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. Journal of Immunology. 2009;183(2):1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Dolganiuc A, et al. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. Journal of Immunology. 2004;173(5):3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Szabo G. Inhibition of lipopolysaccharide-mediated NFkappaB activation by ethanol in human monocytes. International Immunology. 1999;11:1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- Marcondes MC, Watry D, Zandonatti M, et al. Chronic alcohol consumption generates a vulnerable immune environment during early SIV infection in rhesus macaques. Alcoholism: Clinical and Experimental Research. 2008;32(9):1583–1592. doi: 10.1111/j.1530-0277.2008.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall C, Roselle GA, Lybecker LA, et al. Hepatitis B vaccination. Response of alcoholic with and without liver injury. Digestive Diseases and Sciences. 1988;33(3):263–269. doi: 10.1007/BF01535747. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Edsen-Moore M, McGill J, et al. Chronic alcohol consumption increases the severity of murine influenza virus infections. Journal of Immunology. 2008;181(1):641–648. doi: 10.4049/jimmunol.181.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikszta JA, Waltenbaugh C, Kim BS. Impaired antigen presentation by splenocytes of ethanol-consuming C57BL/6 mice. Alcohol. 1995;12(3):265–271. doi: 10.1016/0741-8329(94)00105-m. [DOI] [PubMed] [Google Scholar]
- Minagawa Ml, Deng Q, Liu ZX, et al. Activated natural killer T cells induce liver injury by Fas and tumor necrosis factor-alpha during alcohol consumption. Gastroenterology. 2004;126(5):1387–1399. doi: 10.1053/j.gastro.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Mørland H, Johnsen J, Bjørneboe A, et al. Reduced IgG Fc-receptor-mediated phagocytosis in human monocytes isolated from alcoholics. Alcoholism: Clinical and Experimental Research. 1988;12(6):755–759. doi: 10.1111/j.1530-0277.1988.tb01340.x. [DOI] [PubMed] [Google Scholar]
- Moss M, Bucher B, Moore FA, et al. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA: Journal of the American Medical Association. 1996;275(1):50–54. [PubMed] [Google Scholar]
- Nagy LE. Stabilization of tumor necrosis factor-alpha mRNA in macrophages in response to chronic ethanol exposure. Alcohol. 2004;33(3):229–233. doi: 10.1016/j.alcohol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Nelson S, Bagby GJ, Bainton BG, Summer WR. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. Journal of Infectious Diseases. 1989;160(3):422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- Ness KJ, Fan J, Wilke WW, et al. Chronic ethanol consumption decreases murine Langerhans cell numbers and delays migration of Langerhans cells as well as dermal dendritic cells. Alcoholism: Clinical and Experimental Research. 2008;32(4):657–668. doi: 10.1111/j.1530-0277.2007.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HN, Sun R, Jaruga B, et al. Chronic ethanol consumption inhibits hepatic natural killer cell activity and accelerates murine cytomegalovirus-induced hepatitis. Alcoholism: Clinical and Experimental Research. 2006;30(9):1615–1623. doi: 10.1111/j.1530-0277.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- Perlino CA, Rimland D. Alcoholism, leukopenia, and pneumococcal sepsis. American Reviews of Respiratory Disease. 1985;132(4):757–760. doi: 10.1164/arrd.1985.132.4.757. [DOI] [PubMed] [Google Scholar]
- Poonia B, Nelson S, Bagby GJ, et al. Chronic alcohol consumption results in higher simian immunodeficiency virus replication in mucosally inoculated rhesus macaques. AIDS Research and Human Retroviruses. 2006;22(6):589–594. doi: 10.1089/aid.2006.22.589. [DOI] [PubMed] [Google Scholar]
- Prakash O, Mason A, Luftig RB, Bautista AP. Hepatitis C virus (HCV) and human immunodeficiency virus type 1 (HIV-1) infections in alcoholics. Frontiers in Bioscience 1. 2002;7:e286–e300. doi: 10.2741/A924. [DOI] [PubMed] [Google Scholar]
- Pritchard MT, McMullen MR, Medof ME, et al. Role of complement in ethanol-induced liver injury. Advances in Experimental Medicine and Biology. 2008;632:175–186. [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan R, et al. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol. 2004;33(2):147–155. doi: 10.1016/j.alcohol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Quinton LJ, Nelson S, Zhang P, et al. Effects of systemic and local CXC chemokine administration on the ethanol-induced suppression of pulmonary neutrophil recruitment. Alcoholism: Clinical and Experimental Research. 2005;29(7):1198–1205. doi: 10.1097/01.alc.0000171927.66130.aa. [DOI] [PubMed] [Google Scholar]
- Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50(2):638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RK, Seth A, Sheth P. Recent advances in alcoholic liver disease. I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. American Journal of Physiology Gastrointestinal and Liver Physiology. 2004;286(6):G881–G884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- Romeo J, Wärnberg J, Nova E, et al. Moderate alcohol consumption and the immune system: A review. British Journal of Nutrition. 2007;98(Suppl 1):S111–S115. doi: 10.1017/S0007114507838049. [DOI] [PubMed] [Google Scholar]
- Roychowdhury S, McMullen MR, Pritchard MT, et al. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology. 2009;49(4):1326–1334. doi: 10.1002/hep.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad AJ, Jerrells TR. Flow cytometric and immunohistochemical evaluation of ethanol-induced changes in splenic and thymic lymphoid cell populations. Alcoholism: Clinical and Experimental Research. 1991;15(5):796–803. doi: 10.1111/j.1530-0277.1991.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Sander M, Irwin M, Sinha P, et al. Suppression of interleukin-6 to interleukin-10 ratio in chronic alcoholics: Association with postoperative infections. Intensive Care Medicine. 2002;28(3):285–292. doi: 10.1007/s00134-001-1199-9. [DOI] [PubMed] [Google Scholar]
- Shuper PA, Neuman M, Kanteres F, et al. Causal considerations on alcohol and HIV/AIDS: A systematic review. Alcohol and Alcoholism. 2010;45(2):159–166. doi: 10.1093/alcalc/agp091. [DOI] [PubMed] [Google Scholar]
- Siggins RW, Bagby GJ, Molina P, et al. Alcohol exposure impairs myeloid dendritic cell function in rhesus macaques. Alcoholism: Clinical and Experimental Research. 2009;33(9):1524–1531. doi: 10.1111/j.1530-0277.2009.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. Journal of Clinical Gastroenterology. 2007;41(8):761–772. doi: 10.1097/MCG.0b013e3180381584. [DOI] [PubMed] [Google Scholar]
- Siu L, Foont J, Wands JR. Hepatitis C virus and alcohol. Seminars in Liver Disease. 2009;29(2):188–199. doi: 10.1055/s-0029-1214374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies CD, Lanzke N, Schlichting U, et al. Effects of ethanol on cytokine production after surgery in a murine model of gram-negative pneumonia. Alcoholism: Clinical and Experimental Research. 2008;32(2):331–338. doi: 10.1111/j.1530-0277.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Starkenburg S, Munroe ME, Waltenbaugh C. Early alteration in leukocyte populations and Th1/Th2 function in ethanol-consuming mice. Alcoholism: Clinical and Experimental Research. 2001;25(8):1221–1230. [PubMed] [Google Scholar]
- Stoltz DA, Zhang P, Nelson S, et al. Ethanol suppression of the functional state of polymorphonuclear leukocytes obtained from uninfected and simian immunodeficiency virus infected rhesus macaques. Alcoholism: Clinical and Experimental Research. 1999;23(5):878–884. [PubMed] [Google Scholar]
- Szabo G, Catalano D, White B, Mandrekar P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcoholism: Clinical and Experimental Research. 2004;28(5):824–848. doi: 10.1097/01.alc.0000127104.80398.9b. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Dolganiuc A, et al. Reduced alloreactive T-cell activation after alcohol intake is due to impaired monocyte accessory cell function and correlates with elevated IL-10, IL-13, and decreased IFNgamma levels. Alcoholism: Clinical and Experimental Research. 2001;25(12):1766–1772. [PubMed] [Google Scholar]
- Tang Y, Banan A, Forsyth CB, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcoholism: Clinical and Experimental Research. 2008;32(2):355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- Thiele GM, Klassen LW, Tuma DJ. Formation and immunological properties of aldehyde-derived protein adducts following alcohol consumption. Methods in Molecular Biology. 2008;447:235–257. doi: 10.1007/978-1-59745-242-7_17. [DOI] [PubMed] [Google Scholar]
- Wagner F, Fink R, Hart R, et al. Ethanol inhibits interferon-gamma secretion by human peripheral lymphocytes. Journal of Studies on Alcohol. 1992;53(3):277–280. doi: 10.15288/jsa.1992.53.277. [DOI] [PubMed] [Google Scholar]
- Wang X, Douglas SD, Metzger DS, et al. Alcohol potentiates HIV-1 infection of human blood mononuclear phagocytes. Alcoholism: Clinical and Experimental Research. 2002;26(12):1880–1886. doi: 10.1097/01.ALC.0000042148.50808.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambell KL, Phelan H, Vande Stouwe C, et al. Acute alcohol intoxication during hemorrhagic shock: Impact on host defense from infection. Alcoholism: Clinical and Experimental Research. 2004;28(4):635–642. doi: 10.1097/01.alc.0000122104.85971.55. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Happel KI, et al. Pulmonary host defenses and alcohol. Frontiers in Bioscience. 2002;7:d1314–d1330. doi: 10.2741/A842. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Happel KI, et al. Alcohol abuse, immunosuppression, and pulmonary infection. Current Drug Abuse Reviews. 2008;1(1):56–67. doi: 10.2174/1874473710801010056. [DOI] [PubMed] [Google Scholar]
- Zhang P, Welsh DA, Siggins RW, 2nd, et al. Acute alcohol intoxication inhibits the lineage-c-kit+ Sca-1+ cell response to Escherichia coli bacteremia. Journal of Immunology. 2009;182(3):1568–1576. doi: 10.4049/jimmunol.182.3.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XJ, Marrero L, Song K, et al. Acute alcohol inhibits TNF-alpha processing in human monocytes by inhibiting TNF/TNFalpha-converting enzyme interactions in the cell membrane. Journal of Immunology. 2003;170(6):2923–2931. doi: 10.4049/jimmunol.170.6.2923. [DOI] [PubMed] [Google Scholar]
- Zisman DA, Strieter RM, Kunkel SL, et al. Ethanol feeding impairs innate immunity and alters the expression of Th1- and Th2-phenotype cytokines in murine Klebsiella pneumonia. Alcoholism: Clinical and Experimental Research. 1998;22(3):621–627. doi: 10.1111/j.1530-0277.1998.tb04303.x. [DOI] [PubMed] [Google Scholar]