Abstract
Research advances over the past four decades have demonstrated that a significant proportion of people with alcohol use disorders also suffer from a comorbid mood or anxiety disorder. This article briefly reviews the associations among alcohol dependence, major depressive disorder, and posttraumatic stress disorder. Dysregulation of the brain’s and body’s stress system (i.e., the limbic–hypothalamic–pituitary–adrenal axis) might serve as a common mechanistic link to explain some of the relationships among these frequently comorbid conditions. Finally, the article examines the role of sex differences in stress circuitry. These differences may explain why men and women differ in their risk for developing comorbid alcoholism and stress-related disorders.
Keywords: Alcohol dependence, alcohol use disorder, comorbidity, mental health disorders, mood disorder, anxiety disorder, depressive disorder, posttraumatic stress disorder, stress, psychological stress, gender differences
Although the observation that patients with alcohol abuse and dependence (collectively termed alcohol use disorders [AUDs]) frequently suffer from co-occurring mood and anxiety disorders predates the founding of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) 40 years ago, our improved understanding of the nature of the associations among these common psychiatric disorders largely parallels the growth of NIAAA’s research portfolio in this area. Four decades ago, the psychiatric diagnostic systems in place (i.e., the Feighner [Feighner et al. 1972] and the Research Diagnostic Criteria [RDC] [Spitzer et al. 1978]) used a simple approach to describe the complex nature of co-occurring disorders. As a first step, these systems attempted to classify comorbid conditions based solely on the chronological timing of their occurrence. Thus, a person who was diagnosed with alcohol dependence before the onset of his or her psychiatric disorder was labeled as a “primary” alcoholic, whereas a patient whose alcoholism followed the onset of another diagnosable psychiatric illness was labeled as a “secondary” alcoholic. Indeed, the definitions of the alcohol dependence syndrome also were evolving during this period, further complicating the diagnostic picture (Grant and Harford 1995).
With the advent of the Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM–III) (American Psychiatric Association [APA] 1980) and the incorporation of these and the RDC criteria into structured psychiatric diagnostic interviews (e.g., the Diagnostic Interview Schedule [DIS] [Robins et al. 1981]), researchers were able to make further refinements in the classification of these disorders. For example, recognizing that heavy alcohol consumption dramatically alters brain function—including mood regulation—scientists designated psychiatric disturbances that occurred in the context of heavy drinking or alcohol withdrawal as “organic mental syndromes”(Anthenelli 1997; Nunes and Rounsaville 2006). The result was a catch-all category that was quite generic in nature but which represented a broad attempt to distinguish psychiatric complaints occurring in the context of a medical condition (e.g., depression in a patient with hypothyroidism). Nevertheless, the use of diagnostic criteria sets, whose reliability and validity could be tested by incorporating them into structured psychiatric diagnostic interviews, led to an expanding number of studies on comorbidity and helped researchers to move beyond using strictly clinical samples of alcohol-dependent patients.
Over the past 30 years, several landmark epidemiological surveys have documented high rates of co-occurrence of certain mood and anxiety disorders with AUDs. Using the DSM–III (APA 1980) criteria for mental syndromes, findings from the Epidemiological Catchment Area (ECA) Study (Helzer and Pryzbeck 1988; Regier et al. 1990) first documented that alcohol dependence and stress-related mood and anxiety disorders frequently co-occur. This was followed by the National Comorbidity Survey (NCS) (Kessler et al. 1997), which applied the DSM–III–Revised (DSM–III–R) (APA 1987) diagnostic criteria in a nationally representative household survey and found that roughly 28 percent of people who met the DSM–III–R criteria for alcohol dependence in the past year also reported experiencing a major depressive episode during that same time frame (see table 1). The NCS results also demonstrated high rates of co-occurrence of anxiety disorders with alcohol dependence. Thus, alcoholics were two to three times more likely than nonalcoholics to suffer from a comorbid anxiety disorder.
Table 1.
Prevalence of Comorbid Mood and Anxiety Disorders in Individuals With Alcohol Abuse and Alcohol Dependence: Focus on Major Depressive Disorder (MDD) and Posttraumatic Stress Disorder (PTSD)
| Comorbid Disorder | Alcohol Abuse | Alcohol Dependence | ||
|---|---|---|---|---|
| 1-year rate (%) | Odds ratio | 1 year-rate (%) | Odds ratio | |
| National Comorbidity Survey1 | ||||
| Any Mood Disorder | 12.3 | 1.1 | 29.2 | 3.6a |
| MDD | 11.3 | 1.1 | 27.9 | 3.9a |
| Any Anxiety Disorder | 29.1 | 1.7 | 36.9 | 2.6a |
| PTSD | 5.6 | 1.5 | 7.7 | 2.2a |
| NESARC2 | ||||
| Any Mood Disorder | 11.7 | 1.3b | 27.5 | 4.1b |
| MDD | 8.2 | 1.2 | 20.5 | 3.7b |
| Any Anxiety Disorder | 11.8 | 1.1 | 23.4 | 2.6b |
NOTE:
Adapted from Kessler et al. 1997.
National Epidemiologic Survey on Alcohol and Related Conditions (NESARC); Adapted from Grant et al. 2004.
Odds ratio was significantly different from 1 at 0.05 level. The odds ratio reflects the probability of an individual with an alcohol use disorder (AUD) having the comorbid disorder compared with people without AUD.
As epidemiologists began documenting that alcoholism frequently co-occurs with other psychiatric conditions, continuing advances in the nosology of the disorder and the instruments used to disentangle the various comorbidities further refined these relationships. In 1992, NIAAA conducted the National Longitudinal Alcohol Epidemiologic Survey (NLAES) (Grant et al. 1994), which used the first iteration of a newly structured diagnostic interview named the Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS) (Grant and Hasin 1992). In contrast to the ECA and NCS studies that preceded it, NLAES was the first large-scale, nationally representative household survey to apply DSM–Fourth Edition (DSM–IV) criteria (APA 1994). This included distinguishing alcohol abuse and alcohol dependence as separate syndromes and better pinpointing the onset of these AUD syndromes as being either current (i.e., symptoms and signs clustering within the past 12 months at the time of the interview), prior to the past year, or on a lifetime basis (Grant and Harford 1995). A key finding to emerge from the NLAES data was that a past history of alcohol dependence, even among former drinkers, was associated with a more than fourfold-increased risk for a current or recent (within the past 1 year) major depressive episode (Hasin and Grant 2002). That study, and another one analyzing NLAES data and the relationship between AUDs and major depression (Grant and Harford 1995), found that this association was strongest among people with alcohol dependence (as opposed to alcohol abuse) and among women compared with men. A previous issue of Alcohol Research & Health (Vol. 26, 2002) examined the topic of alcohol and comorbid mental health disorders in greater detail.
With the publication of DSM–IV (APA 1994) and its successor, DSM–IV–Text Revision (DSM–IV–TR) (APA 2000), a new category of “alcohol-induced disorders” was defined. In addition to influencing the sophistication and refinement of several structured and semistructured diagnostic interviews (e.g., the Semi-Structured Assessment for the Genetics of Alcoholism, Version II [SSAGA–II] [Bucholz et al. 1994] and the AUDADIS–DSM–IV Version [AUDADIS–IV] [Grant et al. 2001]), this new nomenclature for discriminating mood and anxiety complaints in alcohol-dependent patients gave researchers the opportunity to better identify subgroups of patients with AUDs and psychiatric disorders that went beyond the simple primary versus secondary distinction. The essential feature of an alcohol-induced disorder is the presence of persistent symptoms that are judged to be the result of the direct physiological effects of alcohol. To warrant this designation, these conditions should occur within 4 weeks of the last use of or withdrawal from alcohol and be clinically significant beyond what is expected from typical alcohol withdrawal or intoxication (APA 1994). As Shivani and colleagues (2002) and others (Nunes et al. 2006) have noted, this designation does not specify whether the alcohol-induced disorder meets the criteria for a full syndrome, per se (e.g., for major depression, at least five symptoms present for 2 or more weeks), or simply represents scattered psychiatric symptoms and signs that do not coalesce over time to warrant labeling as a syndrome. Nevertheless, this diagnostic refinement can help clinicians and researchers to further disentangle the heterogeneous nature of many comorbid presentations.
The NIAAA-sponsored National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) was the first epidemiologic survey to employ the DSM–IV and DSM–IV–TR criteria to specifically examine both independent mood and anxiety disorders and alcohol-induced disorders (Grant et al. 2003). The survey was designed to examine the comorbidity among AUDs (and other substance use disorders [SUDs]) and nine independent mood and anxiety disorders in a nationally representative sample of over 43,000 respondents aged 18 years and older. As depicted in table 1, the 12-month odds of suffering from an independent major depression were nearly four times greater among men and women with alcohol dependence than among people without AUDs (Grant et al. 2004). Although posttraumatic stress disorder (PTSD) was not assessed during Wave 1 of the NESARC and was therefore not considered in this calculation, the odds of an individual with alcohol dependence meeting the criteria for an independent anxiety disorder were increased 2.6 times compared with individuals without alcohol dependence.
In summary, regardless of the labeling systems applied, alcoholism and stress-related mood and anxiety problems frequently go hand-in-hand. The diagnostic criteria used to identify comorbid syndromes and advances in the research tools used to evaluate them have been further refined over the past 40 years, corresponding with NIAAA’s support in this research area. The challenge for the next decade will be to sort out the causal mechanisms underlying these associations, as discussed below.
Common Mechanistic Links Between AUDs and Mood and Anxiety Disorders
A common thread linking AUDs with other comorbid mental health disorders such as depression and PTSD is the role that stress plays in precipitating and maintaining these disorders. A comprehensive review is beyond the scope of this article, but previous editions of Alcohol Research & Health have examined the topics of stress and alcohol in greater detail (see Vol. 22, No. 3, 1998, and Vol. 23, No. 4, 1999). Below is a brief sketch of how a better understanding of the irregularities in the brain’s stress–reward systems might help elucidate the interrelationships among AUDs and some stress-related psychiatric disorders.
Components of the Stress Response
When an organism perceives a threat or when there is a challenge to its normal state (i.e., homeostasis), outgoing signals from multiple parts of the brain funnel input to the hypothalamus. The paraventricular nucleus (PVN) within the hypothalamus contains nerve cells (i.e., neurons) responsible for secreting corticotropin-releasing hormone (CRH) and the hormone arginine vasopressin (AVP) (see the figure). The stress-induced release of CRH and AVP into the portal circulation triggers the release of adrenocorticotropin hormone (ACTH) from the anterior pituitary gland, which, in humans, ultimately stimulates the production of cortisol (i.e., the stress hormone) from the adrenal cortex. A regulatory system is in place to turn off the endocrine stress response (Ulrich-Lai and Herman 2009). Thus, the hormonal response to stress is mediated through the limbic–hypothalamic–pituitary– adrenal (LHPA) axis.
Figure.
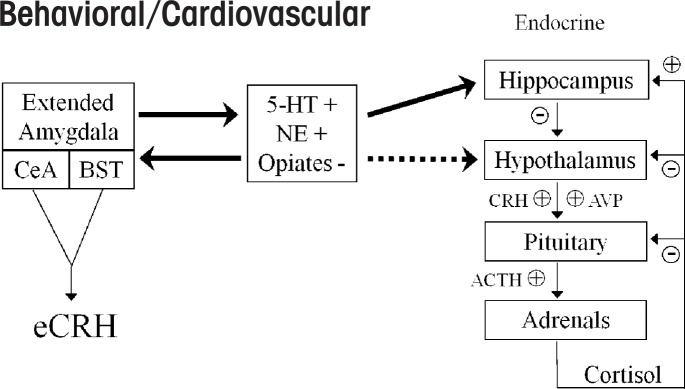
Schematized drawing illustrating crosstalk between the hypothalamic corticotropin-releasing hormone (CRH)-pituitary-adrenal (HPA) and extrahypothalamic CRH (eCRH) stress axes. Brain neurotransmitters such as serotonin (5-hydroxytryptamine or 5-HT) and norepinephrine (NE) stimulate (+) these interconnected stress circuits, whereas endogenous opiates inhibit (–) stress responses. Solid lines indicate direct connections between the brain nuclei where these neurotransmitters are manufactured and the brain structure (e.g., the hippocampus) involved in modulating the stress response, and dashed lines indicate indirect connections between these structures. See text for details.
In addition to this hypothalamic–pituitary–adrenal (HPA) neuroendocrine stress axis, a parallel extrahypothalamic CRH (eCRH) brain stress system exists. This circuitry has its roots in a portion of the brain called the extended amygdalae (Koob 2009). The amygdalae (singular amygdala) are almond-shaped groups of neurons located deep within the medial temporal lobes of the brain. They encompass several nuclei, or structures in the central nervous system, including the central, lateral, and basal nuclei. The extended amygdala is composed of a group of structures, including the central nucleus of the amygdala (CeA) and the bed nucleus of stria terminalis (BNST), a group of neurons in the forebrain. CRH is produced by neurons in the CeA, the BNST, and the brainstem. This eCRH system helps mediate behavioral responses to stress and controls the sympathetic nervous system response to stressors (Koob 2009). It is important to note that although the HPA–CRH and extended amygdalar–eCRH stress circuits are interconnected, the latter system can act independent of the HPA axis.
Several different chemicals that transmit impulses between neurons (i.e., neurotransmitters) regulate the CRH systems in the brain, but interactions between norepinephrine and CRH in the region of the locus coeruleus (a nucleus in the brainstem) are believed to be particularly relevant to alcoholism (Koob 2009) and certain stress-related psychiatric disorders, such as major depression and PTSD (Curtis et al. 2006). Other key neurotransmitters regulating these stress–reward circuits—which are likely to play a role in comorbid alcoholism and stress-related disorders—are serotonin, endogenous opiates, γ-aminobutyric acid (GABA), and glutamate (Anthenelli et al. 2001; Uhart and Wand 2009).
Chronic Alcohol Disrupts the HPA Stress Response
Koob and LeMoal (2005) hypothesized that a key interface between the brain’s stress systems and reward circuits lies in the region of the extended amygdala. It is here that the positive reinforcing effects of drugs, including alcohol, and the negative reinforcing effects of these agents are believed to intersect, with the CRH systems playing a critical modulatory role in this interaction.
In both experimental animals (Heilig and Koob 2007) and humans (Adinoff et al. 2005), alcohol intoxication results in the release of CRH and activation of the HPA axis. Based on evidence (described below) from studies with rodents, it appears that these stress-activating effects may be greater in women than men (Larkin et al. 2010; Ogilvie and Rivier 1997). Although it might seem counterintuitive to consider that consumption of alcohol increases the brain’s and body’s stress hormone levels, it is likely that the brain adapts to repeated bouts of heavy drinking by downregulating responsive elements in the stress circuitry, and it is this process (i.e., neuroadaptation) that characterizes aspects of the addiction process (Richardson et al. 2008).
Effects of Alcohol Withdrawal on the Stress Response
Alcohol’s activation of the stress systems is even more profound during acute withdrawal. Studies in experimental animals (Koob 2009) and humans (Hundt et al. 2001) show marked increases in brain CRH levels and plasma ACTH and cortisol concentrations, respectively, in the hours and days following the last exposure to alcohol. This hyperactivation of the brain’s stress system is accompanied by anxiety-like behaviors in experimental animals and depressed mood in humans. Furthermore, studies in rodents demonstrate that administering drugs that block CRH activity in the CeA (i.e., CRH antagonists) can block these aversive withdrawal effects (Koob 2009).
According to Koob and LeMoal’s theory (2005), the compulsive stage of alcohol dependence is produced, in part, by alcohol- seeking behaviors to relieve a “negative-affect state,” which, according to these authors, may be a hallmark of the protracted abstinence syndrome. Furthermore, the neurobiological underpinnings of this negative emotional state originate in the extended amygdala and are mediated through interactions among the CRH, GABAergic, and norepinephrine systems (Koob 2009). A final piece of the theory postulates that alcohol and other drug addiction is marked by an ongoing adaptation (or allostatic change) in these stress–reward systems (Koob and Le Moal 2001), meaning that alcohol-dependent individuals have adjusted their biological set points and have become more sensitive to stressors and more prone to stress-induced relapse to alcohol.
Indeed, in the days and weeks following the acute withdrawal period, the HPA axis remains dysregulated in alcoholics. However, during this subacute or protracted withdrawal phase, the system is typically blunted. That is, when challenged by a variety of stressors,1 the endocrine response typically is lower than normal. A few studies (Adinoff et al. 2005; Junghanns et al. 2003) have linked these abnormal stress responses with the individual’s risk of relapse to drinking. With lengthier abstinence, however, the HPA axis response to stress appears to normalize. These changes in the HPA axis in relation to a person’s drinking status (i.e., intoxication versus acute and protracted withdrawal) are depicted in table 2.
Table 2.
Differential Patterns of Hypothalamic–Pituitary–Adrenal (HPA) Reactivity Among Individuals With Alcohol Use Disorders (AUDs), Major Depressive Disorder (MDD), and Posttraumatic Stress Disorder (PTSD)
| AUD | MDD | PTSD | |
|---|---|---|---|
| HPA Axis Dysregulation | + | + | + |
| • AUD Intoxication | ACTH and cortisol ↑ | ||
| • AUD Acute Withdrawal | ACTH and cortisol ↑ | ||
| • AUD Protracted Withdrawal | ACTH and cortisol ↓ | ||
| • MDD Melancholic type | Cortisol ↑ | ||
| • MDD Atypical type | Cortisol ↓ | ||
| • PTSD | Cortisol ↓ b | ||
| Dexamethasone Suppression | − | ↓ a | ↑or – c |
NOTE:
Nonsuppression occurs in 25 to 60 percent of patients with MDD using the dexamethasone suppression test (DST) and in upwards of 80 percent of depressives using the combined DST/corticotropin-releasing hormone stimulation test.
Some reports of elevated cortisol levels as well, which may reflect variation in PTSD symptomatology over time (Ehlert et al., 2001).
Negative findings may relate to dosage of dexamethasone used.
Overlapping Mechanisms With Other Stress-Related Disorders
Stress is known to contribute to the development of alcoholism in at-risk individuals and to the maintenance of the disease through stress-induced relapse. It also is a well-known precipitant of stress-related psychiatric disorders such as major depressive disorder (MDD) and PTSD. In fact, the principle of allostasis, which plays a central role in Koob and LeMoal’s (2001) theories on addiction also plays an integral role in McEwen and Wingfield’s (2010) and other scientists’ perspectives on the development of stress-related mood and anxiety disorders. In those hypotheses, chronic stress produces an “allostatic state” (e.g., chronic elevations in steroid hormones [i.e., glucocorticoids]) that can damage brain structures (e.g., the hippocampus, which inhibits the HPA) and cause other compensatory neuroadaptive changes, such as changes to the neurotransmitter binding molecules (i.e., receptors) that lead to desensitization (McEwen 2003). Cumulatively, the dysregulated stress responses produce an “allostatic load” on the organism that contributes to tissue damage, cognitive deficits, and chronic illnesses such as cardiovascular disease.
Just as alcoholics display changes in HPA axis function that vary as a function of disease state, people with stress-related psychiatric disorders such as MDD and PTSD manifest dysregulated HPA axis responsivity. These characteristic response patterns also are depicted in table 2 and are briefly discussed below.
Given that MDD, PTSD, and AUDs so frequently overlap, the challenge confronting researchers in the next decade and beyond is to tease out whether the pattern of altered stress responses seen in comorbid patients shows evidence of greater or lesser dysregulation compared with patients with only one disorder. It is possible that alcohol may exacerbate or counterbalance stress system abnormalities associated with these disorders in the absence of alcoholism.
HPA Axis Abnormalities in Patients With Co-Occurring Psychiatric Disorders
PTSD
In alcohol-dependent patients, resting ACTH and cortisol concentrations appear to vary based on the length of abstinence. Similarly, the stress hormones corticotropin and cortisol in patients with PTSD appear to be influenced by (1) whether the traumatic event occurred only once or multiple times, (2) the duration of time that has elapsed since the traumatic stressor occurred, and (3) the effects of past trauma (Ehlert et al. 2001; Handwerger 2009). On balance, CRH levels appear to be increased in patients with PTSD (Yehuda 2009). Some research also suggests that patients with single-event trauma and PTSD generally display lower cortisol levels than healthy, non–trauma-exposed individuals and those exposed to trauma who did not develop PTSD (Handwerger 2009). This same pattern of lower cortisol concentrations, compared with non-PTSD control subjects, also has been observed among patients with multiple-event trauma. Moreover, PTSD frequently is marked by cortisol hypersuppression following the low-dose dexamethasone suppression test2 (Ehlert et al. 2001; Handwerger 2009).
As illustrated in table 2, the pattern of HPA axis abnormalities observed in patients with PTSD has both similarities and differences with those found in patients with alcohol dependence, and these differential response patterns might have implications for understanding the increased risk of alcoholism in PTSD patients. For example, insufficient cortisol signaling and enhanced negative-feedback inhibition in patients with PTSD may lead some patients to use alcohol for its acute HPA stimulatory effects. Conversely, the hyperarousal symptoms associated with PTSD, which appear to be most related to the extrahypothalamic CRH system, might be ameliorated, at least in the short-term, by drinking and alcohol’s effects on the stress circuitry (Volpicelli et al. 1999). Moreover, patients with PTSD and comorbid AUDs may be more treatment resistant because chronic alcohol-induced changes in brain and stress system functioning interfere with either the cognitive– behavioral or pharmacological approaches commonly used to treat PTSD.
PTSD and Alcohol Dependence
Despite the high co-occurrence rate of PTSD and alcohol dependence and the obvious involvement of the HPA axis in mediating these disorders, only one series of published studies has examined these relationships in comorbid individuals. Brady and colleagues (2006a, b) performed the cold pressor task3 in alcohol-dependent patients with and without comorbid PTSD and compared their findings with control groups of non–alcohol-dependent patients with PTSD and healthy control subjects without either disorder. In this mixed-gender sample, in which alcohol-dependent participants were very recently abstinent, all three patient groups exhibited blunted ACTH responses to the physical stressor compared with control subjects, while at the same time endorsing higher subjective stress levels than controls. The comorbid alcohol–PTSD group had similar endocrine responses to that of the alcohol-only and PTSD-only groups. Among the alcohol-dependent participants, there was a link between stress-induced craving for alcohol and subsequent relapse to drinking. Specifically, in patients who were alcohol dependent only, blunted ACTH responses coupled with a reported increase in craving for alcohol was a predictor for alcohol use in the follow-up period, whereas no such relationship was observed in the comorbid alcohol–PTSD group. Thus, as was the case in the studies conducted by Junghanns and colleagues (2003), there was evidence that blunted stress hormone responses might signal a heightened relapse risk among recently abstinent alcoholics without comorbid PTSD. It remains unknown as to why this potential relapse risk marker was not present in patients with comorbid alcohol dependence and PTSD. However, this dissimilarity in stress reactivity between patients with and without a comorbid stress-related condition is a provocative reminder that results from laboratory stress paradigms performed in non-comorbid patient populations might not translate completely to comorbid patient populations because of important, disease-specific differences in HPA axis dynamics. Clearly, further research is required to disentangle these complex relationships.
MDD
As described above regarding PTSD, patients suffering from MDD show alterations in HPA axis function. Studies show that high levels of cortisol while at rest occur in 40 to 60 percent of patients with MDD (Handwerger 2009). When tested using an overnight dexamethasone suppression test (DST), roughly 40 percent of patients with unipolar nonpsychotic major depression and 65 percent of patients with psychotic depression show cortisol nonsuppression. Under normal circumstances, taking dexamethasone should reduce ACTH levels and lead to decreased cortisol levels. Abnormal DST responses have been linked to a more severe course of illness and a higher likelihood of relapsing after treatment. The combined dexamethasone (Dex)/CRH stimulation test provides a more sensitive assessment of HPA axis irregularities than the DST alone (Heuser et al. 1994). Using a combined Dex/CRH test, 80 percent of patients with depression showed an abnormal response, marked by an exaggerated ACTH and cortisol response (Ehlert et al. 2001; Shea et al. 2005). Thus, although MDD and PTSD both are characterized by elevated brain CRH levels, differences in resting cortisol concentrations (higher in many patients with MDD and lower in patients with PTSD) and the cortisol response to the low-dose DST can be used to distinguish these disorders.
MDD and AUDs
Given that symptoms of depression co-occur with alcohol dependence in about 80 percent of patients and that 30 to 40 percent of alcohol-dependent men and women suffer from an independent major depressive episode during their lifetime (Shivani et al. 2002), it is surprising that relatively few studies have examined HPA reactivity in patients with these comorbidities. In fact, after a burst of activity in the 1980s and 1990s using the DST, no published studies examined stress reactivity in patients with these comorbid disorders. As noted elsewhere (Anthenelli et al. 2009), these first-generation DST studies were either exclusively or predominately conducted in alcohol-dependent men, creating a huge gender gap in the knowledge base for a common comorbidity known mostly to affect women. Moreover, as discussed below, when some women were included in the samples, inadequate attention was paid to confounding variables such as menstrual cycle phase and concomitant use of hormonal contraceptives—factors that markedly influence stress hormone concentrations (Anthenelli et al. 2009). Recognizing these and other inconsistencies (e.g., variable lengths of abstinence, unclear distinctions between alcohol-induced versus independent depressive episodes) with these older studies, it is difficult to interpret their results. However, in general, most studies found that alcohol-dependent study participants had normal responses to the DST and were similar to the comparison groups (Anthenelli et al. 2009). As was the case for patients with comorbid AUDs and PTSD, there clearly is a gap in our understanding of the ways HPA axis reactivity is altered in women and men with AUDs and MDD.
Gender Influences on the Risk for Comorbid Disorders
As alluded to above, the prevalence rates and clinical courses of AUDs, MDD, and PTSD vary among men and women. For example, regarding AUDs, men are twice as likely as women to develop an AUD (Keyes et al. 2008). However, in alcoholic women, these disorders are more likely to follow an accelerated course (Hesselbrock et al. 1985) and to emerge after the onset of a stress-related mood or anxiety disorder (Kessler et al. 1997). Alcoholic women with AUDs also appear to be more susceptible to alcohol’s neurotoxic effects (Mann et al. 2005) compared with alcoholic men. Thus, damage to stress-regulating (e.g., the hippocampus) brain structures influencing stress reactivity and emotional processing may contribute to the higher rates of mood and anxiety disorders in alcohol-dependent women.
In contrast, women are twice as likely as men to suffer from MDD (Kessler et al. 1994), and gender influences the course of that disorder. For example, among outpatients with MDD, women were found to have an earlier age-at-onset to their depression than men (Fava et al. 1996). Women also self-report more depressive symptoms and poorer health status than do men (van Noorden et al. 2010). Patterns of comorbidity in depressives vary according to gender as well. Thus, men with MDD are more likely to have comorbid alcohol and other drug use disorders, along with attention deficit hyperactivity disorder, whereas depressed women are more likely to suffer from comorbid PTSD and bulimia nervosa (Fava et al. 1996; van Noorden et al. 2010).
Women have higher rates of PTSD than men, even after controlling for gender differences in the number and types of traumata suffered (Kessler et al. 1995; Stein et al. 2000). These gender differences are apparent during adolescence (Keller et al. 2010), and, as is the case with adult women and men with comorbid PTSD and AUDs (Sonne et al. 2003), the onset of PTSD typically antedates the onset of AUDs in teenage girls compared with boys.
Do Biological Sex Differences in Stress-Reward Circuits Mediate Comorbidity Risk?
Although sociocultural and environmental factors certainly play an important part in explaining gender-specific disparities in comorbidity risk, recent research conducted by NIAAA-sponsored scientists points to an integral role of biological sex differences in stress–reward systems as primary mediators of these gender effects.4 As reviewed elsewhere (Fox and Sinha 2009; Witt 2007), gender differences in AUD, MDD, and PTSD risk begin to emerge after puberty when adrenal and gonadal steroid levels rise and become more divergent in boys and girls. These changes include reactivation of the hypothalamic–pituitary–gonadal (HPG) axis, which, like the HPA axis described earlier, orchestrates sexual development and reproductive behavior using finely tuned, circadian and circamensual hormone–brain interactions (Solomon and Herman 2009; Witt 2007). Although beyond the scope of this discussion, sex steroids produced by the gonads (testes in boys and ovaries in girls) and adrenal steroids have profound effects on brain development and functioning (see sidebar).
Sex Differences in Stress Responsivity
An important consideration for the next generation of studies identifying the causal mechanisms linking alcohol use disorders (AUDs) with stress-related psychiatric disorders is that, like healthy men and women, female and male alcoholics differ in stress responsivity. Thus, studies attempting to unravel these associations will need to include adequate numbers of alcohol-dependent women and men, or focus on female alcoholics with and without psychiatric comorbidity to decipher these mechanisms.
As reviewed elsewhere (Fox and Sinha 2009; Kajantie and Phillips 2006), sexual dimorphisms in stress reactivity begin early on during pre-natal brain development. At puberty, when sex steroid levels rise and become more divergent between boys and girls, there is further sex-dependent “sculpting” of neural circuits, including those involved with regulating the stress response (Sisk and Zehr 2005). Thus, some of these sex differences appear to involve the brain’s hard wiring.
Gonadal steroids such as estrogen, progesterone, and testosterone also influence various elements of the stress response. For example, estradiol can bind to either of two estrogen receptors (ERs) on brain cells that secrete corticotropin-releasing hormone (CRH) and the hormone arginine vasopressin (AVP). Depending on where in the brain these estrogen-binding events take place, and which of these ERs are bound, CRH secretion may be amplified and AVP release suppressed, respectively (Kajante and Phillips 2006). Thus, cyclical changes in estrogen and progesterone levels that occur during the female menstrual cycle influence stress responsivity, and menstrual cycle phase needs to be adequately controlled in human studies measuring stress hormone reactivity.
Estrogens also affect circulating levels of the protein that binds the stress hormone cortisol in the bloodstream. Circamensual fluctuations in estradiol levels affect the ratio of bound versus unbound cortisol, and because only free (i.e., unbound) cortisol that is metabolically active, this is another reason that controlling for menstrual cycle phase is important. Oral contraceptives that contain estrogens or estrogen–progestin mixtures also affect corticosteroid-binding (CBG) concentrations (Kirschbaum et al. 1999), and these medications can be an important confounding factor in measuring stress hormone responses in premenopausal women.
Finally, sex steroids have profound effects on brain chemical (i.e., neurotransmitter) systems governing central and peripheral stress responses. For instance, synthesis and reuptake of the neurotransmitter serotonin is influenced by fluctuating levels of sex steroids at multiple levels (Anthenelli et al. 2001). Thus, sex matters when it comes to determining the associations between AUDs and stress-related psychiatric disorders.
References
- Anthenelli RM, Maxwell RA, Geracioti TD, Jr, Hauger R. Stress hormone dysregulation at rest and after serotonergic stimulation among alcohol-dependent men with extended abstinence and controls. Alcoholism: Clinical and Experimental Research. 2001;25:692–703. [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress-system changes: Implications for treatment in substance-abusing women. Harvard Review of Psychiatry. 2009;17:103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, et al. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontal Neuroendocrinology. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
These effects on the brain are readily apparent in the stress–reward circuits described previously and also are evident in the ways alcohol stimulates the HPA axis. For example, in rodent models, whether alcohol is administered systemically (Ogilvie and Rivier 1997) or directly into the brain (Larkin et al. 2010), the effects on stress hormone release are sexually dimorphic with female rodents exhibiting greater increases in corticotropin levels than male rodents. Indeed, as elegantly reviewed by Witt (2007), there is compelling animal and human evidence that sex and gonadal steroids influence multiple aspects of alcohol drinking and alcohol-seeking behaviors.
Unraveling Sex Differences in Brain Stress–Reward Circuits Underlying Comorbidity: The Next Decade of Progress
Researchers have suggested that the increased risk for stress-related MDD and/or PTSD in alcohol-dependent women compared with men is both a function of biological sex differences in HPA and eCRH stress systems and environmental gender effects (e.g., childhood adversities including traumatic life events) (Anthenelli et al. 2009; Brady 2006a, b; Fox and Sinha 2009). A fundamental aspect of this hypothesis is that, as is the case in experimental animals, the human stress response is sexually dimorphic and stressor specific. However, unlike studies in rodents or nonhuman primates, in which scientists can manipulate the animal’s hormonal milieu and many other important experimental conditions influencing the stress response, the challenges in human studies are more daunting. Menstrual cycle phase, concomitant use of hormonal contraceptives and other drugs of abuse (e.g., cocaine, cannabinoids, nicotine, and opiates), past trauma exposure, and length of abstinence from alcohol and other drug use are just some of the factors that can confound the results of human experiments (Kudielka et al. 2009), making such studies labor intensive and costly. Moreover, researchers need to be sure to distinguish between mood alterations observed during subacute and protracted withdrawal (i.e., alcohol-induced psychiatric disorders) and independent, stress-related psychiatric conditions, which, themselves, frequently overlap in the same individual (Campbell et al. 2007).
Although the task is formidable, the vast knowledge accrued over the first 40 years of NIAAA’s existence has set the stage to overcome these challenges. Unraveling the effects of alcohol and gender on stress reactivity will offer new avenues for prevention and treatment of these common comorbid mental health disorders.
Conclusion
A significant proportion of people with AUDs also suffer from a coexisting mood or anxiety disorder. Researchers are examining the body’s stress response, and the role of the HPA axis in particular, to explain the mechanisms that may increase the risk for these disorders. Stress and alcohol use initiate similar hormonal responses, and some of the alterations observed in the HPA’s response to stress are similar for patients who are alcohol dependent and those who suffer from depression and PTSD. However, important differences in stress reactivity occur across these conditions, which may be identified using laboratory stress paradigms and exploited to better understand how patients with comorbid conditions differ from those with alcoholism alone. Future research needs to examine how gender differences in traumatic life events and biologically driven sex differences in stress and emotional reactivity influence a person’s vulnerability to alcoholism and other comorbid disorders. Such investigations may allow researchers to determine the specific biological factors contributing to the vulnerability to alcoholism and other mental health disorders and may lead to the development of new and more effective treatments for alcoholism and stress-related psychiatric disorders that are gender-specific. The challenge, then, of the next decade and beyond for NIAAA and its funded investigators is to unravel the causal mechanisms underlying comorbid alcohol dependence and stress-related disorders.
Acknowledgments
The writing of this manuscript was supported, in part, by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant award nos. R01AA013307, R01AA013307–05S1, and R01AA013957; the National Institute on Drug Abuse (NIDA)/VA CSP no. 1022; and by the Department of Veterans Affairs Research Service.
Footnotes
Researchers study this phenomenon in alcohol-dependent patients using laboratory-based stress tests that monitor an individual’s stress hormone concentrations, heart rate, blood pressure, and emotions in response to various stressors. Responses vary based on the type of stressor (i.e., psychogenic versus pharmacologic), the duration of abstinence at the time of testing, and individual characteristics such as gender and age.
Dexamethasone is an exogenous steroid that, like cortisol, provides negative feedback to the pituitary to suppress the secretion of ACTH. Under normal circumstances, taking dexamethasone should reduce ACTH levels and lead to decreased cortisol levels.
The cold pressor task involves placing a hand or forearm in cold water, which produces a stimulus that increases in discomfort from mild to moderate intensity until the subject voluntarily removes his or her limb. This task is used to study a person’s response to pain, as well as autonomic reactivity, and hormonal stress responses.
Scientists make distinctions between their use of the terms “sex” versus “gender.” The former implies biologically mediated differences between men and women that derive from chromosomal (male XY versus female XX) and gonadal steroid effects (e.g., estrogen, progesterone and testosterone), whereas the latter connotes environmental and sociocultural differences between the sexes.
Financial Disclosure
Dr. Anthenelli provides consulting services to Pfizer. The Tri-State Tobacco and Alcohol Research Center receives research support from Eli Lilly, Nabi Biopharmaceuticals, and Pfizer.
References
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: Implications for relapse. Alcoholism: Clinical and Experimental Research. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. Third Edition. Washington, DC: APA; 1980. [Google Scholar]
- APA . Diagnostic and statistical Manual of Mental Disorders. Third Edition, Revised. Washington, DC: APA; 1987. [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: APA; 1994. [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders. 4th Edition, Text Revision. Washington, DC: APA; 2000. [Google Scholar]
- Anthenelli RM. A basic clinical approach to diagnosis in patients with comorbid psychiatric and substance use disorders. In: Miller NS, editor. Principles and Practice of Addictions in Psychiatry. Philadelphia, PA: W.B. Saunders Company; 1997. pp. 119–126. [Google Scholar]
- Anthenelli RM. “Sex Differences in the Stress Hormone Response to the Combined Dexamethasone-Corticotropin Releasing Hormone (Dex-CRH) Stimulation Test”. Paper presented at the Alcohol Stress Interest Group, 32nd Annual Scientific Meeting of the Research Society on Alcoholism; San Diego, California. Jun, 2009. [Google Scholar]
- Anthenelli RM, Maxwell RA, Geracioti TD, Jr, Hauger R. Stress hormone dysregulation at rest and after serotonergic stimulation among alcohol-dependent men with extended abstinence and controls. Alcoholism: Clinical and Experimental Research. 2001;25:692–703. [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, et al. Cold pressor task reactivity: Predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcoholism: Clinical and Experimental Research. 2006a;30:938–946. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, Waldrop AE, McRae AL, et al. The impact of alcohol dependence and posttraumatic stress disorder on cold pressor task response. Journal of Studies on Alcohol. 2006b;67:700–706. doi: 10.15288/jsa.2006.67.700. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Campbell DG, Felker BL, Liu CF, et al. Prevalence of depression-PTSD comorbidity: Implications for clinical practice guidelines and primary care-based interventions. Journal of General Internal Medicine. 2007;22:711–718. doi: 10.1007/s11606-006-0101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: The role of the hypothalamus-pituitary-adrenal axis. Biological Psychology. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Fava M, Abraham M, Alpert J, et al. Gender differences in Axis I comorbidity among depressed outpatients. Journal of Affective Disorders. 1996;38:129–133. doi: 10.1016/0165-0327(96)00004-3. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Robins E, Guze SB, et al. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry. 1972;26:57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress-system changes: Implications for treatment in substance-abusing women. Harvard Review of Psychiatry. 2009;17:103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: Results of a national survey. Drug and Alcohol Dependence. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS. The Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS) Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1992. [Google Scholar]
- Grant BF, Dawson DA, Hasin DS. The Alcohol Use Disorder and Associated Disabilities Interview Schedule: DSM-IV Version. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2001. [Google Scholar]
- Grant BF, Moore TC, Kaplan K. Source and Accuracy Statement: Wave 1 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2003. [Google Scholar]
- Grant BF, Peterson A, Dawson DA, Chou PS. Source and Accuracy Statement for the National Longitudinal Alcohol Epidemiologic Survey. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1994. [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Grant BF. Major depression in 6050 former drinkers: Association with past alcohol dependence. Archives of General Psychiatry. 2002;59:794–800. doi: 10.1001/archpsyc.59.9.794. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in Neuroscience. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer JE, Pryzbeck TR. The co-occurence of alcoholism with other psychiatric disorders in the general population and its impact on treatment. Journal of Studies on Alcohol. 1988;49:219–224. doi: 10.15288/jsa.1988.49.219. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. Journal of Psychiatric Research. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hundt W, Zimmermann U, Pottig M, et al. The combined dexamethasone-suppression/CRH-stimulation test in alcoholics during and after acute withdrawal. Alcoholism: Clinical and Experimental Research. 2001;25:687–691. [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, et al. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol and Alcoholism. 2003;38:189–193. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Keller TE, Salazar AM, Courtney ME. Prevalence and timing of diagnosable mental health, alcohol, and substance use problems among older adolescents in the child welfare system. Children and Youth Services Review. 2010;32:626–634. doi: 10.1016/j.childyouth.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, et al. Lifetime co-occurence of DSM–III–R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM–III–R psychiatric disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Research. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addition. Nature Neuroscience. 2005;8(11):1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Larkin JW, Binks SL, Li Y, Selvage D. The role of oestradiol in sexually dimorphic hypothalamic-pituitary-adrena axis responses to intracere-broventricular ethanol administration in the rat. Journal of Neuroendocrinology. 2010;22:24–32. doi: 10.1111/j.1365-2826.2009.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, et al. Neuroimaging of gender differences in alcohol dependence: Are women more vulnerable? Alcoholism: Clinical and Experimental Research. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Rounsaville BJ. Comorbidity of substance use with depression and other mental disorders: From Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) to DSM-V. Addiction. 2006;101(Suppl. 1):89–96. doi: 10.1111/j.1360-0443.2006.01585.x. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Gender difference in hypothalamic-pituitary-adrenal axis response to alcohol in the rat: Activational role of gonadal steroids. Brain Research. 1997;766:19–28. doi: 10.1016/s0006-8993(97)00525-8. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA: Journal of the American Medical Association. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, et al. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. European Journal of Neuroscience. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Shea A, Walsh C, Macmillan H, Steiner M. Child maltreatment and HPA axis dysregulation: Relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005;30:162–178. doi: 10.1016/j.psyneuen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Shivani R, Goldsmith RJ, Anthenelli RM. Alcoholism and psychiatric disorders: Diagnostic challenges. Alcohol Research & Health. 2002;26:90–98. [Google Scholar]
- Solomon MB, Herman JP. Sex differences in psychopathology: Of gonads, adrenals and mental illness. Physiology and Behavior. 2009;97:250–258. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonne SC, Back SE, Diaz Zuniga C, et al. Gender differences in individuals with comorbid alcohol dependence and post-traumatic stress disorder. American Journal on Addictions. 2003;12:412–423. [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: Rationale and reliability. Archives of General Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- Stein MB, Walker JR, Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behavioural Research and Therapy. 2000;38:619–628. doi: 10.1016/s0005-7967(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: An update of human research. Addiction Biology. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noorden MS, Giltay EJ, den Hollander-Gijsman ME, et al. Gender differences in clinical characteristics in a naturalistic sample of depressive outpatients: The Leiden Routine Outcome Monitoring Study. Journal of Affective Disorders. 2010 doi: 10.1016/j.jad.2009.12.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Volpicelli J, Balaraman G, Hahn J, et al. The role of uncontrollable trauma in the development of PTSD and alcohol addiction. Alcohol Research & Health. 1999;23:256–262. [PMC free article] [PubMed] [Google Scholar]
- Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicology and Teratology. 2007;29:81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Annals of the New York Academy of Sciences. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]


