Abstract
Forty years ago, alcohol was not commonly recognized as a teratogen, an agent that can disrupt the development of a fetus. Today, we understand that prenatal alcohol exposure induces a variety of adverse effects on physical, neurological, and behavioral development. Research supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) has contributed to the identification of the range and prevalence of fetal alcohol spectrum disorders (FASD), as well as methods for prevention and treatment of FASD. The worldwide prevalence and high personal and societal costs of FASD speak to the importance of this research. This article briefly examines some of the ways that NIAAA has contributed to our understanding of FASD, the challenges that we still face, and how this research is translated into changes in public policy.
Keywords: Alcohol consumption, fetal alcohol spectrum disorders, fetal alcohol syndrome, prenatal alcohol exposure, fetus, teratogenesis, human studies, animal studies, prevention research, treatment research
Despite centuries of alcohol use, the first two clinical reports of fetal alcohol syndrome (FAS) in English literature did not appear until 1973, published in the journal Lancet by a group of investigators from the University of Washington, Seattle. The first paper (Jones et al. 1973) described the common dysmorphic and developmental problems in eight children of alcoholic women. The second paper (Jones and Smith 1973) characterized an additional three children and introduced the term “fetal alcohol syndrome” to describe the common pattern of observed deficits.
Within 2 years following these publications, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) initiated its first research projects on FAS. The goals of that early research were to confirm alcohol’s ability to disrupt the development of a fetus (i.e., its teratogenicity) and, if confirmed, to further characterize the syndrome. Among the first grants awarded was one to Dr. Ann Streissguth, a coauthor on the first Lancet paper, to begin an investigation of the effects of alcohol across the full spectrum of dose levels on neurodevelopmental outcomes. Subsequently known as the Seattle 500 Study, based on the number of children recruited, this initial project has been an active research program for more than 30 years. Two other epidemiologic studies also were awarded to examine the relationship between alcohol and fetal outcome: one of these was awarded to Dr. Jan Kuzma, an epidemiologist at Loma Linda University, and the other to Dr. Joel Alpert at the Boston University School of Medicine. One of the coleaders of the Boston University project was Dr. Henry Rosett, a psychiatrist and NIAAA Career Teacher who became an important and prominent leader in research and education on FAS.
Concurrent to these investigations, NIAAA initiated a number of research projects involving animal models. These animal models were critical for addressing the early skepticism that alcohol was a teratogenic agent. The argument was made that if alcohol causes birth defects, surely the medical field would have noted this long before, given the thousands of years of alcohol use. In addition, although the initial cases of FAS all were born to women who had significant alcohol use disorders (AUDs), it was not clear if FAS was the direct result of alcohol on the embryo and fetus or it was caused by some other drug, malnutrition, or even “a deviant lifestyle.” The animal studies enabled scientists to control many of these other variables and thereby truly assess whether FAS was indeed the result of alcohol or some other factor. The initial animal models studied rats, mice, and dogs, among other species, and effects of alcohol were similar to those observed in the offspring of women with AUDs (Abel and Dintcheff 1978; Chernoff 1977; Ellis and Pick 1980; Randall and Taylor 1979; Randall et al. 1977; Riley et al. 1979). Thus, animal studies were critical for the recognition of alcohol as a teratogen.
In February 1977, NIAAA organized the first workshop on FAS. Researchers from epidemiological, clinical, and basic science fields (about 50 in attendance) brought their findings to the forum. Although the intent was primarily to help guide future research directions, the attendees at the meeting were so impressed with the findings that they collectively expressed a need to inform the public that alcohol could be damaging to the developing fetus, potentially resulting in FAS.
NIAAA took responsibility for gaining approval from the Department of Health Education and Welfare (DHEW) for issuing an advisory on alcohol use in pregnancy. After documenting the available evidence in a state-of-the-science report, the Department approved an advisory issuance. It was released on June 1, 1977, in the Food and Drug Administration’s (FDA’s) Drug Bulletin and the Centers for Disease Control and Prevention’s (CDC’s) Morbidity and Mortality Weekly Report, as well as through a press conference at DHEW headquarters. The FDA and CDC bulletins were selected as the appropriate dissemination vehicles because their audiences were physicians and other health care professionals—the primary targets sought at that time. The advisory warned against heavy drinking in pregnancy and recommended a somewhat refrained two-drink-per-day drinking limit.
Following the publication of the advisory in the FDA Drug Bulletin, the Commissioner of the FDA wrote to the Director of the then Bureau of Alcohol Tobacco and Firearms in the Department of the Treasury on the need for warning labels on alcohol-containing beverages to inform drinkers of the potential harm caused by alcohol’s use in pregnancy. The letter, which also was publicly distributed, led to initiation of Senate hearings on FAS and potential beverage alcohol warning labels. Two sets of Senate hearings were held in successive years. The outcome was a legislatively passed requirement for a joint report to be submitted by both the Department of Human Health Services (DHHS; replacing DHEW) and the Department of the Treasury, addressing the health hazards of alcohol, with particular reference to pregnancy and recommendations on what could and should be done to address the problems. In 1980, the joint DHHS and Treasury Report (1980) was sent to Congress. The Report did not immediately call for alcohol beverage labeling but rather requested the Surgeon General to issue a (new) health advisory on FAS. Released in May 1981, the Surgeon General’s advisory went further than the original NIAAA/DHEW 1977 advisory and recommended that both pregnant women and those seeking to become pregnant avoid all alcohol. In 1988, the issue of alcoholic beverage labeling emerged again in Congress. This time, Congress passed legislation requiring all alcohol-containing beverages to carry a warning label. That label addressed both alcohol and pregnancy and other risks associated with alcohol use. The language of the warning label, as well as the size, color, and placement have not been changed since 1989.
Also of note, NIAAA took a lead in developing information on the dangers of alcohol use during pregnancy and disseminated those findings through public information campaigns in both print brochures and radio and television public service announcements, as early as 1978. Thus, NIAAA has been instrumental in affecting public policy and informing the public of the adverse effects of prenatal alcohol exposure. Moreover, over the last 35 years of NIAAA-supported research, our understanding of alcohol’s teratogenic effects has grown substantially, and many questions have been answered in whole or in part. The number of researchers in the field has grown, with more than 400 members of the Fetal Alcohol Spectrum Disorders Study Group and 106 studies currently funded by NIAAA, as important questions surrounding prenatal alcohol exposure continue to be addressed.
The Consequences of Prenatal Alcohol Exposure
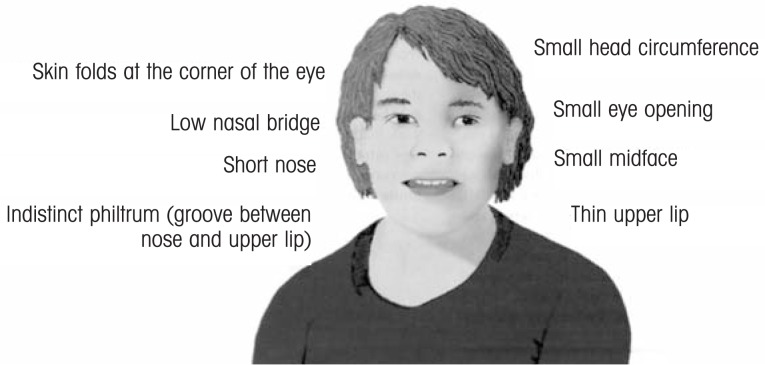
FAS is characterized by three diagnostic criteria: a distinct pattern of facial dysmorphology (see figure 1), pre- and postnatal growth deficiencies, and central nervous system dysfunction. However, it was readily apparent to all involved in the early days of alcohol and pregnancy research that prenatal alcohol exposure could produce a range of effects that fell short of meeting all of the diagnostic criteria for full-blown FAS. Over the years, a number of terms have been used to describe these alcohol-attributed effects, including partial FAS, fetal alcohol effects, alcohol-related birth defects, and alcohol-related neurodevelopmental disorders, with the Institute of Medicine providing some standardization in their 1996 report (Stratton et al. 1996). Subsequently, a general acceptance emerged that the adverse outcomes fall across a spectrum, and an umbrella term was introduced for this full spectrum: fetal alcohol spectrum disorders (FASD) (Streissguth and O’Malley 2000).
Figure 1.
Facial features of FAS.
NIAAA-supported investigations were vital for identifying many of the adverse outcomes of prenatal alcohol exposure, from neurodevelopmental and physical effects to end points such as a heightened risk of miscarriage and stillbirth. Although early research focused only on those individuals most affected by prenatal alcohol exposure, recent research is examining the range of FASD using novel technologies to advance our understanding. For example, early studies of prenatal alcohol-induced neuropathology were derived from postmortem tissue, which showed that prenatal alcohol leads to microencephaly (small brain), as well as neuroglial heterotopias (nerve cells that have migrated to the wrong location) and disrupted development of the corpus callosum and cerebellum (Clarren et al. 1978). However, these reports were derived from cases at the severe end of the FASD continuum.
In the early 1990s, NIAAA funded seminal research by Dr. Edward Riley and colleagues, which used noninvasive imaging to show that multiple areas of the central nervous system are adversely affected by prenatal alcohol exposure. Structural imaging studies confirmed reductions in the volume of overall brain size, with disproportionate reductions in basal ganglia and the anterior vermis of the cerebellum (Mattson et al. 1992). Additional studies have shown alterations in brain shape, changes in cortical thickness, reduced size, and altered shape of the corpus callosum, as well as alterations in the hippocampus (see Norman et al. 2009).
Since those early studies, a variety of imaging techniques have been used, including diffusion tensor imaging, magnetic resonance spectroscopy, functional magnetic resonance imaging, positron emission tomography, and single-photon emission computed tomography (for a review, see Norman et al. 2009). These studies show that prenatal alcohol exposure disrupts development of both gray and white matter (see figure 2) and further illustrate alcohol-related alterations in cerebral blood flow, neurotransmitters, and neuronal activity, even when there are no obvious structural changes. Importantly, it was discovered that individuals exposed to alcohol prenatally could suffer from brain anomalies and dysfunction without exhibiting distinct facial dysmorphology, a finding with significant implications for the identification of children with FASD. More recently, prenatal and neonatal ultrasound are being used to help identify brain anomalies early in development, which may be key for early intervention. NIAAA also is now supporting similar brain-imaging techniques with animal models, in which alcohol exposure parameters can be controlled (Parnell et al. 2009) (see figure 3).
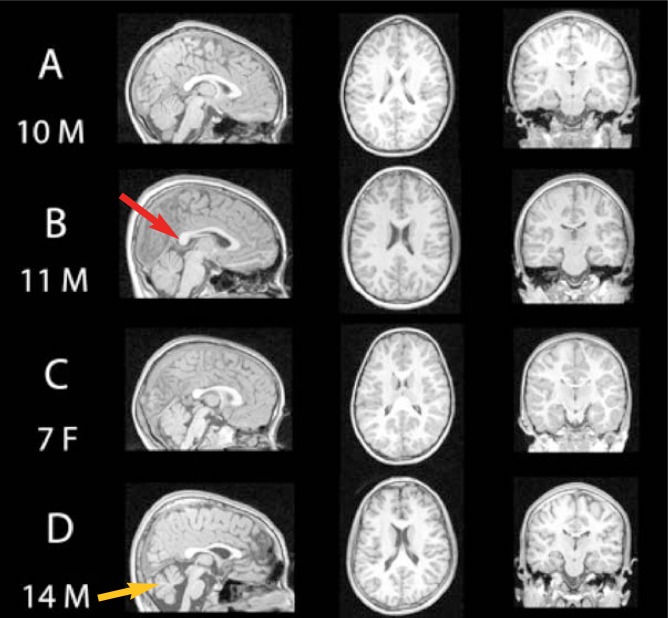
Figure 2.
Magnetic Resonance Imaging (MRI) scans of four children: (A) shows a typically developing 10-year-old boy who has not been exposed to alcohol (B) features an 11-year-old boy with partial fetal alcohol syndrome (pFAS) (C) shows a 7-year-old girl with FAS, and (D) shows a 14-year-old boy with FAS. Notice the variability in brain structures among the individuals with fetal alcohol syndrome disorders, including alcohol-related changes in areas such as the corpus callosum (red arrow) and cerebellum (yellow arrow).
SOURCE: Sowell, E.; Nunez, S.; Roussotte, F. Structural and functional brain abnormalities in fetal alcohol spectrum disorders, Alcohol Research & Health, in press.
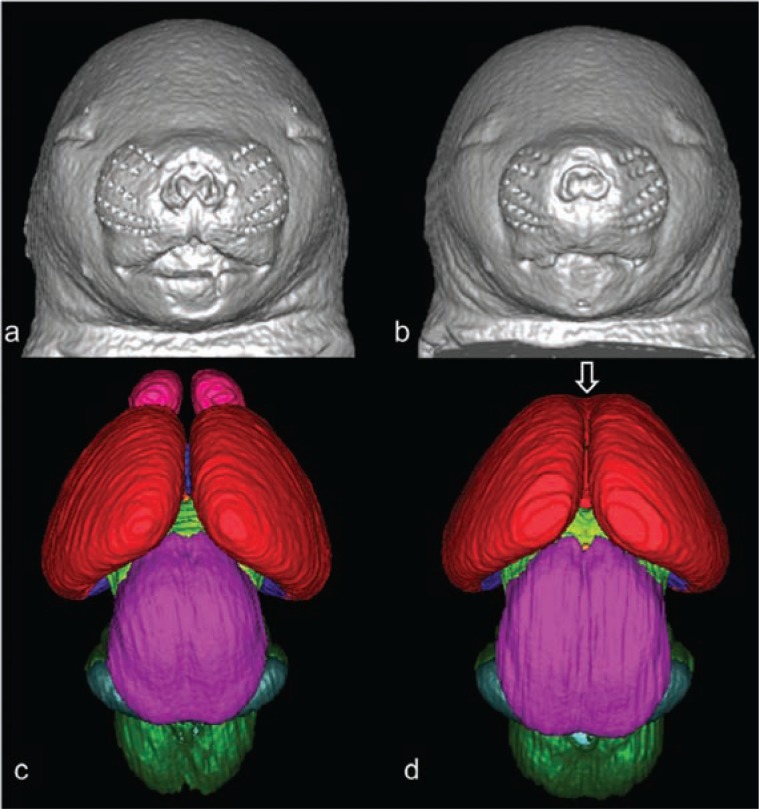
Figure 3.
3D reconstruction of the faces and brains of mice at 17 days of gestation. Control mice are shown in a and c, mice exposed to alcohol are shown in b and d. Mouse fetuses in b and d illustrate dysmorphology resulting from exposure to alcohol at 7 days of gestation. Compared to the control (a), the ethanol-exposed fetus (b) has a smaller head size, a small nose, and an elongated/abnormal philtral portion of the upper lip. These facial features are characteristic of fetal alcohol syndrome. The brain of the ethanol-exposed animal also is dysmorphic (d); the olfactory bulbs are absent and the cerebral hemispheres are united rostrally (open arrow). Color codes: red=cerebral cortex, pink=olfactory bulbs, magenta=mesencephalon, light green=diencephalon, dark green=pons and medulla, teal=cerebellum.
SOURCE: O’Leary-Moore, S.K.; Parnell, S.E.; Godin, E.A.; and Sulik, K.K. Magnetic resonance-based studies of FASD in animal models, Alcohol Research & Health, in press. Modified from Godin, E.A.; O’Leary-Moore, S.K.; Khan, A.A.; et al. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 7. Alcoholism: Clinical and Experimental Research 34(1):98–111, 2010. PMID: 19860813
The neuropathology associated with FASD leads to a range of behavioral effects. Early studies demonstrated general impairments in intelligence (although there is quite a range of IQ scores among individuals exposed to alcohol prenatally), impaired reflex development, deficits in motor coordination, and hyperactivity. More recent studies suggest that deficits in attention, learning and memory, emotional dysregulation, and executive functioning are core deficits, likely reflecting the dysfunction of the frontal lobe (for reviews, see Kodituwakku 2007; Riley and McGee 2005). These behavioral domains also are disrupted with animal models of FASD (Driscoll et al. 1990). Moreover, prenatal alcohol-induced alterations in cognitive functioning and stress responses may contribute to secondary disabilities, including psychiatric comorbidities and vulnerability to addiction. One of the challenges is to determine if there is a pattern of neuropathology and behavioral expression that is unique to prenatal alcohol exposure and therefore useful for diagnosis, as described below.
Prenatal alcohol also leads to physical and physiological changes that are not part of an FAS diagnosis, including alterations in skeletal and organ formation as well as immune function (see Zhang et al. 2005). Prenatal alcohol exposure also may contribute to other disorders. For example, it may disrupt the development of brain structures that contribute to sudden infant death syndrome. NIAAA is addressing this issue jointly with the National Institute on Child Health and Human Development via a consortium. When considering that 40 years ago it was not commonly recognized that alcohol was a teratogen, remarkable strides have been made in understanding the range of ethanol’s adverse effects on the developing embryo and fetus.
Problems in Identifying Individuals Exposed to Alcohol Prenatally
The identification of individuals who have been exposed to alcohol prenatally can be challenging. Accurate maternal drinking histories may not be available and even if the child exhibits the defining features of FAS, they may be missed if the child is not diagnosed by a trained dysmorphologist. It is even more challenging to identify individuals who have been exposed to alcohol prenatally but who do not meet the diagnostic criteria for FAS (i.e., do not exhibit all of the defining facial features). Thus, there is a need for tools to enhance diagnoses, particularly because diagnoses often are necessary for the individual to receive appropriate services. The possibility that more subtle dysmorphic features could aid in a diagnosis of FAS or partial FAS in the absence of information on alcohol exposure in pregnancy (after eliminating genetic disorders otherwise appearing as phenocopies) currently is being investigated by an international consortium funded by NIAAA. Three-dimensional camera imaging is being explored as a means for identifying facial features that represent the full spectrum of FASD. Such images could serve as a tool for sites that do not have access to a trained dysmorphologist and to detect individuals with partial FAS. Moreover, evaluation of the relationship between the dysmorphic facial features, the neuroanatomic brain features, and the neurodevelopmental alterations may not only elucidate the common embryological processes that are disrupted but also can be used to determine if a pattern of injury exists that can serve as a unique signature of prenatal alcohol exposure.
Identification of FASD also can be improved by better detection of women who are drinking alcohol during pregnancy. Clinical research has shown that it is possible in many situations, both prospectively and retrospectively, to obtain good clinical histories on alcohol exposure during pregnancy (Chiodo et al. 2009; Hannigan et al. 2009; Sokol et al. 1989). Screening for such alcohol exposure information has been aided by a number of brief questionnaires that have been developed over the years, such as the T-ACE (Chiodo et al. 2010; Sokol et al. 1989), TWEAK (Russell et al. 1994, 1996), the 10-question AUDIT (Bohn et al. 1995), and the more direct and convenient 3-question AUDIT-C (Dawson et al. 2005), among several others (Chasnoff et al. 2007; Chiodo et al. 2009). Nonetheless, there are occasions during which the attainment of reliable alcohol exposure information is not possible, for example, when maternal memory is poor, drinking is denied, or biological parents are not available.
Therefore, the identification of biomarkers that can reliably reflect fetal alcohol exposure and/or injury is of high priority, especially because such markers may be useful for early case recognition and thereby early intervention. Although metabolites of tobacco or other drugs often are used as biomarkers of their respective use, the prime oxidative metabolites of alcohol are carbon dioxide and water, both of which are present in such abundance that they cannot serve as a marker of alcohol use. However, there also are less prominent nonoxidative routes by which alcohol is eliminated from the body. This includes the formation of esters with the body’s fatty acids to make fatty acid ethyl esters (FAEEs). The FAEEs, which can accumulate in hair, are a potential marker for prior alcohol use (Kulaga et al. 2009). FAEEs also may be found in the meconium from newborns and can indicate exposure to alcohol in the last few weeks or months of pregnancy (Bearer et al. 2003). Other nonoxidative metabolites of ethanol include ethyl glucuronide, which already has been used as a clinical marker to reflect alcohol exposure within the past 3 days, and phosphatidyl ethanol, a marker that may indicate alcohol use for up to 1 to 2 weeks (Litten et al. 2010). Newer technologies in the fields of proteomics and metabolomics (Harrigan et al. 2008; Hiller-Sturmhöfel et al. 2008), and even epigenetic alterations of histone proteins and DNA methylation eventually may provide meaningful indications of alcohol exposure via biological fluids from either maternal or infant sources. Markers that can reliably indicate level and timing of alcohol exposure would greatly improve the ability to identify individuals with FASD.
Defining the Problem
Although the Institute of Medicine (1996) estimates the prevalence of FAS in the United States to be between 0.5 and 2.0 cases per 1,000 live births, this is likely an underestimate. Determining the true prevalence has proven to be a major challenge because children with FASD often are diagnosed with other disorders (such as attention deficit hyperactivity disorder, oppositional defiant disorder, or conduct disorder), or the neurodevelopmental deficits are totally ignored and no educational or clinical diagnosis is given. Obtaining an accurate prevalence of FAS requires the use of an expensive epidemiologic technique called active case ascertainment (May et al. 2009). When this method was applied in South Africa, it revealed an alarmingly high FAS prevalence of 65 to 74 per 1,000 live births (Viljoen et al. 2005). An active case ascertainment study in Italy revealed an FAS prevalence of 3.7 to 7.4 per 1,000 live births (May et al. 2006), a rate similar to a pilot active case ascertainment study in a small western city in the United States (Clarren et al. 2001). Incidence of the full spectrum of FASD is much higher, estimated to affect 1 percent of live births in the United States (Sampson et al. 1997), 2 to 4 percent in Italy (May et al. 2006), and 7 to 9 percent in South Africa (May et al. 2007). Through examination of FASD at various international sites, NIAAA is identifying not only the prevalence of FASD but also the environmental factors that may convey risk or protection against FASD.
The Risk Factors for FASD
Both clinical and preclinical studies have identified a number of risk factors for FASD, including dose, pattern, and timing of alcohol exposure (see Abel and Hannigan 1995; May et al. 2008a; West 1987). For example, using a mouse model, Sulik and colleagues (1981) demonstrated that facial dysmorphology is caused by alcohol exposure during early gestation (i.e., during gastrulation). Heavier drinking and binge drinking increase the likelihood of FASD. But is there a threshold for alcohol’s damaging effects? This is a difficult question and, to date, safe amounts of alcohol during pregnancy have not been established. Moreover, because so many factors influence alcohol’s adverse effects, including polydrug use, prenatal care, nutrition, and genetic factors, it is difficult to predict outcome on an individual basis. In general, low socioeconomic status and education, increased maternal age and number of pregnancies (i.e., gravidity), and poor nutrition are associated with more severe outcomes following prenatal alcohol exposure. One approach to better understanding the relationship between maternal and alcohol exposure factors to fetal outcome is through prospective studies. NIAAA currently is funding several prospective studies to address risk factors in FASD. Understanding protective and provocative factors will allow prevention efforts to be targeted at high-risk populations.
Preventing High-Risk Drinking During Pregnancy
The most desirable route for prevention involves eliminating or significantly reducing alcohol consumption by women during pregnancy. In 1996, the Institute of Medicine report proposed a three-level model for prevention involving components targeted to the general population (universal prevention), to high-risk communities (targeted prevention), and to the specific individuals at greatest risk, which would include women who already have given birth to a child with an FASD or women who meet diagnostic criteria for alcohol dependence (indicated prevention) (Stratton et al. 1996). Whereas universal prevention efforts have failed to provide meaningful success in FASD prevention, targeted and indicated prevention efforts have met with significant success (May et al. 2008b).
One aspect of FASD prevention involves the recognition of high-risk drinking in women in primary-care settings and prenatal clinics. Importantly, research shows that screening and brief interventions in these settings are highly effective in reducing and eliminating risky drinking. Unfortunately, even though such tools are readily available through NIAAA (http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/clinicians_guide.htm), they are not routinely utilized, fostering the need to greatly expand such efforts (Chang et al. 2000, 2005).
FASD is completely preventable if women would no longer drink alcohol during pregnancy. Recognizing that this ideal goal often may not be met, other efforts are underway to explore the possibility of minimizing the damage caused by prenatal alcohol exposure (see sections below).
How Does Alcohol Disrupt Development?
Understanding the mechanisms by which alcohol disrupts development is important, both for the development of better prevention efforts and of novel interventions. Because alcohol exerts multiple actions on the developing embryo and fetus, it induces damage via many mechanisms (Goodlett et al. 2005). For example, studies show that alcohol can disrupt every step in central nervous system development, from proliferation, migration, and differentiation, to synaptogenesis and myelination; in fact, alcohol can directly lead to apoptotic and necrotic cell death.
Neuropathology can be induced both by alcohol actions on neurons as well as by supportive glial cells. The mechanisms will depend on the dose and developmental timing of alcohol exposure and the characteristics of the various cell populations. Early research demonstrated that both alcohol and its metabolite acetaldehyde are directly teratogenic. Alcohol also can have secondary effects, including interference with placental function and nutrient absorption. More recent research supported by NIAAA demonstrates that alcohol disrupts development via some specific actions, including oxidative stress and mitochondrial dysfunction, inhibition of cell adhesion molecules, reduction of neurotrophic factors, and effects on neurotransmitter systems such as glutamate and γ-aminobutyric acid systems. These mechanisms of action have led to the development of a variety of experimental therapeutics, as described below.
Developmental alcohol exposure also alters gene expression. For example, alcohol may affect a number of early gestational genes, including Pax 6, Otx 6, Sox 3, NCAM, TBX 5, and Vax 2 (Peng et al. 2004). Recently, NIAAA, recognizing the importance of epigenetic aspects in alcohol’s actions, has marked this as a key area of research in the Institute’s strategic plan. Epigenetic factors refer to factors that regulate gene expression (through DNA methylation, histone modification, or via micro RNAs) without altering the actual DNA. Recent studies indicate that developmental alcohol may lead to epigenetic changes (for reviews, see Ramsay 2010; Haycock 2009). Such findings may help to explain the results of early studies (Stockard 1913) showing the teratogenic effects of alcohol exposure before conception and on male gametes. Epigenetic effects may contribute to long-lasting changes in physical and behavioral development, including changes in the stress and immune systems that can impact a range of cognitive and emotional effects in offspring.
Intervening With and Treating Individuals With FASD
Despite prevention efforts, many women continue to drink alcohol during pregnancy. Moreover, many countries have yet to acknowledge that FASD occurs in their populations, so policies to reduce alcohol are not in place. Thus, it is vital to identify ways of reducing the severity of effects in children with FASD.
NIAAA began funding studies to examine how nutritional and environmental factors might reduce the severity of FASD within 10 years of the recognition of FAS. To date, NIAAA has funded numerous preclinical and clinical studies that examine methods for protecting against alcohol-related damage at the time of the insult, as well as treatments that may effectively reduce the severity of FASD among individuals who have been exposed to alcohol prenatally.
For example, as noted above, alcohol may lead to oxidative stress. Numerous animal studies have shown that various agents with antioxidant properties, including vitamins C and E, or even the activation of endogenous antioxidants can reduce the adverse physical, neuropathological, and behavioral effects of alcohol. Protection against the physical effects of alcohol exposure also has been observed with administration of a variety of neurotrophic factors, including BDNF, IGF-I, NGF, and neuropeptides derived from activity-dependent neurotrophic factor (ADNF) and activity-dependent neurotrophic peptide (ADNP), as well as their small peptide active fragments SAL and NAP, to name a few. In fact, NAP and SAL, which now are being investigated as a treatment for other disorders such as Alzheimer’s disease, can protect against physical and neuropathological effects of alcohol (i.e., Chen et al. 2005). Serotonin agonists also can protect against alcohol-related developmental damage, as serotonin serves not only as a neurotransmitter but also as a neurotrophic factor (i.e., Druse et al. 2006). Similarly, drugs that block the over activity of N-methyl-d-aspartic acid receptors, which may contribute to brain damage during alcohol withdrawal, also can reduce neuropathology and behavioral deficits (i.e., Thomas et al. 2001). Finally, agents that antagonize alcohol’s blockade of cell adhesion sites are being further refined (Arevalo et al. 2008; Chen et al. 2001).
As noted above, another key risk factor for FASD is poor nutrition. Animal studies have shown that some nutrients may attenuate alcohol’s teratogenic effects, including choline, folate, zinc, and nicotinamide, although not all studies have found beneficial effects. This leads to the intriguing question of whether nutritional supplements can reduce FASD in clinical populations. NIAAA currently is funding the first study to examine the effectiveness of micronutrient supplementation among women who have been drinking alcohol during pregnancy.
In the meantime, what can be done after the child is born and already has sustained the alcohol-induced injury? Early reports suggested that the IQs of individuals exposed to alcohol prenatally were stable and little could be done to improve the cognitive and behavioral abilities of those with FASD. More recently, however, it has been recognized that the brain’s ability to recover (i.e., its plasticity) from early alcohol exposure is much greater than previously thought and, although some studies suggest that prenatal alcohol may limit lifespan neuroplasticity, interventions may indeed improve behavioral function and quality of life.
Animal model studies indicate that various pharmacological, nutritional, and behavioral interventions can improve outcome. For example, drugs like cognitive enhancers (Medina et al. 2006) may enhance plasticity and recovery of function. Nutritional factors, such as choline, also have been shown to effectively improve cognitive functioning, even when administered weeks after developmental alcohol exposure. Choline supplementation can reduce the severity of deficits on a variety of cognitive tasks (i.e. Thomas et al. 2007). Finally, environmental or behavioral interventions, such as communal rearing, environmental enrichment, motor acrobatic training, and exercise also can improve cognitive and motor function (Hannigan et al. 2007; Klintsova et al. 2000; Thomas et al. 2008). These studies suggest that if individuals with FASD are given the appropriate experience, whether social, cognitive, or motor in nature, their performance can improve (see Kelly et al. 2009).
Consistent with preclinical findings, clinical studies show that stable home environments are associated with a better outcome than unstable, stressful environments (Streissguth et al. 1996). Moreover, based on NIAAA-funded studies showing that environmental factors could influence outcome, an increasing number of studies are demonstrating that educational interventions can reduce the severity of FASD. Such interventions can effectively assist families in creating a stable environment, and train individuals with FASD on targeted skills, such as mathematical abilities, social skills, metacognition, and literacy.
These promising studies indicate that multiple approaches may improve the quality of life of people with FASD. NIAAA continues to support research to identify the most effective neurodevelopmental and educational interventions for FASD.
Summary
Forty years ago, alcohol was not commonly recognized as a teratogen. Today, not only has public policy changed, but we also have a better understanding of the consequences of prenatal alcohol exposure and the prevalence and mechanisms of alcohol-related damage. Although we have developed diagnostic, prevention, and treatment strategies, challenges remain. Better identification and diagnosis of the full range of FASD are needed, which could be improved with the development of biomarkers that aid in detection and accurate quantification of prenatal alcohol consumption. Continued development of effective prevention and treatment strategies also is critical. Through support of epidemiological, clinical, and preclinical research; facilitation of scientific exchange through conferences; and by leading public policy, NIAAA will continue to play an important role in understanding the complexities of FASD.
Footnotes
Financial Disclosure
The authors declare that they have no competing financial interests.
References
- Abel EL, Dintcheff BA. Effects of prenatal alcohol exposure on growth and development in rats. Journal of Pharmacology and Experimental Therapeutics. 1978;207(3):916–921. [PubMed] [Google Scholar]
- Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: Provocative and permissive influences. Neurotoxicology and Teratology. 1995;17(4):445–462. doi: 10.1016/0892-0362(95)98055-6. [DOI] [PubMed] [Google Scholar]
- Arevalo E, Shanmugasundararaj S, Wilkemeyer MF, et al. An alcohol binding site on the neural cell adhesion molecule L1. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(1):371–375. doi: 10.1073/pnas.0707815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer CF, Jacobson JL, Jacobson SW, et al. Validation of a new biomarker of fetal exposure to alcohol. Journal of Pediatrics. 2003;143(4):463–469. doi: 10.1067/S0022-3476(03)00442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): Validation of a screening instrument for use in medical settings. Journal of Studies on Alcohol. 1995;56(4):423–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- Chang G, Goetz MA, Wilkins-Haug L, Berman S. A brief intervention for prenatal alcohol use: An in-depth look. Journal of Substance Abuse Treatment. 2000;18(4):365–369. doi: 10.1016/s0740-5472(99)00105-1. [DOI] [PubMed] [Google Scholar]
- Chang G, McNamara TK, Orav EJ, et al. Brief intervention for prenatal alcohol use: A randomized trial. Obstetrics and Gynecology. 2005;105(5 Pt. 1):991–998. doi: 10.1097/01.AOG.0000157109.05453.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, McGourty RF, Bailey LK. Validation of the 4P’s Plus screen for substance use in pregnancy. Journal of Perinatology. 2007;27(12):744–748. doi: 10.1038/sj.jp.7211823. [DOI] [PubMed] [Google Scholar]
- Chen SY, Charness ME, Wilkemeyer MF, Sulik KK. Peptide-mediated protection from ethanol-induced neural tube defects. Developmental Neuroscience. 2005;27(1):13–19. doi: 10.1159/000084528. [DOI] [PubMed] [Google Scholar]
- Chen SY, Wilkemeyer MF, Sulik KK, Charness ME. Octanol antagonism of ethanol teratogenesis. FASEB Journal. 2001;15(9):1649–1651. doi: 10.1096/fj.00-0862fje. [DOI] [PubMed] [Google Scholar]
- Chernoff GF. The fetal alcohol syndrome in mice: An animal model. Teratology. 1977;15(3):223–229. doi: 10.1002/tera.1420150303. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Janisse J, Delaney-Black V, et al. A metric of maternal prenatal risk drinking predicts neurobehavioral outcomes in preschool children. Alcoholism: Clinical and Experimental Research. 2009;33(4):634–644. doi: 10.1111/j.1530-0277.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Sokol RJ, Delaney-Black V, et al. Validity of the T-ACE in pregnancy in predicting child outcome and risk drinking. Alcohol. 2010 doi: 10.1016/j.alcohol.2009.08.009. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarren SK, Alvord EC, Jr, Sumi SM, et al. Brain malformations related to prenatal exposure to ethanol. Journal of Pediatrics. 1978;92(1):64–67. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Randels SP, Sanderson M, Fineman RM. Screening for fetal alcohol syndrome in primary schools: A feasibility study. Teratology. 2001;63(1):3–10. doi: 10.1002/1096-9926(200101)63:1<3::AID-TERA1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcoholism: Clinical and Experimental Research. 2005;29(5):844–854. doi: 10.1097/01.alc.0000164374.32229.a2. [DOI] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: Comparability of effects in humans and animal models. Neurotoxicology and Teratology. 1990;12(3):231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Tajuddin NF, Gillespie RA, Le P. The effects of ethanol and the serotonin(1A) agonist ipsapirone on the expression of the serotonin(1A) receptor and several antiapoptotic proteins in fetal rhombencephalic neurons. Brain Research. 2006;1092(1):79–86. doi: 10.1016/j.brainres.2006.02.065. [DOI] [PubMed] [Google Scholar]
- Ellis FW, Pick JR. An animal model of the fetal alcohol syndrome in beagles. Alcoholism: Clinical and Experimental Research. 1980;4(2):123–134. doi: 10.1111/j.1530-0277.1980.tb05627.x. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: Mechanisms of damage and strategies for intervention. Experimental Biology and Medicine (Maywood) 2005;230(6):394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, O’Leary-Moore SK, Berman RF. Postnatal environmental or experiential amelioration of neurobehavioral effects of perinatal alcohol exposure in rats. Neuroscience and Biobehavioral Reviews. 2007;31(2):202–211. doi: 10.1016/j.neubiorev.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Chiodo LM, Sokol RJ, et al. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol. 2009. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Harrigan GG, Maguire G, Boros L. Metabolomics in alcohol research and drug development. Alcohol Research & Health. 2008;31(1):26–35. [PubMed] [Google Scholar]
- Haycock PC. Fetal alcohol spectrum disorders: The epigenetic perspective. Biology of Reproduction. 2009;81(4):607–617. doi: 10.1095/biolreprod.108.074690. [DOI] [PubMed] [Google Scholar]
- Hiller-Sturmhöfel S, Sobin J, Mayfield RD. Proteomic approaches for studying alcoholism and alcohol-induced organ damage. Alcohol Research & Health. 2008;31(1):36–48. [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302(7836):999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth AP. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Goodlett CR, Hannigan JH. Animal models of fetal alcohol spectrum disorders: Impact of the social environment. Developmental Disabilities Research Reviews. 2009;15(3):200–208. doi: 10.1002/ddrr.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Goodlett CR, Greenough WT. Therapeutic motor training ameliorates cerebellar effects of postnatal binge alcohol. Neurotoxicology and Teratology. 2000;22(1):125–132. doi: 10.1016/s0892-0362(99)00052-5. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: A review. Neuroscience and Biobehavioral Reviews. 2007;31(2):192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Kulaga V, Pragst F, Fulga N, Koren G. Hair analysis of fatty acid ethyl esters in the detection of excessive drinking in the context of fetal alcohol spectrum disorders. Therapeutic Drug Monitoring. 2009;31(2):261–266. doi: 10.1097/FTD.0b013e31819c33b8. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Bradley AM, Moss HB. Alcohol biomarkers in applied settings: Recent advances and future research opportunities. Alcoholism: Clinical and Experimental Research. 2010 Apr 5; doi: 10.1111/j.1530-0277.2010.01170.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, et al. Fetal alcohol syndrome: A case report of neuropsychological, MRI and EEG assessment of two children. Alcoholism: Clinical and Experimental Research. 1992;16(5):1001–1003. doi: 10.1111/j.1530-0277.1992.tb01909.x. [DOI] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Gossage JP, et al. Epidemiology of FASD in a province in Italy: Prevalence and characteristics of children in a random sample of schools. Alcoholism: Clinical and Experimental Research. 2006;30(9):1562–1575. doi: 10.1111/j.1530-0277.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews. 2009;15(3):176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, et al. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug and Alcohol Dependence. 2007;88(2–3):259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, et al. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: A third study. Alcoholism: Clinical and Experimental Research. 2008a;32(5):738–753. doi: 10.1111/j.1530-0277.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- May PA, Miller JH, Goodhart KA, et al. Enhanced case management to prevent fetal alcohol spectrum disorders in Northern Plains communities. Maternal and Child Health Journal. 12(6):747–759. doi: 10.1007/s10995-007-0304-2. 2008b. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Ramoa AS. Restoration of neuronal plasticity by a phosphodiesterase type 1 inhibitor in a model of fetal alcohol exposure. Journal of Neuroscience. 2006;26(3):1057–1060. doi: 10.1523/JNEUROSCI.4177-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Developmental Disabilities Research Reviews. 2009;15(3):209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell SE, O’Leary-Moore SK, Godin EA, et al. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: Effects of acute insult on gestational day 8. Alcoholism: Clinical and Experimental Research. 2009;33(6):1001–1011. doi: 10.1111/j.1530-0277.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Yang PH, Ng SS, et al. A critical role of Pax6 in alcohol-induced fetal microcephaly. Neurobiology of Disease. 2004;16(2):370–376. doi: 10.1016/j.nbd.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Ramsay M. Genetic and epigenetic insights into fetal alcohol spectrum disorders. Genome Medicine. 2010;2(4):27. doi: 10.1186/gm148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Taylor J, Walker DW. Ethanol-induced malformations in mice. Alcoholism: Clinical and Experimental Research. 1977;1(3):219–224. doi: 10.1111/j.1530-0277.1977.tb05876.x. [DOI] [PubMed] [Google Scholar]
- Randall CL, Taylor WJ. Prenatal ethanol exposure in mice: Teratogenic effects. Teratology. 1979;19(3):305–311. doi: 10.1002/tera.1420190305. [DOI] [PubMed] [Google Scholar]
- Riley EP, Lochry EA, Shapiro NR, Baldwin J. Response perseveration in rats exposed to alcohol prenatally. Pharmacology, Biochemistry, and Behavior. 1979;10(2):255–259. doi: 10.1016/0091-3057(79)90097-2. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: An overview with emphasis on changes in brain and behavior. Experimental Biology and Medicine. 2005;230(6):357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Russell M, Martier SS, Sokol RJ, et al. Screening for pregnancy risk-drinking. Alcoholism: Clinical and Experimental Research. 1994;18(5):1156–1161. doi: 10.1111/j.1530-0277.1994.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Russell M, Martier SS, Sokol RJ, et al. Detecting risk drinking during pregnancy: A comparison of four screening questionnaires. American Journal of Public Health. 1996;86(10):1435–1439. doi: 10.2105/ajph.86.10.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, et al. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56(5):317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Martier SS, Ager JW. The T-ACE questions: Practical prenatal detection of risk-drinking. American Journal of Obstetrics and Gynecology. 1989;160(4):863–868. doi: 10.1016/0002-9378(89)90302-5. discussion 868–870. [DOI] [PubMed] [Google Scholar]
- Stratton KR, Howe CJ, Battaglia FC. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL. Final Report to the Centers for Disease Control and Prevention (CDC) Seattle, Washington: University of Washington, Fetal Alcohol & Drug Unit; 1996. Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE) [Google Scholar]
- Streissguth AP, O’Malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Seminars in Clinical Neuropsychiatry. 2000;5(3):177–190. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: Embryogenesis in a mouse model. Science. 1981;214(4523):936–938. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O’Bryan KA, et al. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behavioral Neuroscience. 2007;121(1):120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Fleming SL, Riley EP. MK-801 can exacerbate or attenuate behavioral alterations associated with neonatal alcohol exposure in the rat, depending on the timing of administration. Alcoholism: Clinical and Experimental Research. 2001;25(5):764–773. [PubMed] [Google Scholar]
- Thomas JD, Sather TM, Whinery LA. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behavioral Neuroscience. 2008;122(6):1264–1273. doi: 10.1037/a0013271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of the Treasury and U.S. Department of Health and Human Services . Report to the President and Congress on Health Hazards Associated With Alcohol and Method to Inform the General Public of these Hazards. Washington, DC: U.S. Government Printing Office; 1980. [Google Scholar]
- Viljoen DL, Gossage JP, Brooke L, et al. Fetal alcohol syndrome epidemiology in a South African community: A second study of a very high prevalence area. Journal of Studies on Alcohol. 2005;66(5):593–604. doi: 10.15288/jsa.2005.66.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR. Fetal alcohol-induced brain damage and the problem of determining temporal vulnerability: A review. Alcohol and Drug Research. 1987;7(5–6):423–441. [PubMed] [Google Scholar]
- Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: Effects on neuroendocrine and immune function. Experimental Biology and Medicine. 2005;230(6):376–388. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]