Abstract
Background
Systemic sclerosis (SSc) is associated with a marked economic burden, high treatment costs and decreased productivity. Although treatment strategies for SSc can have a substantial effect on patients’ outcomes, it is not known whether patients with SSc consistently receive such care. Evaluation of process-of-care quality requires specification of quality indicators (QIs), clinically detailed statements of the eligible patients and the care they should receive to achieve a minimal level of quality of care. Our objective was to develop QIs for patients with SSc.
Methods
We performed a comprehensive literature review of diagnosis and treatment of SSc and proposed QIs that were evaluated by a national Expert Panel (n=9) who were asked to review the supporting literature and individually rank the validity of each QI. These rankings formed the basis of discussion at a face-to-face meeting following the RAND/UCLA method to integrate expert opinion with literature review to identify a set of final QIs. We then presented these QIs to members of the Scleroderma Clinical Trials Consortium (SCTC).
Results
Thirty-two QIs for SSc care were judged valid by the Expert Panel. The QI set includes 9 QIs for newly diagnosed with SSc, 12 follow-up QIs for management of SSc, and 11 treatment QIs. The SCTC experts agreed with the validity of each of the 32 QI and agreed that for all but one QI the specified tests, procedures and treatments recommended in the QI were generally available.
Conclusion
We have developed 32 QIs for SSc using a rigorous methodology that can be employed to evaluate and improve care for patients with SSc, as well as inform policy decisions supporting appropriate care for SSc patients.
Keywords: Systemic sclerosis, quality indicator
Introdution
Systemic sclerosis (scleroderma, SSc) is a rheumatic disease with substantial morbidity and mortality (1) and many detrimental effects on health-related quality of life. In addition, SSc is associated with a marked economic burden, with high treatment costs and decreased productivity (2). Although early treatment for SSc can have a substantial effect on patients outcomes (3-5), no studies of the quality of care provided to patients with SSc have been performed. One well-established method of evaluating the care provided for a specific condition is to develop and apply indicators of care quality. MacLean and colleagues (6) developed an Arthritis Foundation set of quality indicators (QI) to assess quality of health care in arthritis, especially for rheumatoid arthritis, osteoarthritis, and analgesic use, and QIs exist concerning gout (7), safety in rheumatologic prescribing (8), and systemic lupus erythematosus (9). However, no QIs exist for the treatment of SSc.
The quality of health care can be assessed in many ways, and is most commonly evaluated by measuring health outcomes or processes of care (6, 10). Process of care describes what health care providers do for patients and includes taking a health history, performing a physical examination, ordering diagnostic tests, prescribing medications, and performing procedures. We chose to develop measures of process because processes of care tend to be under the control of the health care provider or health system and are more efficiently measured than outcomes. Furthermore, performance on process measures can identify specific areas of care that are deficient and hence can be targeted for quality improvement. We chose not to develop outcome measures because clinically important outcomes in SSc may take years to develop, and may be affected by factors outside the control of the health care provider or health care system.
A process-of-care QI is a specific statement that describes care necessary to achieve a minimal level of quality of care. A QI must be measurable; clinically detailed QIs are often measured using information contained in the medical record. QIs are applicable to any physician providing care and not just limited to subspecialists providing the care. Like any other measurement, QIs will have acceptable ranges of misclassification of care (i.e. false positives and negatives with regard to true quality). They are thus most beneficially applied where misclassifications, if random with respect to variables of interest, will cancel each other out. In contrast, clinical guidelines are meant to guide individual clinicians in the care of individual patients. As such they describe a flexible range of diagnostic and therapeutic processes that might be considered for different groups of patients and often advocate best practices. Given their flexibility, guidelines may advocate higher performance than that required by a corresponding a QI. The indicators are not intended to replace existing guidelines, but rather to provide a means of assessing a minimum standard of care.
Methodology
Preparation of the preliminary set of Quality Indicators
A comprehensive search was performed to identify published recommendations and guidelines in SSc and SSc-specific organ involvements (process detailed in Fig. 1). We excluded procedures for the diagnosis and management of other rheumatic diseases, even if these overlapped with SSc, localised scleroderma, or juvenile SSc. The QIs were constructed using an “IF, THEN, BECAUSE” format where “IF” defined the eligible patient for whom the care should be provided, “THEN” described the process of care that should occur, and “BECAUSE” described the relationship between the process and a clinical outcome.
Fig. 1.
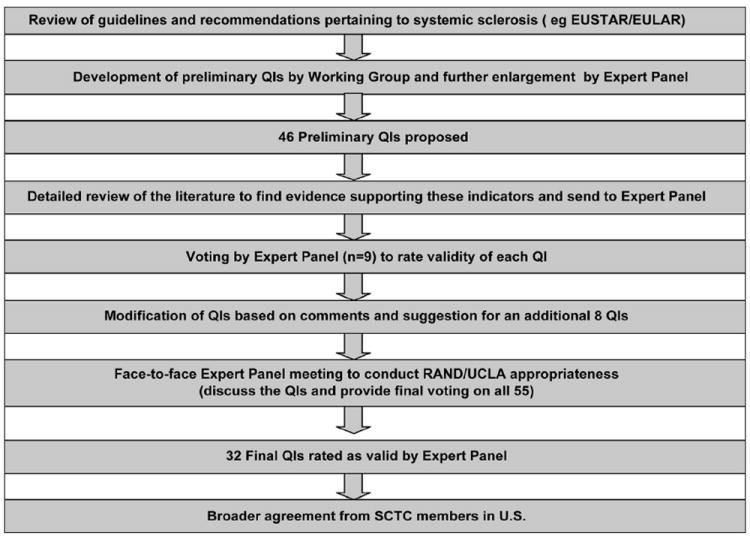
Methodology used to develop the SSc QI set.
Based on the literature search results and clinical experience, 69 preliminary QIs were developed. These QIs were sent to 9 international experts (2 of them were part of the Expert Panel) to provide their comments/ suggestions and to eliminate/edit/add new quality QIs. Based on their comments, 23 QIs were eliminated, leaving 46 preliminary QIs.
Comprehensive literature review
A comprehensive literature review for each of the areas covered by the QIs was performed by three members of the Steering Committee (OKB, PPK, AL). For each procedure (e.g. echocardiography, pulmonary function tests) or treatment, a structured literature search was performed in the PubMed database (1966 to June 2009) using predefined key words which included combination of “systemic sclerosis” OR “scleroderma” OR “CREST” and terms specific for a particular procedure (e.g. forced vital capacity). This search was combined with the recent systematic review of PubMed, EMBASE and Cochrane databases used for developing recommendations for treatment of SSc (11) [available through OKB]. In this systematic review, the majority of articles in the Cochrane database were also captured by PubMed and no relevant non-English article was found. Therefore, our search was limited to PubMed and articles written in English which included adult humans only. The domains/systems and procedures/tools for which a literature search was performed are listed in Table I. To identify other potentially relevant articles, reference lists of recent reviews were examined. In addition, web pages of medical societies and international and national organisations (EULAR, EUSTAR, American College of Rheumatology, American Heart Association, American College of Cardiology, World Gastroenterology Organisation, American Gastroenterological Association, and British Thoracic Society) were screened for particular recommendations and/or guidelines that might apply. Initial selection was done based on screening of the titles and/or abstracts of the identified publications. Then, full-text articles were retrieved for detailed review. The literature search team members (PK, OKB or AL) held weekly teleconferences to reach consensus in 8 domains grouped primarily according to body systems (Table I). Each section consisted of baseline, follow-up, and treatment supportive literature review and references. QIs for osteoporosis and drug safety were excluded as they have recently been developed by the American College of Rheumatology, (ACR) (http://www.rheumatology.org/practice/qmc/drug-safety.asp).
Table I.
| Domain/Organ System | Tools/procedures | |
|---|---|---|
| I | Cardiopulmonary |
|
| II | Pulmonary |
|
| III | Gastrointestinal |
|
| IV | Renal |
|
| V | Musculoskeletal |
|
| VI | Cutaneous |
|
| VII | Health-Related Quality of Life |
|
| VIII | Serologies |
|
| IX | Prevention and Drug Monitoring | |
RAND/ UCLA Appropriateness Panel meeting
We used the RAND/UCLA appropriateness method to quantify expert opinion regarding the proposed indicators. This well-established method allows panelists to extend the scope of the indicators where the supporting evidence is not completely clear. It structures consideration of the literature and efficiently brings all experts’ points of view to attention without forcing consensus. In other applications, it has been shown to predict future randomised controlled trials (12).
The 46 draft QIs with the supporting detailed literature review (available on request from 1st author) were sent to a national Expert Panel (5 rheumatologists, 1 cardiologist, 1 gastroenterologist, 1 general internist, and 1 pulmonologist). Each panelist spends 20–100% of their time in patient care that ranges from outpatient clinics to inpatient consults. All physicians have an interest in management of SSc but also manage other patients in their subspecialties.
Before the panel meeting, each panel member was asked to review the supporting literature and individually rank the validity each QI on a 1–9 scale (1 = completely invalid to 9 = completely valid). A QI was considered valid if 1) there was adequate scientific evidence or professional consensus to support a link between the performance of care specified by the QI and subsequent accrual of health benefit to the patient, and 2) physician or health plan performance of the care processes contained in the QI indicated higher quality care and 3) the care process in the QI was under the control of the physician or health plan. In considering the link between process and outcome, panelists were instructed to use both their clinical experience and expert guidelines as well as more rigorous published scientific evidence like randomised controlled trials and/or observational data. The 9 member panel was invited to suggest changes to the QIs and provide general comments; the panel suggested 9 additional QIs.
The expert panelists were explicitly asked to consider the process to outcome link in rating the indicators. Their judgment about the link was informed by both their clinical experience and expert guidelines as well as more rigorous published scientific evidence like randomised controlled trials, or if these were unavailable/ observational data.
A one-day face-to-face Expert Panel meeting was held in Los Angeles, CA, led by an experienced moderator (SA) to discuss the proposed 55 QIs. Each QI was considered by the group after a brief presentation of the supporting evidence by one of the literature search team members. After reviewing the first set of ratings and having a detailed discussion, the Expert Panel again rated the validity of each QI. QIs with a median rating of ≥7 and no statistical disagreement were accepted as final QIs for SSc. Disagreement was defined as one-third or more panelists rating the QI in the lowest tertile (1-3) and one-third or more rating the same QI in the highest tertile (7-9) (13).
Assessing agreement among other Scleroderma Experts
In order to assess whether there was agreement concerning validity and feasibility of the QIs recommended by the Expert Panel, we surveyed physicians who were US members of Scleroderma Clinical Trials Consortium (SCTC). We only included US members as QIs were developed for US healthcare although is applicable for any country. The members of SCTC include private practitioners and academic clinicians who have a special interest in SSc. However, majority of members also see patients with other rheumatic diseases. The survey asked about validity using the same 1 to 9 scale completed by the Expert Panel and also asked about the availability/feasibility of obtaining the tests/ procedures contained in the care processes in their geographic region. This group did not get the literature review. SCTC raters responded on a 1-9 Likert scale, where “1” was “totally unavailable” and “9” was “routinely available.” The survey was conducted using the internet and was sent to 35 members (all rheumatologists); 20 (57%) returned the survey.
Funding
The QIs were developed from a K23 grant to D. Khanna and unrestricted funds to D. Khanna and D.E. Furst from Actelion Pharmaceuticals Inc and Gilead Pharmaceuticals. Funding agencies did not in any way contribute to the research or influence the content or submission of the manuscript.
Results
Thirty-two of the 55 QIs were judged to be valid by the Expert Panel. Table II presents the final QIs. The QI set includes 9 baseline QIs that apply to patients with newly diagnosed SSc. There are 12 follow-up QIs for treatment of the patient with prevalent SSc and 11 treatment QIs. The QIs are further divided into general measures and by organ system. As an example, for initial assessment of cardio-pulmonary status, a resting echocardiogram with Doppler should be offered within 12 months of diagnosis since echocardiogram with Doppler screens for pulmonary arterial hypertension, diastolic dysfunction, pericardial effusion, and cardiomyopathy (QI no.2). On other hand, for a follow-up visit, echocardiogram with Doppler should be offered within 3 months of a new complaint of dyspnea on exertion and/or a new finding of a DLCO of <65% of predicted (QI no.11).
Table II.
Quality indicators for systemic sclerosis.
|
Offered: offered or performed or reason for non-performance documented
Note: The care process in a quality indicator is considered to have been passed if the care is documented in the medical record or if the care is recommended, even if it is refused by the patient.
ACE: Angiotensin converting enzyme; CPK: creatine phosphokinase; DLCO: diffusion capacity for carbon monoxide; FVC: forced vital capacity; NYHA/WHO: New York Heart Association / World Health Organisation; PDE-5: Phosphodiesterase-5
SCTC experts agreed with the validity of each of the 32 QI with median rating ≥7. They also rated the tests, procedures and treatments with median rating ≥7 with the exception of one QI (QI no.32). This QI assesses availability of treatment (e.g. calcium channel blockers, prostacyclin therapy, topical nitrate therapy, PDE-5 inhibitor) within 3 months of the occurrence of digital tip ulcers.
Discussion
We have developed a new set of QIs for SSc using rigorous and well-established methodology (6). These QIs address important issues in the diagnosis and management of SSc, a multi-system disease.
QIs can be used for public accountability, quality improvement, accreditation, and research (6). For example, the National Committee for Quality Assessment has adopted one of the Arthritis Foundation’s QIs for rheumatoid arthritis – requiring as a minimal standard of care that rheumatoid arthritis patients followed in the outpatient setting be dispensed at least one prescription for a disease modifying anti-rheumatic drug. Physicians and health plans are rated based on the performance of these measures (14). Unlike guidelines or recommendations, QIs are minimum standards of care – they are also measurable actions. As an example, QI no.16 assesses the adherence of spirometry with DLCO in an SSc patient with new onset dyspnea. In real practice, one would not wait for 6 months to investigate new onset dyspnea, but if spirometry was not offered within 6 months this would be considered poor care. Similarly, for new onset SSc, one would not wait for 12 months to initiate work for internal organ involvement but if these are not offered within 12 months, this would be considered suboptimum care. Commonly, clinically-detailed QIs are abstracted on chart review by independent auditors and are presented as the proportion of eligible patients who received the recommended QI at the level of the physician or health plan. This translates into documentation of pertinent history (such as ability to perform ADLs), discussion of adverse events due to medications, or refusal of a recommended procedure or treatment by the patient. For example, in a single-centre study to assess adherence of QIs for RA and drug safety endorsed by the American College of Rheumatology (ACR), 99% of patients with RA were receiving disease-modifying agents (DMARDs), but discussion of potential risks for a new DMARD or glucocorticoids was documented in only 35% of patient records (15). This finding is explained by the requirement to document an action. Although rheumatologists and their staff members routinely counsel patients concerning the risks associated with these therapies and many patients are provided with relevant pamphlets from the Arthritis Foundation, adherence to QIs is based strictly on medical record documentation.
Quality measurement with explicit QIs can be used as a trigger for quality improvement. If performance for a QI is low, this can stimulate a search for the source of the deficit in care, including provider factors, resource constraints or other barriers to access. For example, one of the QIs recommends that a patient with newly diagnosed SSc have a baseline echocardiogram with Doppler performed. Patients with SSc have a high prevalence of pulmonary hypertension (3, 16). The American College of Chest Physicians (ACCP) guidelines recommend baseline echocardiogram with Doppler in high-risk populations (17). However, in a prospective study of 669 patients with SSc and “mixed connective tissue disease” in community rheumatology practices, only 27% had ever had an evaluation for PH with Doppler echocardiogram. Since ordering an echocardiogram with Doppler is a measurable action, it is hoped that the adherence to this QI in newly diagnosed SSc will lead to earlier diagnosis and treatment of cardiopulmonary involvement in SSc and thereby improve health outcomes in these patients. Poor scores on QIs should lead to clinical reminders or the use of other informatic tools (available at Veterans Affairs hospitals), the development of clinical registries and provider education, payment guidelines and other initiatives.
During the development of QIs, it was decided not to divide patients into limited and diffuse subtypes since this condition involves skin examination. Although skin examination (and skin score) is routinely done in scleroderma centers, it is rarely performed in private rheumatology practices. In addition, skin tends to soften over time and patients may have normal texture of skin later in their disease course. Furthermore, since QIs are generalisable to any physician, it was considered that distinguishing between limited vs. diffuse SSc may not be feasible in clinical practice. Therefore, QI no.9 states that patients with early systemic sclerosis (<5 years from first signs or symptoms), should be counseled to perform at least weekly blood pressure measurements. We also want to emphasise that QIs are meant to be achieved by a single physician. In other words, if any physician (not necessarily the one seeing the patient at the time) involved in the care of a patient performs Doppler echocardiogram at baseline visit (no.2) or serum haemoglobin annually (no.11), then the QIs are met for the particular patient.
The EULAR/EUSTAR have recently made 14 recommendations for the treatment of SSc involving 6 organ systems (18). Our panel proposed 6 QIs that are similar to these recommendations. These belong to the gastrointestinal tract (n=3), digital ulcers, cardio-pulmonary, pulmonary, and renal systems (n=1 each). The difference is that our QIs are measurable. As an example, EULAR/EUSTAR stated that “In view of the results from two high-quality RCTs and despite its known toxicity, cyclophosphamide should be considered for treatment of SSc-ILD”, whereas our QI (no.30) provides measurable parameters: “IF a patient has systemic sclerosis-associated interstitial lung disease and documented a >10% decline in FVC during the past 12 months, THEN immunosuppressive treatment (e.g. cyclophosphamide, methotrexate, azathioprine, cyclosporine, mycophenolate mofetil) should be offered within 3 months.”
Certain recommendations from the panelists (such as QI no.1 to test for SSc-specific autoantibodies) concerned us because of questions regarding validity and availability of these tests/ procedures. Therefore, we took the unusual step of externally validating the QI set by presenting them to a separate set of SSc clinical experts using a Delphi exercise performed by US members of the Scleroderma Clinical Trial Consortium (SCTC). The members of the SCTC include academic and practicing rheumatologists with an interest in SSc. Among the rheumatologists who responded, the median ratings were very similar to the ratings of the Expert Panel, thus providing strong external validity for the QIs. In addition, we asked the SCTC investigators about the availability of tests/ procedures in their local area or whether their health plan would cover the costs of the proposed QIs. We felt that this inquiry was important in determining if practicing physicians would have access to the proposed tests and procedures. The median ratings availability was ≥7 (agreement that the tests were routinely available) for all QIs except 1 (QI no.32). This QI, assessing the availability of therapies for digital ulcers, received a median score of 6. This result is probably because expensive therapies (prostacyclin analogues, PDE-5 inhibitors) proposed in this QI are not yet approved for the treatment of digital ulcers and thus are not widely available.
In summary, we developed QIs for SSc using rigorous well-established methodologies. The users of the indicator set are free to include or exclude those indicators at their discretion. It is our hope that these QIs will be employed to improve care and in turn improve health outcomes in patients with SSc, as well as inform policy decisions supporting appropriate care for SSc patients.
Key messages.
We have developed quality indicators for systemic sclerosis that can be employed to evaluate and improve care for patients with SSc
Quality indicators can also be used to inform policy decisions supporting appropriate care for SSc patients
Acknowledgments
We want to thank Drs. Firas Alkassab, Soumya Chatterjee, Lorinda Chung, David Collier, Mittie Doyle, Tracy Frech, Aryeh Fischer, Samina Hayat, Laura Hummers, Monique Hinchcliff, Vivien Hsu, Ted Lally, Peter Merkel, Kristine Phillips, Naomi Rothfield, James Seibold, Lee Shapiro, Richard Silver, and Fred Wigley for participating in the SCTC Delphi exercise.
D. Khanna was supported by a National Institutes of Health Award (NIAMS K23 AR053858-04). P. Khanna was supported by Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant NIAMS 1 T32 AR053463 and American College of Rheumatology Research and Education Foundation Clinical Investigator Fellowship Award 2009-2011.
Footnotes
Disclosure of interest
Dr D. Khanna has received consultancy fees from Actelion, Gilead, United Therapeutics, MedImmune and Pfizer, and grant support from Actelion, Gilead and NIH;
Dr M.D. Mayes has received consulting fees, speaking fees, and/or research grants from Actelion, Gilead, United Therapeutics and Novartis;
Dr D. Furst has received grant support and consultancy fees from Actelion and Gilead; the other co-authors have declared no competing interests.
References
- 1.ALTMAN RD, MEDSGER TA, Jr, BLOCH DA, MICHEL BA. Predictors of survival in systemic sclerosis (scleroderma) Arthritis Rheum. 1991;34:403–13. doi: 10.1002/art.1780340405. [DOI] [PubMed] [Google Scholar]
- 2.BERNATSKY S, HUDSON M, PANOPALIS P, et al. The cost of systemic sclerosis. Arthritis Rheum. 2009;61:119–23. doi: 10.1002/art.24086. [DOI] [PubMed] [Google Scholar]
- 3.WIGLEY FM, LIMA JA, MAYES M, McLAIN D, CHAPIN JL, WARD-ABLE C. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study) Arthritis Rheum. 2005;52:2125–32. doi: 10.1002/art.21131. [DOI] [PubMed] [Google Scholar]
- 4.KHANNA D, YAN X, TASHKIN DP, et al. Impact of oral cyclophosphamide on healthrelated quality of life in patients with active scleroderma lung disease: Results from the scleroderma lung study. Arthritis Rheum. 2007;56:1676–84. doi: 10.1002/art.22580. [DOI] [PubMed] [Google Scholar]
- 5.KHANNA D, HAYS RD, MARANIAN P, et al. Reliability and validity of the University of California, Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument. Arthritis Rheum. 2009;61:1257–63. doi: 10.1002/art.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLEAN CH, SAAG KG, SOLOMON DH, MORTON SC, SAMPSEL S, KLIPPEL JH. Measuring quality in arthritis care: methods for developing the Arthritis Foundation’s quality indicator set. Arthritis Rheum. 2004;51:193–202. doi: 10.1002/art.20248. [DOI] [PubMed] [Google Scholar]
- 7.MIKULS TR, MacLEAN CH, OLIVIERI J, et al. Quality of care indicators for gout management. Arthritis Rheum. 2004;50:937–43. doi: 10.1002/art.20102. [DOI] [PubMed] [Google Scholar]
- 8.SAAG KG, OLIVIERI JJ, PATINO F, MIKULS TR, ALLISON JJ, MacLEAN CH. Measuring quality in arthritis care: the Arthritis Foundation’s quality indicator set for analgesics. Arthritis Rheum. 2004;51:337–49. doi: 10.1002/art.20422. [DOI] [PubMed] [Google Scholar]
- 9.YAZDANY J, PANOPALIS P, GILLIS JZ, et al. A quality indicator set for systemic lupus erythematosus. Arthritis Rheum. 2009;61:370–7. doi: 10.1002/art.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KHANNA D, ARNOLD EL, PENCHARZ JN, et al. Measuring process of arthritis care: the Arthritis Foundation’s quality indicator set for rheumatoid arthritis. Semin Arthritis Rheum. 2006;35:211–37. doi: 10.1016/j.semarthrit.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 11.AVOUAC J, KOWAL-BIELECKA O, LANDEWÉ R, et al. European League Against Rheumatism (EULAR) Scleroderma Trial and Research group (EUSTAR) recommendations for the treatment of systemic sclerosis: methods of elaboration and results of systematic literature research. Ann Rheum Dis. 2009;68:629–34. doi: 10.1136/ard.2008.095299. [DOI] [PubMed] [Google Scholar]
- 12.BROOK RH, McGLYNN EA, SHEKELLE PG. Defining and measuring quality of care: a perspective from US researchers. Int J Qual Health Care. 2000;12:281–95. doi: 10.1093/intqhc/12.4.281. [DOI] [PubMed] [Google Scholar]
- 13.BROOK R. Methodology Perspectives. Rockville, Md: Public Health Service; 1994. The RAND/UCLA Appropriateness Method; pp. 59–70. AHCPR no. 95-0009. [Google Scholar]
- 14.SOLOMON DH, GABRIEL SE. Moving forward with quality: pay for reporting meets rheumatology. Arthritis Rheum. 2007;57:703–4. doi: 10.1002/art.22769. [DOI] [PubMed] [Google Scholar]
- 15.KHANNA D, BROWN A. Quality of Care for Veterans Diagnosed with Rheumatoid Arthritis. Arthritis Rheum. 2007;56:S112. abstract. [Google Scholar]
- 16.HACHULLA E, GRESSIN V, GUILLEVIN L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum. 2005;52:3792–800. doi: 10.1002/art.21433. [DOI] [PubMed] [Google Scholar]
- 17.McGOON M, GUTTERMAN D, STEEN V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 18.KOWAL-BIELECKA O, LANDEWÉ R, AVOUAC J, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR) Ann Rheum Dis. 2009;68:620–8. doi: 10.1136/ard.2008.096677. [DOI] [PubMed] [Google Scholar]


