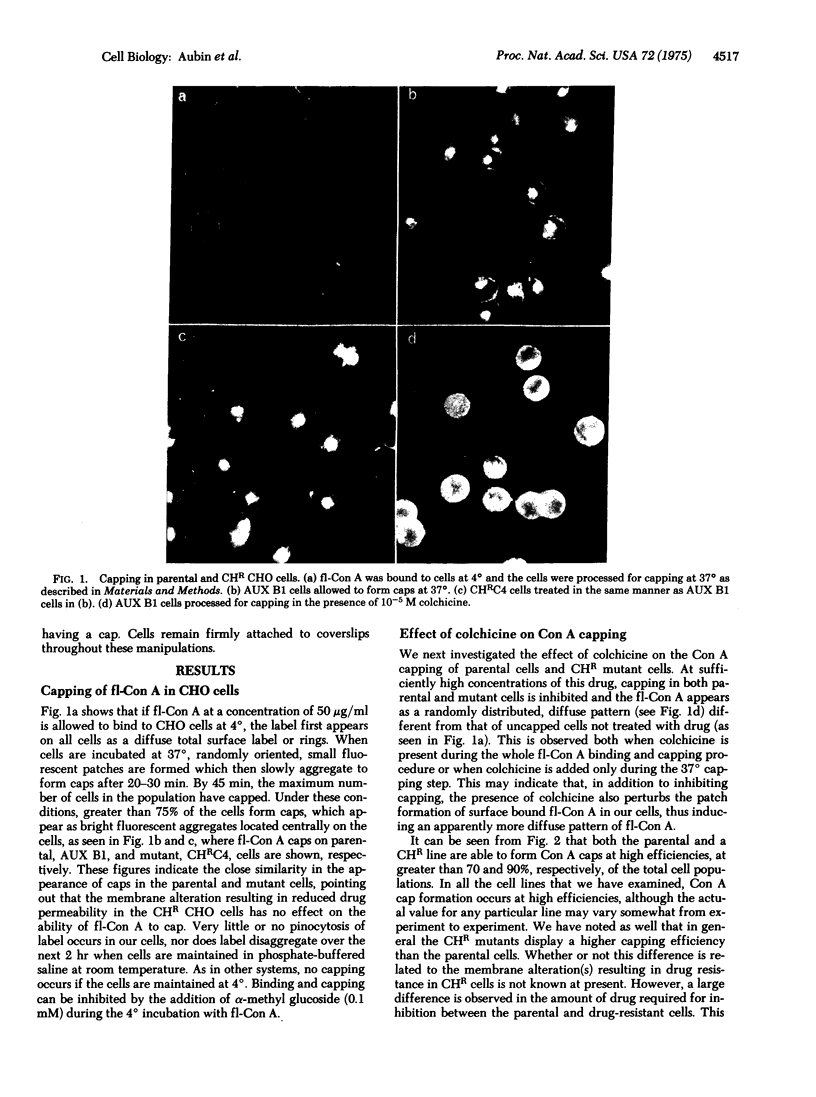
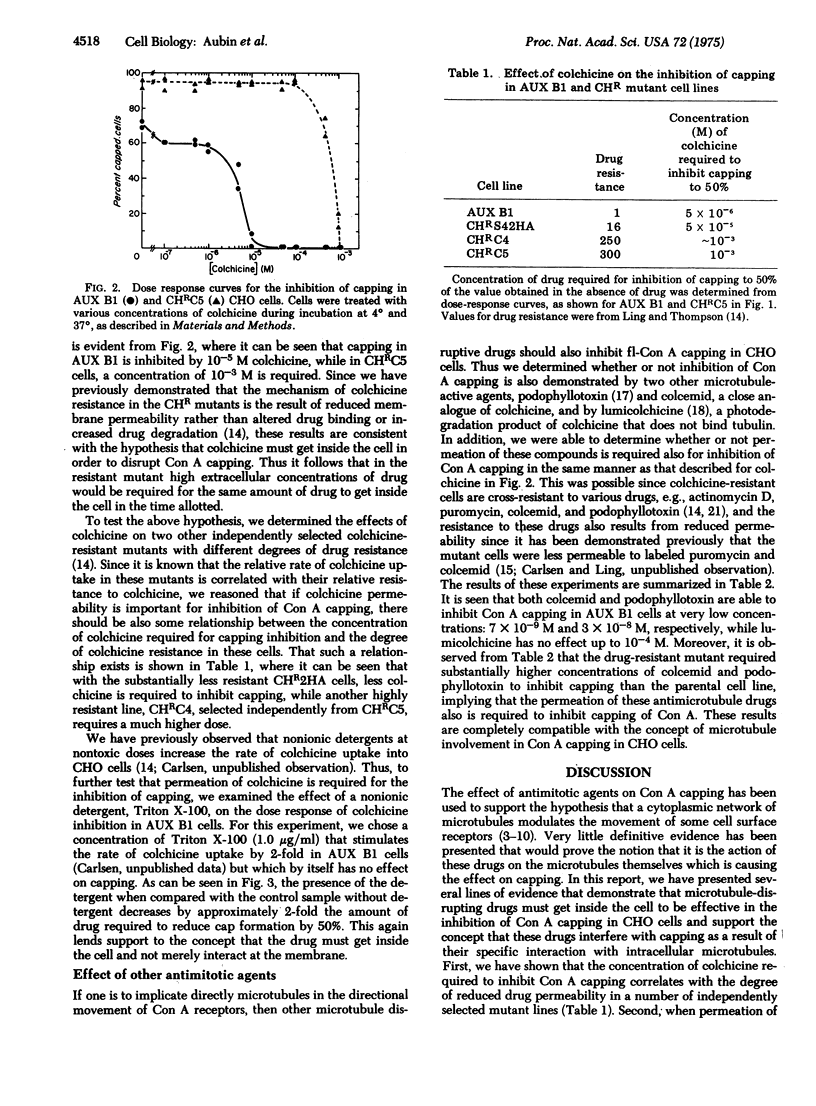
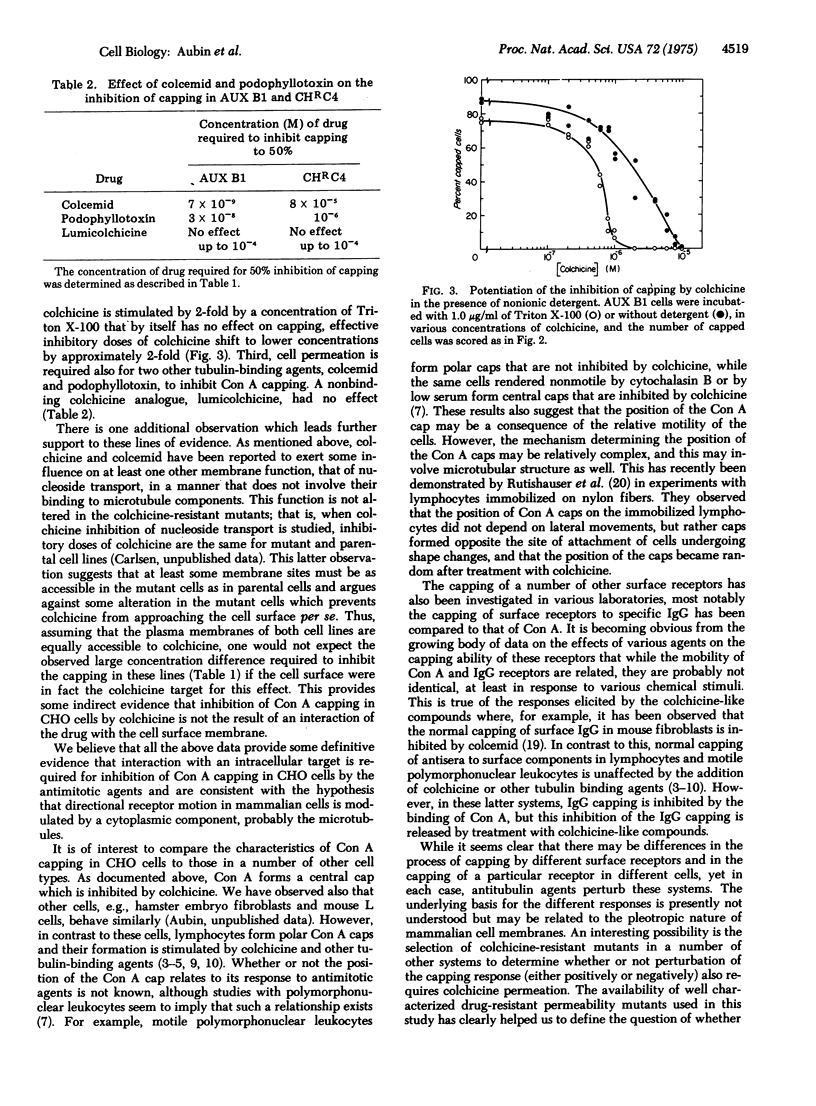
Abstract
Several antimitotic, tubulin-binding agents, such as colchicine, colcemid, and podophyllotoxin, inhibit the capping of fluorescent-labeled concanavalin A in Chinese hamster ovary cells. By comparing the effects of these agents on parental cell lines on several independently selected colchicine-resistant mutants with decreased drug permeability, we have demonstrated that permeation of these drugs in required for inhibition of capping. These data support the hypothesis that these antimitotic agents interact with an intracellular component, probably microtubules, to prevent the directional movement of concanavalin A receptors on the surface membranes of Chinese hamster ovary cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Petris S. Inhibition and reversal of capping by cytochalasin B, vinblastine and colchicine. Nature. 1974 Jul 5;250(461):54–56. doi: 10.1038/250054a0. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Weiss A. Antigen cap formation in cultured fibroblasts: a reflection of membrane fluidity and of cell motility. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2456–2459. doi: 10.1073/pnas.69.9.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Yahara I., Edelman G. M. Morphology, motility, and surface behavior of lymphocytes bound to nylon fibers. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1149–1153. doi: 10.1073/pnas.71.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Borysenko J. Z., Karnovsky M. J. Factors affecting the redistribution of surface-bound concanavalin A on human polymorphonuclear leukocytes. J Cell Biol. 1974 Aug;62(2):351–365. doi: 10.1083/jcb.62.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Unanue E. R., Karnovsky M. J. Inhibition of surface capping of macromolecules by local anaesthetics and tranquillisers. Nature. 1974 Jul 5;250(461):56–57. doi: 10.1038/250056a0. [DOI] [PubMed] [Google Scholar]
- See Y. P., Carlsen S. A., Till J. E., Ling V. Increased drug permeability in Chinese hamster ovary cells in the presence of cyanide. Biochim Biophys Acta. 1974 Dec 10;373(2):242–252. doi: 10.1016/0005-2736(74)90148-5. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Ukena T. E., Borysenko J. Z., Karnovsky M. J., Berlin R. D. Effects of colchicine, cytochalasin B, and 2-deoxyglucose on the topographical organization of surface-bound concanavalin A in normal and transformed fibroblasts. J Cell Biol. 1974 Apr;61(1):70–82. doi: 10.1083/jcb.61.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Karnovsky M. J. Ligand-induced movement of lymphocyte membrane macromolecules. V. Capping, cell movement, and microtubular function in normal and lectin-treated lymphocytes. J Exp Med. 1974 Nov 1;140(5):1207–1220. doi: 10.1084/jem.140.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. I. Synthesis and properties of colchicine labeled with tritium in its acetyl moiety. Biochemistry. 1966 Jul;5(7):2463–2468. doi: 10.1021/bi00871a042. [DOI] [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. II. The in vivo and in vitro binding of colchicine in grasshopper embryos and its possible relation to inhibition of mitosis. Biochemistry. 1967 Oct;6(10):3126–3135. doi: 10.1021/bi00862a021. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Electron microscopic analysis of the modulation of lymphocyte receptor mobility. Exp Cell Res. 1975 Mar 1;91(1):125–142. doi: 10.1016/0014-4827(75)90150-0. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Modulation of lymphocyte receptor mobility by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1579–1583. doi: 10.1073/pnas.72.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Petris S. Concanavalin A receptors, immunoglobulins, and theta antigen of the lymphocyte surface. Interactions with concanavalin A and with Cytoplasmic structures. J Cell Biol. 1975 Apr;65(1):123–146. doi: 10.1083/jcb.65.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]