Summary
BAFF is a soluble factor required for B cell maturation and survival. BAFF-R signals via the non-canonical NF-κB pathway regulated by the TRAF3/NIK/IKK1 axis. We show that deletion of Ikk1 during early B cell development causes a partial impairment in B cell maturation and BAFF-dependent survival, but inactivation of Ikk1 in mature B cells does not affect survival. We further show that BAFF-R employs CD19 to promote survival via PI3K, and that co-inactivation of Cd19 and Ikk1 causes a profound block in B cell maturation at the transitional stage. Consistent with a role for PI3K in BAFF-R function, inactivation of PTEN mediates a partial rescue of B cell maturation and function in Baff−/− animals. Elevated PI3K signaling also circumvents BAFF-dependent survival in a spontaneous B cell lymphoma model. These findings indicate that the combined activities of PI3K and IKK1 drive peripheral B cell differentiation and survival in a context-dependent manner.
Introduction
BAFF is the most critical soluble factor for peripheral B cell maturation and survival, and dysregulated BAFF expression is associated with lupus-like autoimmunity and B cell non-Hodgkin (B-NHL)-like lymphoma (Mackay et al., 2010; Rickert et al., 2011). BAFF-R expression is induced on newly-formed B cells poised to egress from the bone marrow and enter the spleen, and is further up-regulated as transitional B cells mature to become follicular or marginal zone (MZ) B cells (Hsu et al., 2002; Meyer-Bahlburg et al., 2008; Stadanlick et al., 2008). Consistent with the pattern of BAFF-R expression, BAFF or BAFF-R deficiency imposes a block at the transitional T1 – T2 maturation step due to failed survival, while follicular and MZ B cells are reduced >90% and do not recover with age (Miller and Hayes, 1991; Schiemann et al., 2001; Thompson et al., 2001). Provision of a survival signal in the form of forced Bcl-2 expression rescues the transitional B cell block, leading to the generation of follicular B cells; however, MZ B cell formation remains impaired, indicating that BAFF-R engagement also imparts essential differentiation signals (Rahman and Manser, 2004; Sasaki et al., 2004; Tardivel et al., 2004).
In early work distinguishing the canonical (IKK2/Nemo-dependent) and non-canonical (IKK1-dependent) NF-κB pathways, it was observed that BAFF-R engagement efficiently induced the cleavage of p100 (encoded by NF-κB2) into p52, allowing it to pair with RelB to drive gene expression (Claudio et al., 2002; Kayagaki et al., 2002; Senftleben et al., 2001). Cleavage of p100 is enabled by IKK1-dependent phosphorylation, which requires upstream activation by NIK (Senftleben et al., 2001; Xiao et al., 2001). In unstimulated B cells, cytosolic TRAF3 is bound to NIK and mediates its continual ubiquitination and degradation (Vallabhapurapu et al., 2008; Zarnegar et al., 2008b). BAFF-R engagement relieves this suppression by redirecting the ubiquitin-mediated degradation machinery to target TRAF3, allowing for newly-formed NIK to persist (Chan et al., 2010). Consistently, gene-targeted mice lacking TRAF3 in B cells (Gardam et al., 2008; Xie et al., 2007), or mice expressing a mutated NIK molecule that cannot interact with TRAF3 (Sasaki et al., 2008), exhibit BAFF-independent B cell accumulation. The canonical NF-κB pathway has been shown to prime the non-canonical pathway by driving the expression of NF-κB2 (Dejardin et al., 2002). In this regard, studies have shown that the BCR induces p100 to facilitate BAFF-R signaling (Stadanlick et al., 2008). In addition, BAFF-R has some intrinsic capacity to activate canonical NF-κB signaling (Hildebrand et al., 2010). While inhibition of RelB by p100 is relieved by cleavage of p100 into p52, p100 has recently been shown to aggregate and act as an inhibitor of p50:p65 (Basak et al., 2007). Moreover, NIK was recently shown to be destabilized by IKK1 phosphorylation (Razani et al., 2010). Thus, there are both positive and negative feedback mechanisms regulating the NF-κB pathways in B cells.
The majority of studies of BAFF-R signaling have focused on signaling via the TRAF/IKK/NF-κB pathway. However, the phosphatidyl inositol (PtdIns) 3-kinase (PI3K) pathway has also been implicated in BAFF-R function (Baracho et al., 2011). The class IA PI3Ks consist of three catalytic isoforms (p110α, β, and δ) that form heterodimers with adaptor subunits (p85α, p55α, p50α, p85β, and p55γ) that regulate the location and enzymatic activity of the PI3K heterodimer. PtdIns(3,4,5)P3 is also the primary substrate for the phosphoinositide 3-phosphatase, PTEN, which directly antagonizes PI3K activity. Activation of downstream pathways is initiated by the recruitment of effector molecules such as PDK1, Akt, Btk, and PLCγ2 that bear pleckstrin homology (PH) domains that directly bind PtdIns(3,4,5)P3 (Baracho et al., 2011). p110δ-deficient B cells exhibit impaired BAFF-induced survival (Henley et al., 2008), while combined inactivation of p110α/δ results in failed B cell generation or accumulation (Ramadani et al., 2010). Using Akt phosphorylation as a surrogate readout, it has been observed that BAFF induces PI3K activity with both rapid and delayed kinetics (Otipoby et al., 2008; Patke et al., 2006). Thus, there is experimental evidence supporting a role for the PI3K pathway in BAFF-R function, but it is unclear whether this is a primary or ancillary role relative to the non-canonical NF-κB signaling pathway.
Here, we report the surprising finding that acute mature B cell survival is unaffected by the inducible loss of Ikk1, while early deletion of Ikk1 results in an incomplete block in B cell maturation and BAFF responsiveness. We also provide evidence that CD19-dependent activation of the PI3K pathway is an important contributor to BAFF-mediated B cell survival. Thus, PI3K activity is pivotal for both BCR and BAFF-R signaling, underscoring its significance as a therapeutic target in autoimmune disease and B cell malignancy.
MATERIALS AND METHODS
Mice
hCD20TamCre animals (Khalil et al., 2012) were intercrossed with mice carrying the rosa26-flox-STOP-YFP allele (Srinivas et al., 2001), in which YFP is expressed upon Cre activation. Ikk1L/LCD20TamCre and control animals were injected i.p. with 1 mg tamoxifen (Sigma-Aldrich, St. Louis, MO) + 10% ethanol in olive oil on 3 subsequent days. PtenL/LCd19Cre mice (Anzelon et al., 2003) were crossed to Baff−/− (Schiemann et al., 2001) mice to generate a mouse line with B cell-specific deletion of Pten and absence of Baff expression in all tissues (PtenL/LBaff−/−Cd19Cre). Ikk1L/L and Cd19Cre mouse lines were intercrossed to obtain IKK1 deficient (Ikk1L/LCd19Cre), and IKK1 and CD19 double deficient mice (Ikk1L/LCd19Cre/Cre). All animals were maintained in the animal facility of the Sanford-Burnham Medical Research Institute (SBMRI). All protocols were approved by the Institutional Animal Care and Use Committee at SBMRI and were carried out in accordance with institutional guidelines and regulations.
Histology
Spleens were embedded in Tissue-Tek O.C.T. (Sakura Finetek U.S.A., Torrance, CA) and frozen at −80°C. Acetone fixed sections were blocked for 1 hr with 1% BSA + 5% FBS in PBS, and stained with a combination of the following antibodies: Moma-1-bio, CD3-APC, B220-PE, B220-FITC, or PNA-FITC for 2 h at RT or overnight at 4°C and SA-Cy3 in a second staining step. Images were acquired using a Zeiss Axio ImagerM1 microscope (Zeiss, Thornwood, NY).
Flow Cytometry and Antibodies
Single cell suspensions were prepared, counted, and stained with antibodies according to standard procedures. The following antibody clones were used: (eBioscience, San Diego, CA): CD3 (145-2C11), IgM (II/41), IgD (11-26), CD19 (ID3), B220 (RA3-6B2), CD11b (M1/70), CD43 (S7), CD21 (4E3), CD23 (B3B4), CD4 (GK1.5), and CD8 (53-6.7). Biotinylated reagents were detected with streptavidin (SA) conjugated to a fluorescent marker (BD Biosciences, San Jose, CA). All data were collected on a FACSCanto flow cytometer (BD Biosciences).
Immunizations and Enzyme-Linked Immunosorbent Assay (ELISA)
Mice were immunized i.p. with 100 μg NP-KLH precipitated in alum (Imject, Pierce, Rockford, IL) and serum was collected 0, 7, and 14 days post-immunization. Costar® EIA/RIA plates (Corning, Corning, NY) were coated with 10 μg/mL NP23-BSA (Biosearch Technologies, Novato, CA) in PBS containing 0.05% sodium azide. Following blocking with 0.25% BSA in PBS, serial dilutions of the indicated serum samples were added. AP-labeled anti-mouse IgM or IgG antibody (Southern Biotech, Birmingham, AL) and PNPP substrate (Sigma-Aldrich) were used for colorimetric detection at 405nm using an ELx808 plate reader with KC4 software (BioTek Instruments, Winoosky, VT).
Cell Culture, Survival, and Proliferation Assays
B cell purification and in vitro stimulation were performed as described (Miletic et al., 2010). For survival assays, purified splenic or lymph node B cells were plated at a concentration of 1×106 cells/mL in 10% media. Survival was determined by flow cytometry either analyzing FSC/SSC properties of the cells or using the AnnV-FITC Apoptosis Detection Kit (BioVision Incorporated, Milpitas, CA) according to manufacturer’s instructions. For inhibition of PI3K p110δ, cells were pretreated with 10mM IC87114 in DMSO (ICOS Corporation, Bothell, WA).
Immunoblotting and Immunoprecipitations
Purified B cells were stimulated with 1 μg/mL anti-IgM F(ab′)2 or with 25 ng/mL BAFF for the indicated time points, and then lysed on ice with RIPA buffer (PBS, 1% NP40, 0.5% deoxycholate, 0.1% SDS, 10 mM EDTA) supplemented with a protease inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany), 10 mM NaF, and 1 mM Na3VO4, and PMSF. Equal protein amounts were resolved on 10% Bis-Tris gels (Bio-Rad, Hercules, CA or Invitrogen, Carlsbad, CA) followed by western blotting for the indicated proteins. Antibodies raised against total IKK1, phospho-Akt (S473), total Akt, p100/p52, phospho-CD19 (Y513), total CD19, phospho-GSK-3β (S9), actin, and Bim were obtained from Cell Signaling Technology (Beverly, MA). Anti-Mcl-1 was purchased from Rockland Immunochemicals (Gilbertsville, PA). Primary antibodies were detected using horseradish peroxidase (HRP)-labeled donkey anti-rabbit (Jackson Immunoresearch, West Grove, PA) or anti-mouse antibodies (Amersham, Piscataway, NJ).
For co-immunoprecipitation, B cells were lysed in lysis buffer for 20 mins on ice. Clarified lysates were incubated with 2 μg anti-Mcl-1 or control IgG antibodies overnight at 4°C. Protein A/G beads (GE Healthcare) were added for 1 h at 4°C. Immunoprecipitates were washed as described in (Maurer et al., 2006), and western blotting performed as described above.
BrdU incorporation
Mice were provided 0.5mg/ml bromodeoxyuridine (BrdU, Sigma) + 2% sucrose in drinking water for up to 21 days. Bone marrow and splenic cells were isolated on d7, d14 and d21 and stained with antibodies as indicated. After surface staining, cells were fixed with BD Cytofix/Cytoperm™ (BD Biosciences), permeabilized with permeabilization buffer (eBioscience), followed by permeabilization with 0.1% TritonX-100 (Sigma), second fixation and DNase (Sigma) treatment. The cells were then stained with an anti-BrdU (Invitrogen) antibody.
Software and Statistical Analysis
Gimp (GNU image manipulation program) and GraphPad Prism (GraphPad Software, La Jolla, CA) were used for image editing and for statistical evaluation, respectively. The significance of observed differences was evaluated using unpaired t test. Obtained p values are marked as follows: *** = p<0.001 ** = p<0.005 * = p<0.05.
Results
BAFF-mediated mature B cell survival is IKK1-independent
While both NF-κB and PI3K pathways are activated downstream of BAFF-R engagement by BAFF, and loss of either Baff or Baff-r expression results in a block at the transitional stage of B cell maturation, it is unclear whether mature B cells still require IKK1 and/or PI3K for maintenance and survival. To address this issue, we generated a novel mouse strain in which IKK1 expression can be inducibly ablated in mature B cells by intercrossing mice containing a loxP-flanked Ikk1 allele (Ikk1L) (Liu et al., 2008) with the recently described hCD20TamCre strain (Khalil et al., 2012) bearing a loxP-regulated EYFP reporter cassette (Srinivas et al., 2001). Following administration of tamoxifen, Cre recombinase is rapidly activated with concomitant expression of EYFP and deletion of Ikk1 in B cells expressing Cre. Strikingly, we found that deletion of Ikk1 in mature B cells did not result in depletion of mature B cells one or two weeks following induction of Cre with tamoxifen (Figure 1A). Flow cytometric analysis showed that in Ikk1L/LhCD20TamCre mice, on average, over 70% of cells were YFP+ (and thus deleted Ikk1) (Figure 1B). Separation of CD21intCD23hi follicular cells and CD21hiCD23int/low MZ B cells seven days post-tamoxifen injection showed that both subsets of YFP+ B cells persisted equally well in the spleens of Ikk1L/LhCD20TamCre mice (Figure 1B).
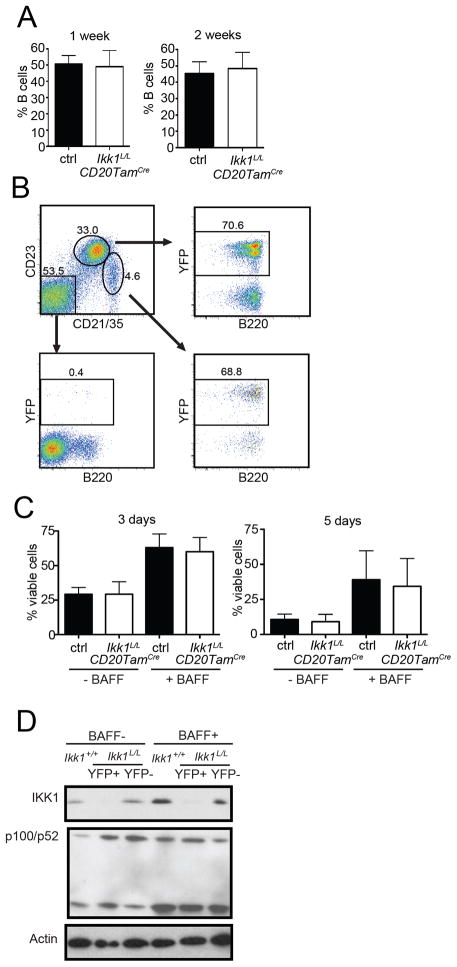
Figure 1. IKK1-deficient mature B cells show normal in vivo survival and BAFF-mediated survival in vitro.
(A) Ikk1 deletion was induced in mature B cells by tamoxifen injection of Ikk1L/LhCD20TamCre+ mice on 3 consecutive days. Ikk1L/LhCD20TamCre− or Ikk1+/+ hCD20TamCre+ mice were used as controls (ctrl). Mice were sacrificed 1 week or 2 weeks after the last tamoxifen injection and the percentage of B cells in the spleen was determined by flow cytometry. Graphs show means +SD from 3 independent experiments. (B) The percentage of YFP+ B cells 7 days after tamoxifen injection was comparable between CD21intCD23hi follicular B cells and CD21hiCD23int/low MZ B cells. YFP expression was not detected in non-B cells (B220−). Shown is a representative of n=2 experiments. (C) To study BAFF-mediated survival in vitro, mice were sacrificed after the last tamoxifen injection, and B cells were purified and stimulated with 10 ng/mL BAFF. The percentage of viable B cells 3 days or 5 days after culture was determined by flow cytometry. Graphs show mean +SD from 3 independent experiments. (D) Splenic B cells from tamoxifen-treated Ikk1L/LhCD20TamCre+ mice were stimulated over night with 25 ng/mL BAFF or incubated in medium alone. p100 cleavage and p52 generation were visualized by western blotting. Absence of IKK1 in Cre+ cells (YFP+) was confirmed by western blot analysis. Actin was used as loading control. Shown is a representative of n=2 experiments.
Consistent with our in vivo observations, in vitro survival assays showed that B cells isolated from Ikk1L/LhCD20TamCre mice survived as well as control B cells in media alone or with BAFF stimulation (Figure 1C). We confirmed that survival of Ikk1L/LhCD20TamCre B cells was not due to residual expression of IKK1 protein by immunoblotting of whole cell lysates from sorted YFP+ and YFP− B cells (Figure 1D). Interestingly, we also found that p52 was present in similar amounts in YFP+ and YFP− B cells from Ikk1L/LhCD20TamCre mice, and could be generated de novo upon BAFF stimulation (Figure 1D).
Since the hCD20TamCre inducible system does not account for the contribution of p100 cleavage that occurred before tamoxifen-induced Ikk1 inactivation, we intercrossed Ikk1L/L mice with Cd19Cre mice to eliminate IKK1 prior to the onset of BAFF-R expression. Ikk1L/LCd19Cre mice exhibited a 40–50% reduction in mature B cells (Figure 2A), but B cell development is not blocked at the T1 – T2 maturation stage as observed in mice lacking BAFF/BAFF-R (Figure 2A, B) (Sasaki et al., 2004), or mice reconstituted with Ikk1−/− fetal liver cells (Kaisho et al., 2001). BrdU-labeling experiments revealed that phenotypically mature splenic B cells in Ikk1L/LCd19Cre mice exhibited a more rapid turnover, whereas mature recirculating B cells analyzed from the bone marrow of Ikk1L/LCd19Cre and control mice had similar rates of turnover (Figure 2C). Ikk1L/LCd19Cre B cells responded to BAFF, albeit less effectively than control B cells (Figure 2D). At the biochemical level, splenic B cells from Ikk1L/LCd19Cre mice showed efficient ablation of IKK1 and impaired but not absent cleavage of p100 (Figure 2E). Moreover, p100 cleavage reached completion following in vitro BAFF stimulation of Ikk1L/LCd19Cre B cells (Figure 2E). Altogether, these findings indicate that the loss of IKK1 imposes a bottleneck at the transitional B cell stage, but B cells that successfully traverse this stage become long-lived mature recirculatng B cells that do not strictly require IKK1 for tonic BAFF-R signaling. Moreover, results of the in vitro stimulation assays raise the possibility that another ser/thr kinase can partially compensate for the loss of IKK1 in the processing of p100 to generate p52.
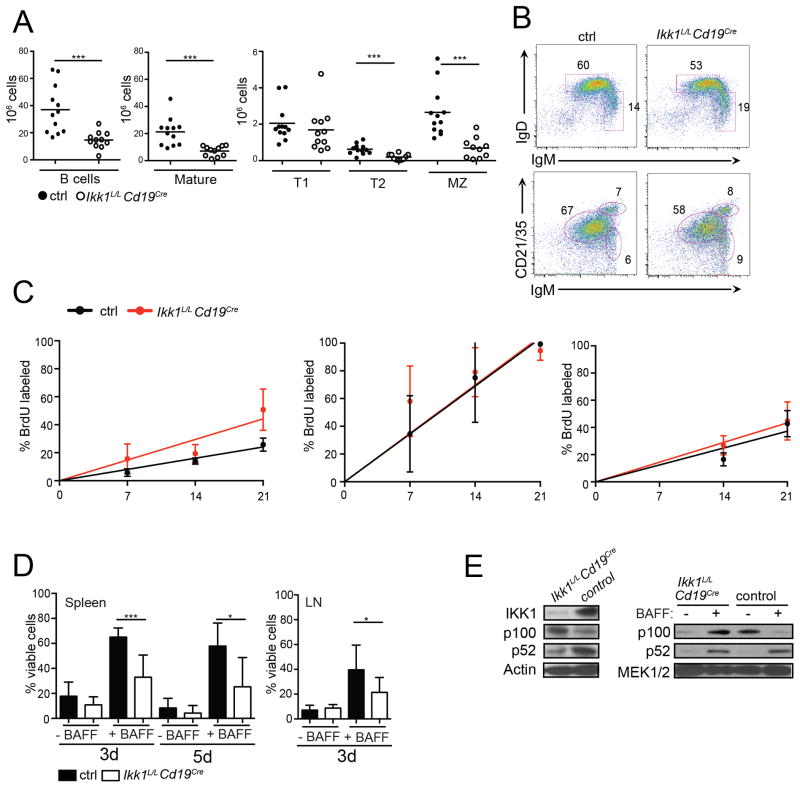
Figure 2. IKK1 deletion early in B cell development results in an incomplete block in B cell maturation.
(A) Graphs show total cell numbers of B cells (left panel) and B cell subsets (middle and right panel) in spleens obtained from Ikk1L/LCD19Cre+ and control mice. Ikk1L/LCD19Cre− or Ikk1+/+ CD19Cre+ mice were used as controls (ctrl). B cell subsets were identified by cell surface markers: B220+=total B cells, B220+CD21intIgMlow=mature B cells, B220+CD21lowIgMhi=T1 B cells, B220+CD21hiCD23hiIgMhi=T2 B cells, B220+CD21hiCD23int/low=MZ B cells. (B) B cell maturation in the spleen was analyzed by flow cytometry. Plots are representative of >11 mice analyzed. (C) Mice were continuously provided BrdU in the drinking water and euthanized after 7d, 14d or 21d of treatment. Cells were harvested from the spleen and the bone marrow and stained with an anti-BrdU antibody and for surface markers as follows: (left) splenic follicular (B220+, IgM+, CD23hi, CD21lo) B cells; (center) bone marrow B cell progenitors (B220+, IgM−, IgD−); (right) recirculating mature B cells (B220+, IgD+, IgMlo) in the bone marrow. Four experimental and CD19Cre control mice (10–15w old) were used per time point and rates of turnover calculated by linear regression analysis. (D) B cells from spleens enriched for mature B cells (CD23+CD43−), or from LNs (B220+CD43−) were stimulated with 10 ng/mL BAFF and the percentage of viable cells was determined by flow cytometry after 3 days and/or 5 days in culture. The graph summarizes n=7 samples for each genotype and time point. (E) Protein lysates from freshly-isolated splenic B cells were assayed for p100 cleavage by western blotting (left panel). p100 processing to p52 in LN B cells stimulated overnight with 25 ng/mL BAFF versus unstimulated cells (right panel).
Sustained PtdIns(3,4,5)P3 signaling restores B cell development in Baff−/− mice
Since IKK1-dependent signaling events cannot solely account for BAFF-R function, we sought to identify additional pathways that may complement IKK1 activity. Several reports have shown that BAFF-R can engage the PI3K pathway (Henley et al., 2008; Otipoby et al., 2008; Patke et al., 2006; Woodland et al., 2008). We confirmed these findings, showing that BAFF induced rapid activation of Akt (Figure S1A). Addition of the p110δ-specific inhibitor IC87114 blocked Akt activation and impaired BAFF-dependent B cell survival (Figure S1B). To address the physiologic significance of BAFF-dependent PI3K activity, we bred PtenL/LCd19Cre mice onto the BAFF-deficient background (PtenL/LBaff−/−Cd19Cre). In PtenL/LBaff−/−Cd19Cre B cells, the absence of PTEN results in sustained activation of the PI3K pathway due to impaired hydrolysis of the PI3K lipid product PI(3,4,5)P3. Consistent with previous reports (Anzelon et al., 2003; Suzuki et al., 2003), B cell-specific deletion of Pten resulted in a skewing towards the MZ B cell fate (Figure 3A, B). In contrast, Baff−/− mice exhibited a dramatic reduction in all peripheral B cell subsets, owing to a block at the transitional stage of maturation (Figure 3A, B). Strikingly, in BAFF-deficient mice lacking expression of Pten, we observed a significant recovery in B cell maturation with no apparent bias towards the MZ B cell subset (Figure 3A, B). In this regard, the size of the CD21/35hiCD1d+ and CD9+ B cell subsets was comparable in wild type and PtenL/LBaff−/−Cd19Cre mice (data not shown). BCR signaling promotes Baff-r expression and (Rowland et al., 2010; Smith and Cancro, 2003), in turn, BAFF signaling up-regulates surface expression of CD21/35 and CD23 on B cells (Gorelik et al., 2004). Here we found that constitutive-activation of the PI3K pathway restored CD21/35 but not CD23 expression in PtenL/LBaff−/−Cd19Cre splenic B cells (Figure 3C and data not shown). These data indicate that downstream of BAFF-R signaling, PI3K supports CD21/35 surface expression (Figure 3C), while CD23 expression is up-regulated by BAFF-R signaling in a PI3K-independent manner or down-regulated by elevated PI3K signaling. Consistent with flow cytometric analyses, histological staining of spleen sections from Pten+/+Baff+/+Cd19Cre, PtenL/LBaff+/+Cd19Cre, Pten+/+Baff−/−Cd19Cre, and PtenL/LBaff−/−Cd19Cre mice confirmed that PtenL/LBaff−/−Cd19Cre mice did not have an expansion of MZ B cells as was observed in PtenL/LBaff+/+Cd19Cre mice, and that overall splenic architecture in PtenL/LBaff−/−Cd19Cre mice was similar to wild type controls (Figure S2A).
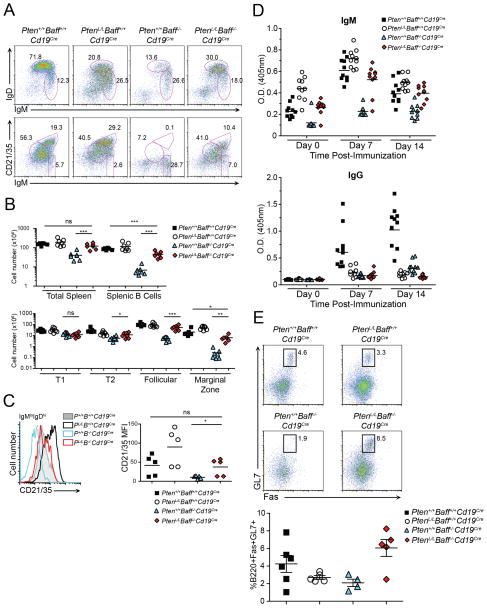
Figure 3. Constitutively-active PI3K restores B cell development in Baff−/− mice.
(A) Flow cytometry of B220+ splenic cells from Pten+/+Baff+/+Cd19Cre, PtenL/LBaff+/+Cd19Cre, Pten+/+Baff−/−Cd19Cre, and PtenL/LBaff−/−Cd19Cre mice. Data are representative of n>8 mice per group. (B) Absolute numbers of splenocytes and splenic B220+ B cells (top panel), and splenic B cell subsets (bottom panel). Shown is n=7 mice per group from n=5 experiments; small horizontal lines indicate mean. (C) Expression of CD21/35 on B220+-gated IgMloIgDhi splenic B cells from Pten+/+Baff+/+Cd19Cre, PtenL/LBaff+/+Cd19Cre, Pten+/+Baff−/−Cd19Cre, and PtenL/LBaff−/−Cd19Cre mice. MFI=mean fluorescence intensity. (D) ELISA of nitrophenol-specific IgM (top) or IgG (bottom) in the sera of Pten+/+Baff+/+Cd19Cre, PtenL/LBaff+/+Cd19Cre, Pten+/+Baff−/−Cd19Cre, and PtenL/LBaff−/−Cd19Cre mice prior to immunization (day 0), and 7 or 14 days post-immunization with 100 μg NP-KLH in alum. (E) Flow cytometric analysis of splenic GC B cells (B220+ gated) from immunized mice (top). Graph summarizes the percent of B220+GL7+Fas+ B cells 14 days post-immunization (bottom).
Antigen-specific immune responses and germinal center (GC) formation are intact in PtenL/LBaff−/−Cd19Cre mice
Despite the paucity of mature B cells in mice lacking expression of BAFF or BAFF-R, small germinal centers (GC) are formed and some IgG is produced (Miller and Hayes, 1991; Rahman et al., 2003; Vora et al., 2003); however, the GC response is transient with impaired proliferation and an associated failure to form mature follicular dendritic cell (FDC) networks (Rahman and Manser, 2004; Rahman et al., 2003; Vora et al., 2003). As in the case of MZ B cell formation, ectopic expression of Bcl-2 does not rescue the GC response in Baff−/− mice, resulting in the accumulation of B cells bearing an immature phenotype and disrupted follicular architecture (Rahman and Manser, 2004). Thus, BAFF signaling is critical for the survival of transitional and mature recirculating B cells, and to promote MZ and GC B cell differentiation.
Although sustained PtdIns(3,4,5)P3 signaling in Baff−/− mice lacking Pten allowed for B cell development beyond the transitional stage, we sought to determine if the mature B cells found in PtenL/LBaff−/−Cd19Cre mice were functional. To this end, PtenL/LBaff−/−Cd19Cre mice and control animals were immunized with nitrophenol-keyhole limpet hemocyanin (NP-KLH) in alum and relative levels of NP-specific serum IgM and IgG antibody were measured at 7 and 14 days post-immunization. PtenL/LBaff−/−Cd19Cre mice produced elevated levels of anti-NP IgM antibody at 7 and 14 days post-immunization as compared to Pten+/+Baff−/−Cd19Cre mice, and responses were statistically indistinguishable from normal Pten+/+Baff+/+Cd19Cre controls (Figure 3D, top). Consistent with previously published studies (Anzelon et al., 2003; Suzuki et al., 2003), PtenL/LBaff+/+Cd19Cre mice displayed a significant reduction in anti-NP IgG antibodies, likely due to the fact that sustained and elevated PtdIns(3,4,5)P3 signaling inhibits class switch recombination by terminating Foxo1-dependent Aicda transcription (Dengler et al., 2008; Omori et al., 2006) (Figure 3D, bottom). Correspondingly, in spite of robust antigen-specific IgM production, PtenL/LBaff−/−Cd19Cre mice showed a virtual absence of NP-specific IgG and resembled PtenL/LBaff+/+Cd19Cre mice in this respect (Figure 3D).
To confirm that the absence of NP-specific IgG was not due to defective GC formation in PtenL/LBaff−/−Cd19Cre mice, we assessed the presence of GCs in immunized control mice and PtenL/LBaff−/−Cd19Cre mice. Flow cytometric analysis of splenocytes from immunized mice showed that unlike Pten+/+Baff−/−Cd19Cre mice, PtenL/LBaff−/−Cd19Cre mice produced abundant B220+PNA+GL7+ GC B cells (Figure 3E). In fact, PtenL/LBaff−/−Cd19Cre mice harbored a greater percentage of GC B cells than normal or PTEN-deficient counterparts. In addition, staining of spleen sections with B220 and PNA showed robust GCs in immunized PtenL/LBaff−/−Cd19Cre mice, consistent with flow cytometric data (Figure S2B). Thus, antigen-driven B cell responses are recovered in PtenL/LBaff−/−Cd19Cre mice, while repression of class switch recombination remains a dominant effect of Pten inactivation.
PTEN-deficient B cells from Baff−/− mice are responsive to extracellular stimuli and BCR engagement
Given the robust in vivo responses of PtenL/LBaff−/−CD19Cre B cells following immunization, we next determined whether PtenL/LBaff−/−Cd19Cre B cells displayed the activation and proliferative properties of mature B cells responding to specific stimuli. To this end, purified splenic Pten+/+Baff+/+CD19Cre, PtenL/LBaff+/+CD19Cre, Pten+/+Baff−/−CD19Cre, and PtenL/LBaff−/−CD19Cre B cells were cultured in the presence of BAFF, anti-IgM F(ab′)2 (with or without BAFF), agonistic anti-CD40 antibody, or LPS. Consistent with previous reports (Anzelon et al., 2003; Suzuki et al., 2003), expression of the activation markers CD69 and CD86 was augmented on PTEN-deficient B cells (Figure 4A, B). In contrast, expression of CD69 and CD86 was significantly reduced or absent on B cells from BAFF-deficient animals following treatment with various stimuli (Figure 4A, B). Notably, constitutive activation of the PI3K pathway by the loss of PTEN expression in BAFF-deficient B cells restored B cell responsiveness and induction of CD69 and CD86 expression under all conditions examined. In this regard, PtenL/LBaff−/−CD19Cre B cells resembled control B cells (Figure 4A, B). Consistent with these data, PtenL/LBaff−/−CD19Cre B cells also proliferated robustly following stimulation with numerous mitogenic stimuli and were comparable to PtenL/LBaff+/+CD19Cre B cells (Figure 4C). Since inhibition of PI3K impairs BAFF-R signaling (Figure S1A, B), we also confirmed that sustained activation of the PI3K pathway in PTEN-deficient B cells promotes BAFF-induced survival (Figure 4D). Thus, the competence of B cells from PtenL/LBaff−/−CD19Cre mice to respond productively to BCR engagement and co-stimulation supports the strong antibody responses in vivo.
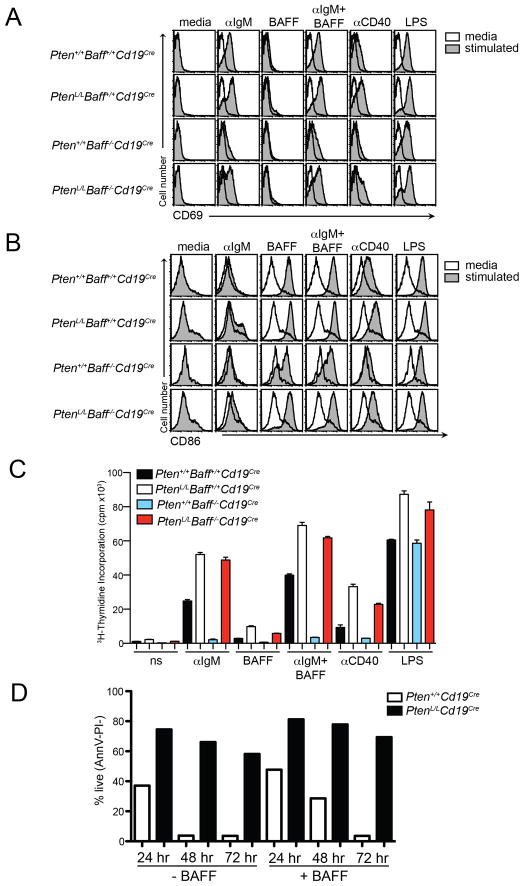
Figure 4. Upregulation of activation markers and proliferation are restored in Baff−/− B cells lacking Pten.
(A) Flow cytometric analysis of CD69 expression on Pten+/+Baff+/+Cd19Cre, PtenL/LBaff+/+Cd19Cre, Pten+/+Baff−/−Cd19Cre, and PtenL/LBaff−/−Cd19Cre B cells following stimulation with indicated mitogens. (B) As in (A), expression of CD86. (C) Purified splenic B cells from Pten+/+Baff+/+Cd19Cre, PtenL/LBaff+/+Cd19Cre, Pten+/+Baff−/−Cd19Cre, and PtenL/LBaff−/−Cd19Cre mice were stimulated as indicated. Proliferation was determined at 48 hours by 3H-thymidine incorporation. All assays were conducted in triplicate and SDs are shown as error bars. Data are representative of 3 independent experiments with n=2 mice per group per experiment. (D) Pten+/+Cd19Cre or PtenL/LCd19Cre mature lymph node B cells were left untreated or were cultured in the presence of BAFF, and cell viability was assessed by Annexin V (AnnV) and propidium iodide (PI) staining. Graph shows the percent of live (AnnV−PI−) cells at each time point. Data are representative of n=3 experiments with 5 mice/group total.
PI3K-driven B lymphomagenesis is unperturbed in the absence of BAFF
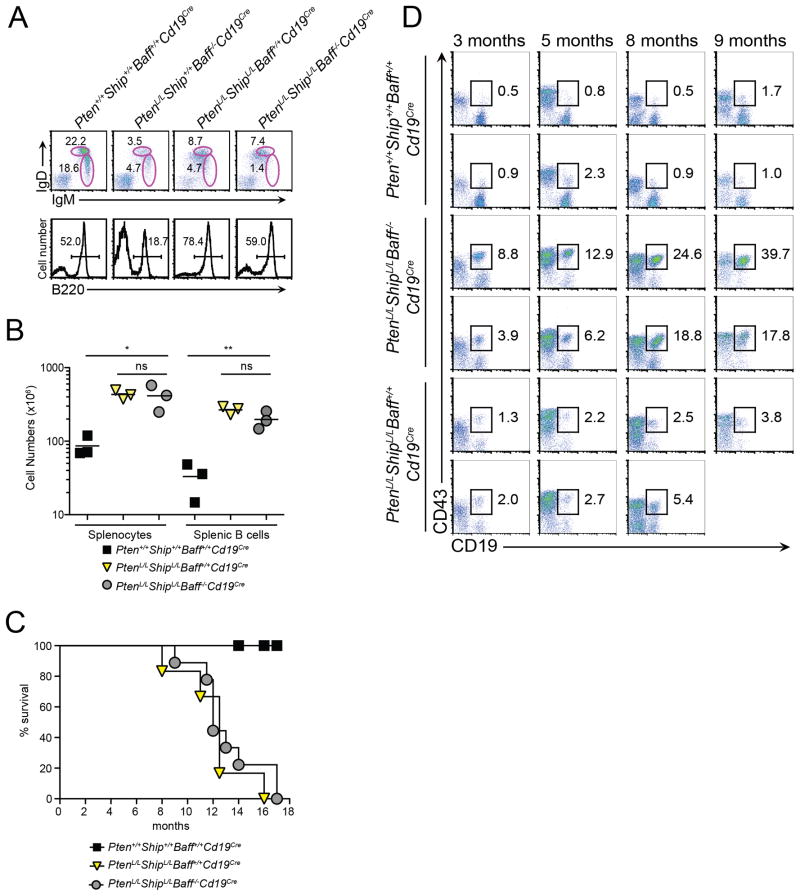
We recently reported a novel model of spontaneous B cell lymphoma in mice harboring B cell-specific deletion of genes encoding PTEN and SHIP phosphatases (Miletic et al., 2010). This model demonstrated not only enhanced survival of PtenL/LShipL/LCD19Cre lymphoma cells in the presence of BAFF, but a proliferative response to BAFF as well. Moreover, PtenL/LShipL/LCD19Cre lymphoma B cells continued to expand upon adoptive transfer into sub-lethally irradiated Baff−/− recipients. Here, we sought to determine whether BAFF is required for B lymphoma initiation as well as progression in PtenL/LShipL/LCD19Cre mice. To this end, we crossed PtenL/LShipL/LCD19Cre mice onto the Baff−/− background (PtenL/LShipL/LBaff−/−CD19Cre). B cell development in PtenL/LShipL/LBaff−/−CD19Cre mice was comparable to that observed in BAFF-expressing PtenL/LShipL/LCD19Cre mice, with B cells numbers similar to wild type controls (Figure 5A, B). Strikingly, PtenL/LShipL/LBaff−/−CD19Cre mice developed lethal lymphoma with a similar onset and penetrance as BAFF-sufficient PtenL/LShipL/LCD19Cre mice (Figure 5C). Moreover, the lymphoma cells that expanded in PtenL/LShipL/LBaff−/−CD19Cre mice were phenotypically similar (B220loCD5+CD11b+) to lymphoma B cells from PtenL/LShipL/LCD19Cre mice (Figure 5D). Collectively, these data indicate that BAFF is not required for B lymphomagenesis when PI3K signaling is highly dysregulated.
Figure 5. Lymphoma development in PtenL/LShipL/LCd19Cre mice occurs in a BAFF-independent manner.
(A) Flow cytometric analysis of B220+-gated splenic cells from Pten+/+Ship+/+Baff+/+Cd19Cre, Pten+/+Ship+/+Baff−/−Cd19Cre, PtenL/LShipL/LBaff+/+Cd19Cre, and PtenL/LShipL/LBaff−/−Cd19Cre mice. Data are representative of 2 independent experiments with n≥2–3 mice per group. (B) Absolute numbers of splenocytes and splenic B cells (n=3 mice per group; small horizontal lines indicate mean). (C) Kaplan-Meier survival curve of Pten+/+Ship+/+Baff+/+Cd19Cre (n=7), PtenL/LShipL/LBaff+/+Cd19Cre (n=6), and PtenL/LShipL/LBaff−/−Cd19Cre (n=9) mice. (D) Expansion of B220−/lowCD19+ lymphoma B cells in peripheral blood of Pten+/+Ship+/+Baff+/+Cd19Cre, PtenL/LShipL/LBaff+/+Cd19Cre, and PtenL/LShipL/LBaff−/−Cd19Cre mice determined by flow cytometry at indicated time points. Data shown are from two representative animals for each group. The PtenL/LShipL/LBaff+/+Cd19Cre animal shown in bottom row died before 9 months of age.
Augmented PI3K signaling by BAFF-R does not affect the non-canonical NF-κB pathway and promotes Mcl-1 function
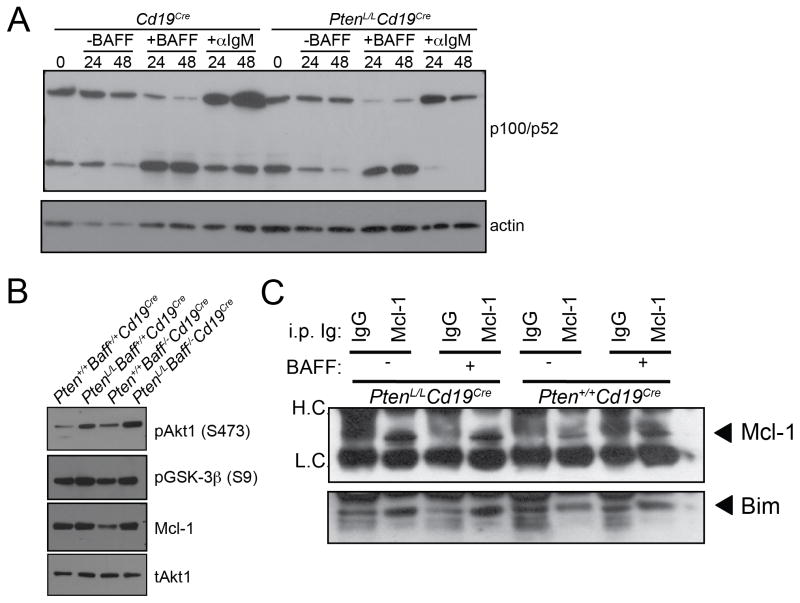
To determine if there is biochemical crosstalk or synergy between the PI3K and NF-κB pathways downstream of BAFF-R, we examined p100 expression and p52 generation in Pten+/+Cd19Cre and PtenL/LCd19Cre B cells. Freshly-isolated B cells from both mouse lines exhibited similar amounts of p100 and the p52 cleavage product, indicating similar in vivo responses to endogenous BAFF (Figure 6A). Accordingly, exposure to BAFF in vitro resulted in efficient conversion of p100 to p52 in control and in PTEN-deficient B cells (Figure 6A). Induction of p100 by BCR stimulation was also similar in control and PTEN-deficient B cells, indicating that canonical NF-κB signaling was not augmented by heightened activation of the PI3K pathway.
Figure 6. Augmented PI3K signaling by BAFF-R does not affect the non-canonical NF-κB pathway and promotes Mcl-1 function.
(A) Pten+/+Baff+/+Cd19Cre and PtenL/LBaff+/+Cd19Cre B cells were left untreated, or were cultured in the presence of BAFF or anti-IgM F(ab′)2 fragments. Activation of non-canonical NF-κB was determined by western blotting with antibodies against p100/p52. Membranes were stripped and reprobed for actin as a loading control. (B) Western blots of protein lysates from freshly-isolated Pten+/+Baff+/+Cd19Cre, PtenL/LBaff+/+Cd19Cre, Pten+/+Baff−/−Cd19Cre, or PtenL/LBaff−/−Cd19Cre splenic B cells probed with anti-pAkt1 (S473), anti-GSK-3β (S9), anti-Mcl-1, or anti-Akt1 antibodies. (C) Pten+/+Cd19Cre and PtenL/LCd19Cre B cells were left untreated or were treated with BAFF. Lysates were generated and Mcl-1 was immunoprecipitated. Immunoprecipitates were resolved by SDS-PAGE and membranes probed with antibodies against Bim and Mcl-1.
BAFF has been characterized chiefly as a pro-survival factor. The targets of BAFF-dependent survival have yet to be identified, but we focused on the pro-survival Bcl-2 family member Mcl-1, which is regulated primarily in a post-translational manner that requires PI3K signaling and has been previously implicated in BAFF-R signaling (Maurer et al., 2006; Woodland et al., 2008). Mcl-1 is phosphorylated by GSK-3β leading to degradation of Mcl-1. The loss of Mcl-1 is countered by Akt-mediated phosphorylation and subsequent inactivation of GSK-3β (Maurer et al., 2006). To examine this regulatory cascade, we measured levels of pAkt (Ser473), pGSK-3β (Ser9), and Mcl-1 in freshly-isolated splenic B cells from Pten+/+Baff+/+Cd19Cre, PtenL/LBaff+/+Cd19Cre, Pten+/+Baff−/−Cd19Cre, and PtenL/LBaff−/−Cd19Cre mice. Both PtenL/LBaff+/+Cd19Cre and PtenL/LBaff−/−Cd19Cre B cells showed elevated levels of phosphorylated Akt as well as phosphorylation of GSK-3β on inhibitory serine 9, the site that is phosphorylated by Akt, as compared to control Pten+/+Baff+/+Cd19Cre or Pten+/+Baff−/−Cd19Cre B cells (Figure 6B). Consistent with these results, we also found elevated levels of Mcl-1 in both PtenL/LBaff+/+Cd19Cre and PtenL/LBaff−/−Cd19Cre B cells (Figure 6B). It is possible that some of these differences reflect the altered distribution of B cell subsets between these strains (Figure 3A, B). That said, PtenL/LBaff−/−Cd19Cre mice displayed reduced total splenic B cells numbers, but a similar subset distribution compared to normal Pten+/+Baff+/+Cd19Cre control mice (Figure 3A, B).
Mcl-1 promotes cell survival through the direct binding and sequestration of the pro-apoptotic BH3 family member, Bim (Maurer et al., 2006). Correspondingly, we found an elevated amount of Bim associated with Mcl-1 in Pten-deficient B cells as compared to control B cells (Figure 6C). Together, these data suggest that activation of PI3K downstream of BAFF-R may promote B cell survival in part via maintenance of Mcl-1 expression and sequestration of Bim by Mcl-1.
BAFF-R signaling employs both the IKK1 and CD19/PI3K pathways
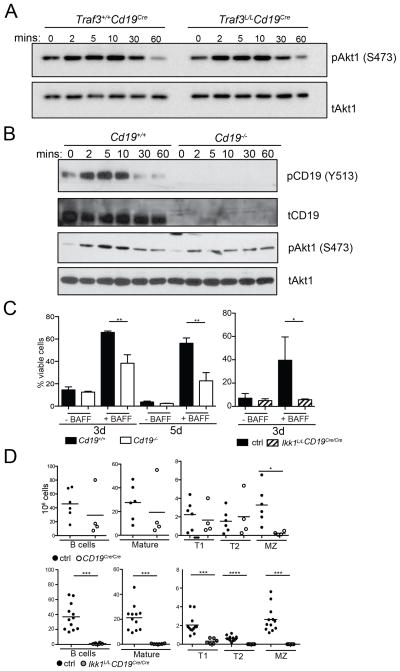
While it is known that PI3K is activated in B cells downstream of the BAFF-R, how PI3K is recruited to the BAFF-R remains unclear. Unlike non-canonical NF-κB signaling, which has been shown to be dependent upon TRAF3 for activation downstream of the BAFF-R (Rickert et al., 2011), we found that Akt activation was not affected in a positive or negative manner in mice lacking TRAF3 in B cells (Traf3L/LCd19Cre) (Figure 7A). Thus, while TRAF3 ablation permits BAFF-independent B cell survival (Gardam et al., 2008; Xie et al., 2007), this effect is apparently not due to augmented PI3K signaling.
Figure 7. BAFF-induced signaling is attenuated in B cells lacking expression of CD19.
(A) Western blots of protein lysates from Traf3+/+Cd19Cre or Traf3L/LCd19Cre splenic B cells treated for indicated time points with BAFF were probed with anti-pAkt1 (S473) or anti-tAkt1 antibodies. (B) Western blots of protein lysates from Cd19+/+ or Cd19−/− splenic B cells treated for indicated time points with 25 ng/mL BAFF were probed with anti-pCD19 (Y513), anti-CD19, anti-pAkt1 (S473), or anti-tAkt1 antibodies. (C) Cd19+/+ or Cd19−/− LN B cells were left untreated or were cultured in the presence of 25ng/mL BAFF and the percentage of viable cells was determined by flow cytometry after 3 days and/or 5 days in culture (left panel). Graphs summarize data from 3 individual mice in technical triplicates per genotype. LN B cells from Ikk1L/LCD19Cre/Cre (IKK1 and CD19 double deficient) and control mice were treated with 10ng/mL BAFF or were cultured in medium alone and cell viability was assessed 3 days later (right panel). Graphs summarize results from 3 independent experiments with 7 control samples and 3 Ikk1L/LCD19Cre/Cre samples in total. These measurements were part of the experiments described in Figure 2C, therefore, results shown for Ikk1L/LCD19Cre/Cre samples can be directly compared with Ikk1L/LCD19Cre/+ samples shown in Figure 2C. (D) Total splenic B cells numbers and cell numbers of indicated B cell subsets from CD19−/− and control mice are shown in the top panel. B cell subsets were defined as in Figure 2A. Analysis of total cell numbers for Ikk1L/LCD19Cre/Cre and control mice is shown in the bottom panel. These mice were analyzed in parallel with mice presented in Figure 2A, therefore, results shown for Ikk1L/LCD19Cre/Cre mice can be directly compared with data from Ikk1L/LCD19Cre/+ mice shown in Figure 2A.
Downstream of the BCR, PI3K p110δ can act on membrane substrates via p85α-mediated recruitment to the transmembrane adaptor CD19 as well as to cytosolic adaptor BCAP (Baracho et al., 2011; So and Fruman, 2012). To determine if CD19 may also act as a co-receptor for BAFF-R signaling, Cd19+/+ and Cd19−/− (aka Cd19Cre/Cre) splenic and lymph node (data not shown) B cells were treated with BAFF and examined for phosphorylation of CD19 on p85-binding site Y513 and Akt S473. We found that BAFF-R binding induced robust phosphorylation of CD19 (Y513) and that expression of CD19 augmented Akt activation (Figure 7B). Impaired BAFF-R signaling correlated with reduced survival of Cd19−/− B cells cultured in the presence of BAFF (Figure 7C). Together, these results indicate that CD19 is a critical component of BAFF-R signaling that may recruit PI3K to BAFF-R in a manner analogous to its role in BCR signaling.
In agreement with earlier findings, Cd19−/− mice display a modest reduction in mature B cells and a near absence of MZ B cells (Figure 7D). However, unlike Baff−/− mice, the T2 population is unaffected (Figures 3A, B and 7D (top)). Thus, to determine if BAFF-R may differentially utilize the IKK1 and CD19/PI3K pathways in transitional, mature, and MZ B cells subsets, we generated mice lacking both CD19 and IKK1 in B cells (Ikk1L/LCd19Cre/Cre). Strikingly, these mice exhibited a strong block in peripheral B cell maturation that was comparable to that observed in Baff−/− mice (Figures 3A, B and 7D (bottom)). Indeed, Ikk1L/LCd19Cre/Cre B cells were non-responsive to BAFF stimulation in vitro (Figure 7C). These findings suggest that the IKK1 and CD19/PI3K pathways act in parallel to mediate BAFF-R signaling in newly formed B cells.
Discussion
It has been shown in numerous studies that BAFF depletion causes the rapid loss of transitional, mature, and GC B cells. BAFF-R signaling via the non-canonical NF-κB pathway is thought to occur similarly in these B cell subsets. Early studies showed that fetal liver-derived B cells from Ikk1−/− mice presented a block at the late transitional (T2) B cell stage (Kaisho et al., 2001), but we found that Ikk1 inactivation in early B cells only resulted in a partial block at the T2 stage. This apparent discrepancy might be explained by the recent discovery of a role for IKK1 in early B cell generation, and perhaps a greater dependence on IKK1 activity for fetal versus bone marrow-derived B cells (Balkhi et al., 2012). BrdU labeling studies revealed that splenic B cells bearing a mature phenotype exhibited a higher turnover in Ikk1L/LCd19Cre mice, suggesting that they were relatively short-lived. However, turnover of mature recirculating B cells in the bone marrow of Ikk1L/LCd19Cre mice was unaffected by the loss of IKK1, consistent with results from the inducible loss of IKK1 using the hCD20TamCre system. One possibility to explain these findings is that inhibitory p100 accumulates in transitional B cells and descendent mature B cells in the spleens of Ikk1L/LCd19Cre mice, predisposing them to apoptosis and failed entry into the long-lived mature recirculating B cell pool.
Lack of a role for IKK1 in mature B cell survival is consistent with our previous observation of intact B cell maturation and survival in knock-in mice expressing a mutant IKK1 molecule that cannot be phosphorylated by NIK (IKKAA) (Mills et al., 2007). In contrast, the IKKAA mice exhibit a complete block in GC B cell differentiation (Mills et al., 2007), suggesting that BAFF-R/NIK/IKK1 signaling may be important for priming the survival and differentiation pathways that are set into place after antigen encounter. Expression of a constitutively-active form of IKK2 or disruption of NIK degradation also allows for BAFF-independent B cell maturation (Sasaki et al., 2008; Sasaki et al., 2006). Elevated NIK activity has been shown to activate the canonical NF-κB pathway as well as the non-canonical pathway (Zarnegar et al., 2008a). Thus, the B cell phenotypes observed in mice expressing constitutively-active IKK2 or NIK may have similar biochemical underpinnings in abnormally augmenting canonical NF-κB-dependent gene transcription.
Given that inactivation of the non-canonical NF-κB pathway is insufficient to explain the biologic effects of BAFF depletion on mature B cells, we focused on the PI3K pathway, which has been previously implicated in BAFF-R function (Baracho et al., 2011). The PI3K pathway serves multiple functions in cell growth, proliferation, survival, and differentiation. Correspondingly, BAFF stimulation also primes B cells for cell cycle entry and protein synthesis (Huang et al., 2004; Patke et al., 2006). These effector pathways likely account, in part, for the observed defects in the MZ and GC B cell compartments in mice bearing defects in PI3K/Akt signaling (Calamito et al., 2010; Clayton et al., 2002; Zhang et al., 2012).
The majority of studies of PI3K function in B cells have focused on BCR-induced PI3K activity, including the recruitment of CD19 as a co-receptor. In this regard, inactivation of CD19 or p110δ yields similar defects in the generation of MZ, B-1, and GC B cells (Clayton et al., 2002; Engel et al., 1995; Okkenhaug et al., 2002; Rickert et al., 1995); however, dual ablation of p110α/δ leads to a nearly complete block in B cell development at the pro-B cell stage (Ramadani et al., 2010). Here, we show that CD19 contributes to BAFF-mediated survival, consistent with BAFF-induced CD19 phosphorylation and Akt activation. Intriguingly, this finding suggests that BAFF-R employs signaling components associated with the BCR in a “co-receptor” capacity. This assertion is supported by the recent work of Schweighoffer et al., who reported a role for Syk in BAFF-R signaling (Tusche et al., 2009). Findings that BAFF activates Btk also supports the possible linkage of the BCR and PtdIns(3,4,5)P3 signaling downstream of BAFF-R (Shinners et al., 2007). Thus, previous studies showing that the BCR is required for continued B cell survival may incorporate homeostatic signaling by BAFF-R (Lam et al., 1997).
To further evaluate the PI3K pathway in BAFF-R signaling, we performed gain-of-function studies by inactivating Pten in B cells. This alteration is similar to, yet distinct from, expressing constitutively active PI3K (p110) in that PTEN loss leads to the sustained presence of PtdIns(3,4,5)P3, but does not affect the amplitude of PtdIns(3,4,5)P3 induction. We have previously shown that PTEN loss leads to the preferential expansion of the MZ and B-1 B cell compartments, and complements CD19 deficiency (Anzelon et al., 2003). Here we show that loss of PTEN supports B cell maturation and function in BAFF-deficient mice. Interestingly, the distribution of peripheral B cell subsets in PtenL/LBaff−/−Cd19Cre mice is more similar to wild type animals than to PtenL/LBaff+/+Cd19Cre mice, suggesting that PTEN loss is not masking residual B cell defects in Baff−/− mice. Moreover, unlike ectopic Bcl-2 expression (Rahman and Manser, 2004; Tardivel et al., 2004), the partial rescue of the BAFF defect is not confined to enhanced B cell survival, but also extends to B cell differentiation and antigen-dependent responses. That said, a full restoration of the mature recirculating B cell pool is not observed in PtenL/LBaff−/−Cd19Cre mice, likely reflecting the importance of IKK1 activity at the transitional B cell stage. This hypothesis is further supported by the phenotype of Cd19Cre/CreIkk1L/L double-deficient mice, underscoring a synergistic relationship of CD19/PI3K and IKK1 signaling.
BAFF induces the transcription of the pro-survival factors, A1, Bcl-xL, and Pim2 (Enzler et al., 2006; Hatada et al., 2003; Hsu et al., 2002). Consistent with the role of BAFF in the generation of T2 B cells, early studies of Bcl-xL−/− mice revealed a reduced percentage of IgM+IgD− B cells (Motoyama et al., 1995). However, the B cells that overcome this bottleneck exhibit normal survival as mature recirculating cells (Motoyama et al., 1995), which may be similar to the phenotype we observed in Ikk1L/LCd19Cre mice. While Pim2−/− and NF-kB2−/− B cells showed similar defects in BAFF-mediated survival in vitro (Enzler et al., 2006), inactivation of all three Pim genes resulted in only a subtle defect in peripheral B cells in younger mice (Mikkers et al., 2004). Induction of A1 transcription by BAFF is not strictly correlated with increased protein expression (Hatada et al., 2003). Moreover, A1 represents a quartet of highly similar genes – of which A1a has been shown to be dispensable for BAFF-mediated survival, suggesting that A1 induction by BAFF may not be critical (Hatada et al., 2003).
Mcl-1 has been linked to BAFF signaling (Giltiay et al., 2010; Woodland et al., 2008), but it is not a transcriptional target of NF-κB. Mcl-1 protein is extremely labile and earlier studies have shown that it is essential for early B cell generation (Opferman et al., 2003). More recently, Vikstrom et al. have demonstrated that Mcl-1 is essential for GC and, to a lesser extent, follicular B cell survival (Vikstrom et al., 2010). By contrast, loss of Bcl-xL is inconsequential for GC B cell differentiation and survival (Vikstrom et al., 2010). We show that PTEN loss promotes Mcl-1 expression, likely due to inactivation of GSK-3 by Akt and resultant disruption of GSK-3-dependent Mcl-1 degradation (Maurer et al., 2006). Thus, our data suggest that Mcl-1 regulation is an important target of PI3K-mediated survival in mature B cells.
Inhibition of the PI3K pathway is of broad interest for applications in oncology, including the treatment of B cell malignancies. The first-in-class small molecule inhibitor GS-1101 is selective for p110δ and has met with considerable success in the clinic, now entering phase 3 clinical trials for the treatment of B cell chronic lymphocytic leukemia (CLL). In addition, phase 2 trials are underway for the use of GS-1101 in the treatment of indolent B-cell non-Hodgkin lymphoma (B-NHL) (follicular lymphoma, small lymphocytic lymphoma, lymphoplasmacytoid lymphoma, marginal zone lymphoma). The efficacy of these inhibitors is largely attributed to the inhibition of BCR-mediated signaling. However, our findings suggest a reappraisal of the molecular basis of these BCR-targeting strategies as possibly reflecting the consequences of impaired BAFF-R signaling that may nonetheless be acting through the BCR complex. As such, BAFF depletion regimens may be effective in combined therapies with small molecule inhibitors targeting BCR signaling. Based upon the mouse lymphoma studies presented here, we would also predict that BAFF-depletion therapy would not be effective in lymphoma cases where PI3K signaling is elevated.
Supplementary Material
Highlights.
IKK1 loss creates a bottleneck but not an impasse in transitional B cell maturation
Acute BAFF-dependent survival of mature B cells is IKK1-independent
Co-inactivation of Cd19 and Ikk1 prevents the generation of mature B cells
Activation of the PI3K pathway partially rescues B cell defects in Baff−/− mice
Acknowledgments
We thank the members of the Rickert laboratory for discussions, and Dr. D. Nemazee (TSRI, La Jolla, CA) for facilitating the transfer of the Baff−/− mice. Work was supported by NIH AI041649, HL088686, and RR026280 (to R.C.R); AI043603 and AR44077 (to M.J.S.); and AI49993 (to G.A.B). G.A.B. received resources and use of facilities from the Iowa City VAMC. A.V.M. was supported by NIH F32 fellowship CA132350. J.J. was supported by fellowships from the Deutsche Forschungsgemeinschaft and the Arthritis Nartional Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4:287–294. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- Balkhi MY, Willette-Brown J, Zhu F, Chen Z, Liu S, Guttridge DC, Karin M, Hu Y. IKKalpha-mediated signaling circuitry regulates early B lymphopoiesis during hematopoiesis. Blood. 2012;119:5467–5477. doi: 10.1182/blood-2012-01-401547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracho GV, Miletic AV, Omori SA, Cato MH, Rickert RC. Emergence of the PI3-kinase pathway as a central modulator of normal and aberrant B cell differentiation. Curr Opin Immunol. 2011;23:178–183. doi: 10.1016/j.coi.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S, Kim H, Kearns JD, Tergaonkar V, O’Dea E, Werner SL, Benedict CA, Ware CF, Ghosh G, Verma IM, Hoffmann A. A fourth IkappaB protein within the NF-kappaB signaling module. Cell. 2007;128:369–381. doi: 10.1016/j.cell.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamito M, Juntilla MM, Thomas M, Northrup DL, Rathmell J, Birnbaum MJ, Koretzky G, Allman D. Akt1 and Akt2 promote peripheral B-cell maturation and survival. Blood. 2010;115:4043–4050. doi: 10.1182/blood-2009-09-241638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TD, Gardam S, Gatto D, Turner VM, Silke J, Brink R. In vivo control of B-cell survival and antigen-specific B-cell responses. Immunological Reviews. 2010;237:90–103. doi: 10.1111/j.1600-065X.2010.00942.x. [DOI] [PubMed] [Google Scholar]
- Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-[kappa]B2 in maturing B cells. Nat Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, Humphries LA, Rawlings D, Reynolds H, Vigorito E, Turner M. A Crucial Role for the p110{delta} Subunit of Phosphatidylinositol 3-Kinase in B Cell Development and Activation. J Exp Med. 2002;196:753–763. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- Dengler HS, Baracho GV, Omori SA, Bruckner S, Arden KC, Castrillon DH, DePinho RA, Rickert RC. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nat Immunol. 2008;9:1388–1398. doi: 10.1038/ni.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P, Zhou LJ, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39–50. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- Enzler T, Bonizzi G, Silverman GJ, Otero DC, Widhopf GF, Anzelon-Mills A, Rickert RC, Karin M. Alternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25:403–415. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Giltiay NV, Lu Y, Allman D, Jorgensen TN, Li X. The adaptor molecule Act1 regulates BAFF responsiveness and self-reactive B cell selection during transitional B cell maturation. J Immunol. 2010;185:99–109. doi: 10.4049/jimmunol.0903312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L, Cutler AH, Thill G, Miklasz SD, Shea DE, Ambrose C, Bixler SA, Su L, Scott ML, Kalled SL. Cutting edge: BAFF regulates CD21/35 and CD23 expression independent of its B cell survival function. J Immunol. 2004;172:762–766. doi: 10.4049/jimmunol.172.2.762. [DOI] [PubMed] [Google Scholar]
- Hatada EN, Do RKG, Orlofsky A, Liou HC, Prystowsky M, MacLennan ICM, Caamano J, Chen-Kiang S. NF-{kappa}B1 p50 Is Required for BLyS Attenuation of Apoptosis but Dispensable for Processing of NF-{kappa}B2 p100 to p52 in Quiescent Mature B Cells. J Immunol. 2003;171:761–768. doi: 10.4049/jimmunol.171.2.761. [DOI] [PubMed] [Google Scholar]
- Henley T, Kovesdi D, Turner M. B-cell responses to B-cell activation factor of the TNF family (BAFF) are impaired in the absence of PI3K delta. European Journal of Immunology. 2008;38:3543–3548. doi: 10.1002/eji.200838618. [DOI] [PubMed] [Google Scholar]
- Hildebrand JM, Luo Z, Manske MK, Price-Troska T, Ziesmer SC, Lin W, Hostager BS, Slager SL, Witzig TE, Ansell SM, et al. A BAFF-R mutation associated with non-Hodgkin lymphoma alters TRAF recruitment and reveals new insights into BAFF-R signaling. The Journal of Experimental Medicine. 2010;207:2569–2579. doi: 10.1084/jem.20100857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting Edge: BLyS Enables Survival of Transitional and Mature B Cells Through Distinct Mediators. Journal of Immunology. 2002;168:5993–5996. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- Huang X, Di Liberto M, Cunningham AF, Kang L, Cheng S, Ely S, Liou HC, Maclennan IC, Chen-Kiang S. Homeostatic cell-cycle control by BLyS: Induction of cell-cycle entry but not G1/S transition in opposition to p18INK4c and p27Kip1. Proc Natl Acad Sci U S A. 2004;101:17789–17794. doi: 10.1073/pnas.0406111101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisho T, Takeda K, Tsujimura T, Kawai T, Nomura F, Terada N, Akira S. I{{kappa}}B Kinase {{alpha}} Is Essential for Mature B Cell Development and Function. J Exp Med. 2001;193:417–426. doi: 10.1084/jem.193.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French DM, Grewal IS, Cochran AG, Gordon NC, Yin J, et al. BAFF/BLyS Receptor 3 Binds the B Cell Survival Factor BAFF Ligand through a Discrete Surface Loop and Promotes Processing of NF-[kappa]B2. Immunity. 2002;17:515–524. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- Khalil AM, Cambier JC, Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012;336:1178–1181. doi: 10.1126/science.1213368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Liu B, Xia X, Zhu F, Park E, Carbajal S, Kiguchi K, DiGiovanni J, Fischer SM, Hu Y. IKK[alpha] Is Required to Maintain Skin Homeostasis and Prevent Skin Cancer. Cancer Cell. 2008;14:212–225. doi: 10.1016/j.ccr.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunological Reviews. 2010;237:205–225. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med. 2008;205:155–168. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkers H, Nawijn M, Allen J, Brouwers C, Verhoeven E, Jonkers J, Berns A. Mice Deficient for All PIM Kinases Display Reduced Body Size and Impaired Responses to Hematopoietic Growth Factors. Molecular and Cellular Biology. 2004;24:6104–6115. doi: 10.1128/MCB.24.13.6104-6115.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletic AV, Anzelon-Mills AN, Mills DM, Omori SA, Pedersen IM, Shin DM, Ravetch JV, Bolland S, Morse HC, 3rd, Rickert RC. Coordinate suppression of B cell lymphoma by PTEN and SHIP phosphatases. J Exp Med. 2010;207:2407–2420. doi: 10.1084/jem.20091962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Hayes CE. Phenotypic and genetic characterization of a unique B lymphocyte deficiency in strain A/WySnJ mice. Eur J Immunol. 1991;21:1123–1130. doi: 10.1002/eji.1830210506. [DOI] [PubMed] [Google Scholar]
- Mills DM, Bonizzi G, Karin M, Rickert RC. Regulation of late B cell differentiation by intrinsic IKKalpha-dependent signals. Proc Natl Acad Sci U S A. 2007;104:6359–6364. doi: 10.1073/pnas.0700296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, Rickert RC. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25:545–557. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- Otipoby KL, Sasaki Y, Schmidt-Supprian M, Patke A, Gareus R, Pasparakis M, Tarakhovsky A, Rajewsky K. BAFF activates Akt and Erk through BAFF-R in an IKK1-dependent manner in primary mouse B cells. Proceedings of the National Academy of Sciences. 2008;105:12435–12438. doi: 10.1073/pnas.0805460105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC{beta}- and Akt-dependent mechanism. The Journal of Experimental Medicine. 2006;203:2551–2562. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman ZS, Manser T. B cells expressing Bcl-2 and a signaling-impaired BAFF-specific receptor fail to mature and are deficient in the formation of lymphoid follicles and germinal centers. J Immunol. 2004;173:6179–6188. doi: 10.4049/jimmunol.173.10.6179. [DOI] [PubMed] [Google Scholar]
- Rahman ZS, Rao SP, Kalled SL, Manser T. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med. 2003;198:1157–1169. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadani F, Bolland DJ, Garcon F, Emery JL, Vanhaesebroeck B, Corcoran AE, Okkenhaug K. The PI3K Isoforms p110{alpha} and p110{delta} Are Essential for Pre-B Cell Receptor Signaling and B Cell Development. Sci Signal. 2010;3:ra60. doi: 10.1126/scisignal.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Zarnegar B, Ytterberg AJ, Shiba T, Dempsey PW, Ware CF, Loo JA, Cheng G. Negative feedback in noncanonical NF-kappaB signaling modulates NIK stability through IKKalpha-mediated phosphorylation. Sci Signal. 2010;3:ra41. doi: 10.1126/scisignal.2000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunological Reviews. 2011;244:115–133. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert RC, Rajewsky K, Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- Rowland SL, Leahy KF, Halverson R, Torres RM, Pelanda R. BAFF Receptor Signaling Aids the Differentiation of Immature B Cells into Transitional B Cells following Tonic BCR Signaling. The Journal of Immunology. 2010;185:4570–4581. doi: 10.4049/jimmunol.1001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Calado DP, Derudder E, Zhang B, Shimizu Y, Mackay F, Nishikawa S-i, Rajewsky K, Schmidt-Supprian M. NIK overexpression amplifies, whereas ablation of its TRAF3-binding domain replaces BAFF:BAFF-R-mediated survival signals in B cells. Proceedings of the National Academy of Sciences. 2008;105:10883–10888. doi: 10.1073/pnas.0805186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol. 2004;173:2245–2252. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-[kappa]B Activity, Dispensable for B Cell Development, Replaces BAFF-Receptor Signals and Promotes B Cell Proliferation upon Activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Shinners NP, Carlesso G, Castro I, Hoek KL, Corn RA, Woodland RT, Scott ML, Wang D, Khan WN. Bruton’s tyrosine kinase mediates NF-kappa B activation and B cell survival by B cell-activating factor receptor of the TNF-R family. J Immunol. 2007;179:3872–3880. doi: 10.4049/jimmunol.179.6.3872. [DOI] [PubMed] [Google Scholar]
- Smith SH, Cancro MP. Cutting Edge: B Cell Receptor Signals Regulate BLyS Receptor Levels in Mature B Cells and Their Immediate Progenitors. The Journal of Immunology. 2003;170:5820–5823. doi: 10.4049/jimmunol.170.12.5820. [DOI] [PubMed] [Google Scholar]
- So L, Fruman DA. PI3K signalling in B- and T-lymphocytes: new developments and therapeutic advances. Biochem J. 2012;442:465–481. doi: 10.1042/BJ20112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William C, Tanabe Y, Jessell T, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Developmental Biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, III, Brezski RJ, Treml LS, Jordan KA, Monroe JG, et al. Tonic B cell antigen receptor signals supply an NF-[kappa]B substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, Mak TW, Nakano T. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. Journal of Experimental Medicine. 2003;197:657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardivel A, Tinel A, Lens S, Steiner QG, Sauberli E, Wilson A, Mackay F, Rolink AG, Beermann F, Tschopp J, Schneider P. The anti-apoptotic factor Bcl-2 can functionally substitute for the B cell survival but not for the marginal zone B cell differentiation activity of BAFF. Eur J Immunol. 2004;34:509–518. doi: 10.1002/eji.200324692. [DOI] [PubMed] [Google Scholar]
- Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- Tusche MW, Ward LA, Vu F, McCarthy D, Quintela-Fandino M, Ruland J, Gommerman JL, Mak TW. Differential requirement of MALT1 for BAFF-induced outcomes in B cell subsets. The Journal of Experimental Medicine. 2009;206:2671–2683. doi: 10.1084/jem.20091802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DAA, Bergsagel PL, Karin M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-[kappa]B signaling. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikstrom I, Carotta S, Luthje K, Peperzak V, Jost PJ, Glaser S, Busslinger M, Bouillet P, Strasser A, Nutt SL, Tarlinton DM. Mcl-1 is essential for germinal center formation and B cell memory. Science. 2010;330:1095–1099. doi: 10.1126/science.1191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora KA, Wang LC, Rao SP, Liu ZY, Majeau GR, Cutler AH, Hochman PS, Scott ML, Kalled SL. Cutting edge: germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. J Immunol. 2003;171:547–551. doi: 10.4049/jimmunol.171.2.547. [DOI] [PubMed] [Google Scholar]
- Woodland RT, Fox CJ, Schmidt MR, Hammerman PS, Opferman JT, Korsmeyer SJ, Hilbert DM, Thompson CB. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood. 2008;111:750–760. doi: 10.1182/blood-2007-03-077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnegar B, Yamazaki S, He JQ, Cheng G. Control of canonical NF-kappaB activation through the NIK-IKK complex pathway. Proc Natl Acad Sci U S A. 2008a;105:3503–3508. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh W-c, Mak TW, et al. Noncanonical NF-[kappa]B activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008b;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TT, Makondo KJ, Marshall AJ. p110delta phosphoinositide 3-kinase represses IgE switch by potentiating BCL6 expression. J Immunol. 2012;188:3700–3708. doi: 10.4049/jimmunol.1103302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.