Abstract
Background There is limited evidence for an association between the pattern of lifetime alcohol use and cause-specific risk of death.
Methods Multivariable hazard ratios were estimated for different causes of death according to patterns of lifetime alcohol consumption using a competing risks approach: 111 953 men and 268 442 women from eight countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study were included. Self-reported alcohol consumption at ages 20, 30, 40 or 50 years and at enrolment were used for the analysis; 26 411 deaths were observed during an average of 12.6 years of follow-up.
Results The association between lifetime alcohol use and death from cardiovascular diseases was different from the association seen for alcohol-related cancers, digestive, respiratory, external and other causes. Heavy users (>5 drinks/day for men and >2.5 drinks/day for women), regardless of time of cessation, had a 2- to 5-times higher risk of dying due to alcohol-related cancers, compared with subjects with lifetime light use (≤1 and ≤0.5 drink/week for men and women, respectively). Compared with lifetime light users, men who used <5 drinks/day throughout their lifetime had a 24% lower cardiovascular disease mortality (95% confidence interval 2-41). The risk of death from coronary heart disease was also found to be 34–46% lower among women who were moderate to occasionally heavy alcohol users compared with light users. However, this relationship was only evident among men and women who had no chronic disease at enrolment.
Conclusions Limiting alcohol use throughout life is associated with a lower risk of death, largely due to cardiovascular disease but also other causes. However, the potential health benefits of alcohol use are difficult to establish due to the possibility of selection bias and competing risks related to diseases occurring later in life.
Keywords: Prospective study, lifetime alcohol use, cause-specific mortality, EPIC
Introduction
Alcohol use has been linked to greater risk of death from cancer, diseases of the digestive tract, injuries and violence, and to a lower risk of death from cardiovascular diseases (CVD).1.2 Studies have suggested that the death rate among moderate alcohol users is lower due to fewer deaths resulting from coronary heart disease (CHD).3 The majority of these findings are based on alcohol consumption at the time of enrolment into the study, and only a few studies have collected information on lifetime use of alcohol.3,4 Additionally, it has been suggested that the lower risk seen in alcohol users, compared with non-users, may arise because the non-users comparison group includes never and former users who stopped alcohol consumption because of illness (termed ‘sick–quitters’), as well as those who have other high-risk profiles for illness that may have caused low alcohol tolerance (e.g. family history of alcohol dependency, pre-existing conditions, medications or mental illnesses.5–7). Unmeasured confounding may also explain some of these findings, as people who use moderate amounts of alcohol have multiple favourable characteristics (as compared with non- or heavy users).8–11 Others argue in favour of a causal relationship between alcohol consumption and CHD risk.3 A recent meta-analysis however, showed that quitting the use of alcohol was only associated with higher CHD mortality but not CHD morbidity, compared with abstention from alcohol.4 It is therefore still not well understood whether the cause of death varies according to the magnitude or variability of alcohol use over the life span.
The European Prospective Investigation into Cancer and Nutrition (EPIC) study collected information on the habitual use of alcoholic beverages from participants at ages 20, 30, 40, 50 years and at the time of enrolment into the study. The relationship between patterns of lifetime alcohol use with the risk of death from leading causes (i.e. CVD, cancer, diseases of the digestive or respiratory system, external and other causes) was integrated in a competing risks approach.
Methods
Study population
EPIC is an ongoing multi-centric cohort study. The study has been described in detail previously.12 In brief, the EPIC population consists of sub-cohorts recruited in 23 centres in 10 European countries. From 1992 to 2000, 519 978 men and women, mainly aged 25–70 years, were recruited from the general population residing in surroundings of the study centres. Exceptions were the cohorts of France (female members of a health insurance for school employees), Utrecht (breast cancer screening attendees), Spain (mainly blood donors and their spouses), and Oxford (mainly vegetarian and health-conscious people). Those who accepted the invitation to participate in the study gave informed consent and completed questionnaires on diet, lifestyle and medical history, and most visited an examination centre where a blood sample and body measurements were taken.
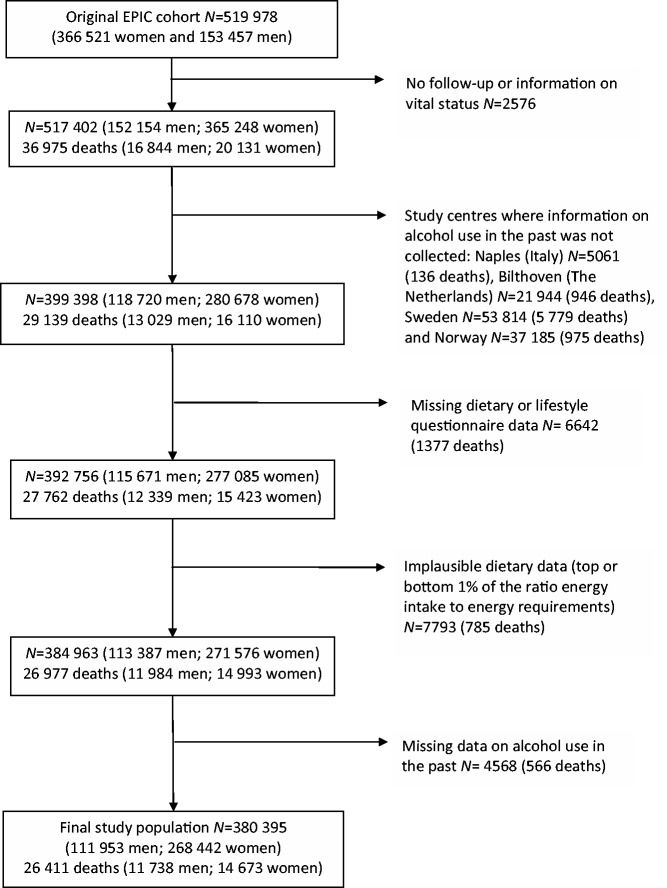
Of the 519 978 participants, a total of 139 583 were excluded (see Figure 1 for more detail), leaving 380 395 available for analysis. These included participants from Denmark (Aarhus, Copenhagen), France, Germany (Heidelberg, Potsdam), Greece, Italy (Florence, Varese, Ragusa, Turin), The Netherlands (Utrecht), Spain (Asturias, Granada, Murcia, Navarra, San Sebastian), and the UK (Cambridge, Oxford) with an average follow-up duration of 12.6 (±2.6) years.
Figure 1.
Study population of the European Prospective Investigation into Cancer and Nutrition study and exclusions in the current analysis
The study was approved by ethical review boards of each of the participating study centres and the International Agency of Research on Cancer in Lyon, France.
Assessment of lifetime alcohol use
Participants reported the number of glasses of wine, beer/cider, fortified wine and spirits they habitually consumed when they were 20, 30, 40 or 50 years old in a standardised lifestyle questionnaire. Further, a semi-quantitative centre-specific dietary questionnaire included questions on alcoholic beverages consumed during 12 months prior to enrolment. Glasses of alcoholic beverages reported in both questionnaires were converted into grams of alcohol per day (g/day) by applying empirically derived definitions of standard drinks for each beverage and country of EPIC. This approach was adopted because the application of common standard drinks does not take gender and the variation of alcohol content, glass size and filling level into account, leading to less accurate individual estimates of alcohol consumption.13 The calculation of beverage-specific standard drinks was based on detailed information from 36 181 24-hour dietary recalls14 of which 17 978 (from 8223 men and 9755 women) contained data on consumed alcoholic beverages. For each point in time, the sum of g/day alcohol from wine, beer and spirits was calculated based on the alcohol content of one glass of each alcoholic beverage multiplied by the reported frequency of consumption per week. This was divided by seven to give grams per day and gender-specific cut-off points were applied to each point in time (Figure 2). The cut-off points were chosen to facilitate interpretation of the gram-based alcohol categories (as the number of drinks assuming that an average alcohol content of one glass of any alcoholic beverage is 12 grams15) and according to recommended upper limits of daily alcohol consumption of one drink/day for women and two drinks/day for men.16–18 Participants were then categorised as never users, former users or lifetime users of alcohol. The exclusive categories characterising the pattern of lifetime alcohol use was then defined based on the variation of alcohol consumption over time (Figure 2).
Figure 2.
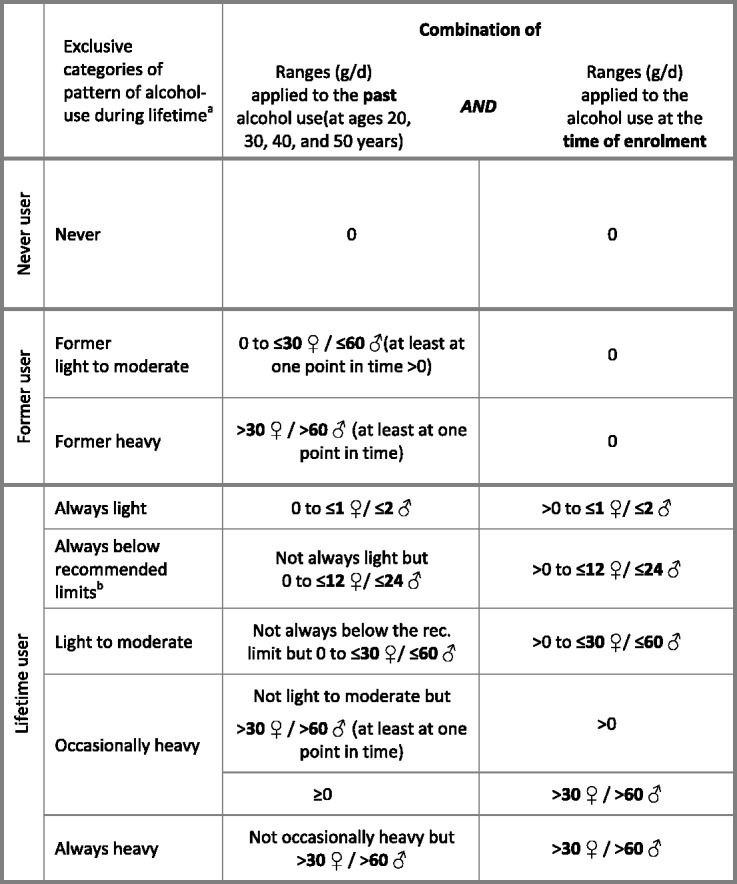
Definition of exclusive categories of pattern of alcohol use during lifetime a(American Heart Association; World Cancer Research Fund/American Institute for Cancer Research; National Institute of Alcoholism and Alcohol Abuse)
Assessment of other lifestyle variables
Information on smoking status and duration, non-occupational physical activity, medical history and educational attainment was obtained using standardised questions in all study centres. Body measures (height, weight and circumferences) were mostly taken with individuals wearing light clothing and no shoes, or were self-reported (Oxford, France).19 Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Dietary data were collected with centre-specific questionnaires and calibrated across centres using 24-hour dietary recall information.20
Assessment of causes of death
Participants were followed from study entry until death, emigration, withdrawal or the end of the follow-up period, as appropriate. Vital status and information on cause and date of death were ascertained using record linkages with cancer registries, boards of health, death registries (Denmark, Italy, The Netherlands, Spain, the UK), or by active follow-up (France Germany, Greece). Germany and Greece identified deceased individuals from undeliverable follow-up mailings and subsequent enquiries to municipality registries, regional health departments, physicians or hospitals. In France, information on deceased participants was obtained using the database of health insurance for school employees and the national death index. Data were coded using the 10th Revision of the International Classification of Diseases, Injuries and Causes of Death (ICD-10) where the underlying cause is the official cause of death. Due to variations in procedures used to check vital status in each of the participating centres, follow-up dates were truncated at the date at which more than 80% of the causes were known. Among the 380 395 eligible participants, 26 411 deaths were identified during the study period. Of these: 2587 were from CHD (I20-I25); 3322 from CVD other than CHD (I00-I99 excl. I20-I25); 2764 from alcohol-related cancers (C01-C06, C09-C15, C18-22; C32; C50 in women); 7789 from other neoplasms (malignant, uncertain, benign: C00-D48 excl. C01-C06, C09-C15, C18-22; C32); 1125 from diseases of the respiratory tract (J00-J99); 572 from diseases of the digestive system [K00-K93 (excl. K292, K70, K860)]; 972 from external causes, such as accidents, suicide, and homicide (S00-T98, V01-Y98) and 2404 from other causes, among them diseases of the nervous system [F00-F99 (excl. F10), G00-G99 (excl. G312, G4051, G621, G721): N = 706] or conditions directly related to alcohol (F10, G312, G4051, G621, G721, I426, K292, K70, K860, R78, X45, X65, Y15, Y919: N = 266). A total of 340 deaths of unknown cause (R96-R99) and 4536 deaths where the information on cause was missing (coded as ‘cause missing’) were combined into one category. Results for this group are not presented.
Statistical analysis
Hazard ratios (HR) of death across categories of lifetime alcohol use were estimated using Cox proportional hazards regression; extended analyses were done using a competing risks approach. Here, a single statistical model was fitted assuming the association between alcohol use and each cause of death is different, based on the approach described by Lunn and McNeil,21 which stratifies on event type and allows for estimation of the separate associations of each lifetime alcohol use with the relative hazard of each outcome under a proportional hazards assumption. In contrast, the relationship between each confounding variable was assumed to be homogeneous across different causes of death. To test for differences across different causes of death, the competing risks model was compared with a model where the association of alcohol with cause-specific death was held constant across outcomes. Models were compared using the likelihood-ratio test, with P-values based on the chi-square distribution, <0.05 considered statistically significant.
To investigate the shape of the relationship between alcohol use among consumers at enrolment and risk of each type of cause of death, we used restricted cubic spline regression with knots at g/day alcohol of 0.86, 2, 24, 60 (men) or 0.35, 1, 12, 30 (women). The first knot (based on the 5th percentile) represented very low alcohol consumption, whereas the other knots corresponded to the cut-off points as applied to the pattern variables (see Figure 2). To test the robustness of the shape of the association, models were also done using knots based on 5th, 25th, 75th and 95th percentiles (men: 0.86, 6.84, 35.5, 71.0 g/day; women: 0.35, 1.81, 13.6, 36.0 g/day); the shapes of the curves remained unchanged (data not shown).
Both types of models used light alcohol users as the reference group and were stratified by age at enrolment (in 1-year categories) and study centre to reduce the likelihood of potential violations to the proportional hazards assumption. Age was the underlying time variable in all analyses. Entry time was defined as the participant’s age at enrolment and exit time as age at death or censoring, whichever came first. All models were further adjusted for: smoking status [never smokers, smokers at enrolment of <15, 15–24, >24 cigarettes/day, other smoking (pipe, cigar etc.), former smokers for ≤10, 11–20, >20 years, unknown]; smoking duration (≤10, 10–20, 20–30, 31–40, >40 years, unknown); educational attainment (no graduation or primary school, technical or professional school, secondary school, university degree, unknown); household and recreational physical activity [gender-specific quartiles of metabolic equivalent (MET) h/week: low, medium, high, very high, unknown]; BMI (kg/m2, continuous); height (cm, continuous); waist circumference (cm, continuous); and intake of red meat and meat products, fruit and vegetables and dietary fibre (g/day, continuous). In women, the models were additionally adjusted for the number of live births (0, 12, 3–4, 5–6, ≥7), menopausal status (pre-, post-, peri-, surgical postmenopausal), and menopausal hormone use (yes, no, unknown). The main models on lifetime alcohol use were not adjusted for prevalent diseases (self-reported myocardial infarction, stroke, cancer, diabetes, and hypertension), although we conducted subgroup analyses of specific causes of death (i.e. CVD, cancer, other) by disease status at enrolment (yes/no). Differently from models with lifetime alcohol use, alcohol consumption at enrolment was modelled in two ways: first adjusting for prevalent diseases (to account for lower alcohol use due to sickness) and secondly by excluding diseased participants (to be consistent with previous studies using information on alcohol use at enrolment). We additionally investigated the association of alcohol consumption at the time of enrolment and risk of death by stratifying by past alcohol use and using recommended limits as cut-off points (men ≤24 g/day, women ≤12 g/day):16–18 alcohol consumption, below the limit, both at enrolment and in the past (reference); below the limit at enrolment and above in the past; above the limit at enrolment and below in the past; and above the limit both at enrolment and in the past. These models used the same stratification by age and adjustments as previous models (including adjustment for prevalent diseases).
To rule out a potential reporting bias of past alcohol use due to bad health at enrolment, a shorter lifetime period of potential alcohol exposure, or different cultural backgrounds of alcohol use in European regions included in this analysis, we conducted a sensitivity analysis that excluded deaths that occurred (i) during the first 3 years of follow-up, (ii) participants ≤50 years of age and (iii) stratifying by geographical region [North Europe including Denmark, Germany, The Netherlands and the UK vs South Europe including France (women only), Italy, Spain, Greece].
Finally, we calculated the rate advancement periods (RAP) for all-cause mortality applying similar (fully adjusted) Cox models with follow-up period as the underlying time variable and age at recruitment as a covariate.22 The RAP is defined as the average time period (in years) by which the death rate is advanced (i.e. number of years lost, compared with lifetime light alcohol use), conditional on survival to the age at enrolment.
The analyses were conducted separately for men and women because they metabolise alcohol differently and have different levels of alcohol consumption and associated socio-demographic factors, which may modify the relationship between alcohol use and death.23,24 A test for interaction by gender was performed by comparing the difference of log-likelihood of models with and without the interaction term with the chi-square distribution with degrees of freedom equal to the number of terms. The test for trend across categories of lifetime alcohol use was based on the median alcohol consumption at enrolment in each category. Analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
Results
Description of alcohol users
Among men, 98% had used alcohol at some point during their lifetime (N = 110 162) and of those the majority (96%) were alcohol users at the time of enrolment (Table 1). Most of these men (80%) were always light users (3%), always below the recommended limit (39%) or light to moderate users (38%). About one-fifth of alcohol users reported heavy alcohol use at some point in their lifetime. Of the non-users at enrolment (N = 6718), 73% were former alcohol users. Men who were lifetime light, below the recommended limit, or light to moderate alcohol users were more highly educated than lifetime or former heavy users. Lifetime heavy users were more likely to be younger, a long-term smoker, to have a higher meat intake and to have more abdominal obesity than lifetime light, below the recommended limit, or light to moderate alcohol users. Comparably, former heavy users were more likely to be heavy smokers and to report a prevalent disease.
Table 1.
Frequency distribution and description of characteristics at enrolment across categories of lifetime pattern of alcohol use among men participating in the EPIC study
| Formerb |
Lifetimec |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Exclusive categories of pattern of alcohol use | TOTAL | Nevera | Former light to moderated | Former heavye | Lightf | Below rec. limitg | Light to moderatee | Occasionally heavye | Heavye |
| Number | 111 953 | 1791 | 3476 | 1451 | 3325 | 41 396 | 40 286 | 18 168 | 2060 |
| Follow-up time (mean, (SD), years) | 11.8 (2.7) | 11.8 (3.3) | 12.1 (3.4) | 11.1 (3.5) | 11.8 (3.0) | 11.8 (2.6) | 11.8 (2.5) | 11.7 (2.8) | 12.0 (2.9) |
| Age (mean, (SD), years) | 53.2 (9.5) | 53.9 (11.0) | 56.5 (9.9) | 54.5 (8.7) | 53.4 (13.4) | 53.5 (10.0) | 52.7 (8.9) | 53.0 (8.4) | 51.1 (8.0) |
| BMI (mean, (SD)) | 26.8 (3.7) | 27.3 (3.7) | 27.0 (3.8) | 27.7 (4.4) | 25.9 (4.0) | 26.4 (3.5) | 26.7 (3.5) | 27.6 (3.8) | 28.5 (4.1) |
| Waist circumference (mean, (SD) cm) | 95.1 (10.1) | 96.3 (10.3) | 95.9 (10.4) | 97.7 (11.4) | 92.7 (10.5) | 93.9 (9.8) | 94.9 (9.7) | 97.7 (10.3) | 100.1 (10.7) |
| Fruit and vegetable intake (median, g/d) | 388.6 | 491.2 | 429.7 | 446.7 | 443.5 | 362.1 | 387.2 | 419.1 | 477.9 |
| Intake of red meat and meat products (median, g/d) | 109.2 | 88.2 | 94.2 | 124.0 | 64.2 | 101.7 | 112.4 | 125.0 | 143.9 |
| Fibre intake (mean, (SD), g/d) | 24.6 (4.8) | 24.3 (4.7) | 23.7 (5.4) | 24.9 (5.0) | 25.9 (5.4) | 24.7 (4.9) | 24.6 (4.6) | 24.3 (4.7) | 24.7 (4.4) |
| University degree (%) | 27.9 | 19.7 | 13.1 | 11.8 | 32.4 | 31.9 | 29.8 | 20.7 | 10.3 |
| High/very high physical activityh(%) | 49.9 | 41.7 | 47.6 | 49.9 | 49.4 | 50.6 | 50.6 | 48.8 | 41.1 |
| Smokers (%) | 30.1 | 27.9 | 26.2 | 43.2 | 18.8 | 23.7 | 30.8 | 42.5 | 53.4 |
| >20 years of smoking (%) | 46.3 | 37.1 | 48.3 | 68.6 | 25.9 | 38.2 | 48.1 | 61.0 | 66.1 |
| Prevalent diseasesi (%) | 26.3 | 26.4 | 34.7 | 43.0 | 23.6 | 23.5 | 25.7 | 31.1 | 31.0 |
| Alcohol at enrolment (median, g/d) | 15.1 | 0 | 0 | 0 | 0.7 | 8.5 | 27.0 | 47.3 | 82.6 |
| Alcohol at age 20 y (median, g/d) | 11.3 | 0 | 9.6 | 77.8 | 0.0 | 5.6 | 18.1 | 45.9 | 99.8 |
| Number of deaths | 11 738 | 240 | 698 | 320 | 441 | 4061 | 3486 | 2235 | 257 |
| Age at death (mean (max.), years) | 69.0 (102.4) | 73.4 (97.5) | 72.8 (94.5) | 66.5 (87.0) | 76.3 (102.4) | 70.6 (100.0) | 67.5 (100.8) | 66.1 (91.4) | 63.2 (87.1) |
BMI, body mass index; MET, metabolic equivalent; rec., recommended.
a Report of no use of alcohol at all points in time (age 20, 30, 40, 50 and at recruitment).
b Report of alcohol use in the past but not at the time of enrolment.
c Report of use of alcohol at past points in time and at the time of recruitment.
d ≤60 g/d.
e >60 g/d.
f ≤2 g/d.
g ≤24 g/d (American Heart Association; World Cancer Research Fund/American Institute for Cancer Research, National Institute on Alcohol Abuse and Alcoholism).
h High physical activity based on METS of recreational and housekeeping activities (>56.78).
i Self-reported cancer, myocardial infarction, stroke, diabetes mellitus or hypertension.
In women, 15% were non-users of alcohol at enrolment, most of whom (68%) were never users (Table 2). Among lifetime alcohol users (N = 227 705), the vast majority (88%) were light (15%), below the recommended limit (44%), or light to moderate alcohol users (29%). Women with heavy alcohol consumption were on average younger, more likely to be highly educated, to be a smoker and to have a low intake of fruits and vegetables, and were less likely to report a prevalent disease than all other types of alcohol users. Among women who had used alcohol at some point during their lifetime (N = 240 767), 12% had used it heavily at least at one of these points. Similarly to men, a higher proportion of women who were former heavy alcohol users reported a prevalent disease or long-term smoking.
Table 2.
Frequency distribution and description of characteristics at enrolment across categories of lifetime pattern of alcohol use among women participating in the EPIC study
| Formerb |
Lifetimec |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Exclusive categories of pattern of alcohol use | TOTAL | Nevera | Former light to moderated | Former heavye | Lightf | Below rec. limitg | Light to moderated | Occasionally heavye | Heavye |
| Number | 26 8442 | 27 675 | 12 428 | 634 | 34 009 | 100 711 | 65 787 | 26 207 | 991 |
| Follow-up time (mean, (SD), years) | 13.0 (2.4) | 13.1 (2.7) | 13.0 (2.6) | 12.1 (3.1) | 13.0 (2.6) | 12.9 (2.4) | 13.0 (2.2) | 13.0 (2.3) | 12.7 (2.1) |
| Age (mean, (SD), years) | 51.6 (9.9) | 52.6 (9.5) | 54.5 (9.4) | 53.3 (8.4) | 51.6 (11.0) | 52.0 (9.6) | 50.6 (10.0) | 50.8 (9.5) | 43.8 (11.1) |
| BMI (mean, (SD)) | 25.0 (4.5) | 26.7 (5.4) | 26.8 (5.0) | 25.7 (4.9) | 25.1 (4.8) | 25.0 (4.4) | 24.4 (4.0) | 24.3 (4.0) | 24.2 (4.1) |
| Waist circumference (mean, (SD) cm) | 80.2 (11.5) | 85.7 (12.6) | 85.1 (12.2) | 83.6 (12.7) | 79.8 (11.8) | 79.9 (11.1) | 78.4 (10.5) | 78.8 (10.6) | 77.3 (10.6) |
| Fruit and vegetable intake (median, g/d) | 417.9 | 454.6 | 441.6 | 416.9 | 428.1 | 412.6 | 409.7 | 391.7 | 387.6 |
| Intake of red meat and meat products (median, g/d) | 65.6 | 64.0 | 62.2 | 66.6 | 57.5 | 66.2 | 67.5 | 69.4 | 63.3 |
| Fibre intake (mean, (SD), g/d) | 20.3 (3.7) | 19.3 (3.4) | 19.8 (3.8) | 20.3 (4.2) | 20.1 (3.7) | 20.5 (3.8) | 20.5 (3.7) | 20.2 (3.8) | 19.6 (3.4) |
| University degree (%) | 24.3 | 14.1 | 11.5 | 18.5 | 22.8 | 23.4 | 29.4 | 33.2 | 36.4 |
| High/very high physical activityh(%) | 49.8 | 62.1 | 64.1 | 56.3 | 49.5 | 49.4 | 46.2 | 41.1 | 47.0 |
| Smokers (%) | 16.6 | 12.4 | 15.9 | 39.6 | 11.7 | 15.2 | 18.5 | 27.3 | 38.3 |
| >20 years of smoking (%) | 20.2 | 11.1 | 19.4 | 47.4 | 12.9 | 18.9 | 23.8 | 34.4 | 32.5 |
| Prevalent diseasesi (%) | 23.3 | 28.3 | 32.1 | 32.3 | 23.4 | 23.1 | 20.5 | 21.6 | 16.0 |
| Pre-menopausal (%) | 33.9 | 34.1 | 27.0 | 26.0 | 35.1 | 32.6 | 36.1 | 33.9 | 60.4 |
| Nulliparous (%) | 18.6 | 12.6 | 14.1 | 22.4 | 18.7 | 16.7 | 21.8 | 24.7 | 42.8 |
| Hormone use (HRT) (%) | 25.1 | 16.0 | 19.6 | 27.4 | 22.4 | 26.7 | 27.1 | 29.8 | 16.0 |
| Alcohol at enrolment (median, g/d) | 4.3 | 0 | 0 | 0 | 0.7 | 4.3 | 14.2 | 33.3 | 45.2 |
| Alcohol at age 20 y (median, g/d) | 0.5 | 0 | 2.4 | 18.3 | 0.0 | 1.2 | 3.7 | 5.1 | 39.2 |
| Number of deaths | 14 673 | 1693 | 1136 | 65 | 1992 | 5123 | 3103 | 1522 | 39 |
| Age at death (mean (max.), years) | 68.6 (104.3) | 69.8 (98.2) | 71.0 (91.7) | 63.4 (84.0) | 70.5 (104.3) | 68.7 (100.5) | 66.9 (99.4) | 66.0 (91.0) | 60.9 (91.0) |
BMI, body mass index; HRT, hormone replacement therapy; MET, metabolic equivalent, rec., recommended.
a Report of no use of alcohol at all points in time (age 20, 30, 40, 50 and at recruitment).
b Report of alcohol use in the past but not at the time of enrolment.
c Report of use of alcohol at past points in time and at the time of recruitment.
d ≤30 g/d.
e >30 g/d.
f ≤1 g/d.
g ≤12 g/d; (American Heart Association; World Cancer Research Fund/American Institute for Cancer Research, National Institute on Alcohol Abuse and Alcoholism).
h High physical activity based on METS of recreational and housekeeping activities (>82.43).
i Self-reported cancer, myocardial infarction, stroke, diabetes mellitus or hypertension.
Cause-specific mortality according to lifetime alcohol use
Compared with men who had used alcohol lightly throughout their lifetime up to enrolment, men who were former heavy users had higher relative risks of death for the largest number of causes, and this was most pronounced for alcohol-related cancers (5-fold increased risk), other causes (3.1-fold), diseases of the digestive (2.7-fold) or respiratory system (2.4-fold), but also for other neoplasms (1.8-fold), CHD and other CVD (1.7- and 1.6-fold, respectively) (Table 3). Former light to moderate users also had a higher risk of death from alcohol-related and other cancers and other causes (1.4–2.4-fold). Among lifetime alcohol users, men who were heavy or occasional heavy users had a higher risk of death from alcohol-related and other cancers, digestive diseases and external and other causes.
Table 3.
Hazard rate ratios of cause specific deaths across categories of lifetime pattern of alcohol use among men participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study
| Exclusive categories of pattern of alcohol use |
Formerb |
Lifetimec |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cause of death | Nevera | Former light to moderated | Former heavye | Lightf | Below rec. limitsg | Light to moderated | Occasionally heavye | Heavye | P for trendh |
| Person-years | 21 087 | 41 918 | 16 108 | 39 168 | 490 252 | 477 358 | 212 821 | 24 645 | |
| CHDk | |||||||||
| Deaths N = 1755 | 43 | 103 | 48 | 92 | 665 | 502 | 278 | 24 | |
| HRi | 1.39 | 1.45 | 2.49 | REF | 1.01 | 1.05 | 1.34 | 1.26 | 0.001 |
| (95% CI) | (0.95; 2.02) | (1.06; 1.98) | (1.71; 3.62) | (0.79; 1.28) | (0.82; 1.35) | (1.03; 1.75) | (0.78; 2.04) | ||
| HRj | 1.25 | 1.18 | 1.66 | REF | 0.90 | 0.86 | 0.92 | 0.77 | 0.462 |
| (95% CI) | (0.85; 1.83) | (0.86; 1.61) | (1.13; 2.42) | (0.71; 1.15) | (0.67; 1.11) | (0.70; 1.20) | (0.48; 1.25) | ||
| CVDl | |||||||||
| Deaths N = 1506 | 36 | 103 | 46 | 86 | 484 | 443 | 277 | 31 | |
| HRi | 1.13 | 1.59 | 2.31 | REF | 0.80 | 0.92 | 1.26 | 1.66 | <0.001 |
| (95% CI) | (0.76; 1.69) | (1.16; 2.19) | (1.57; 3.41) | (0.62; 1.03) | (0.71; 1.19) | (0.96; 1.66) | (1.07; 2.59) | ||
| HRj | 1.00 | 1.28 | 1.62 | REF | 0.72 | 0.76 | 0.91 | 1.10 | 0.007 |
| (95% CI) | (0.68; 1.49) | (0.93; 1.77) | (1.10; 2.40) | (0.56; 0.92) | (0.59; 0.98) | (0.69; 1.20) | (0.70; 1.71) | ||
| Alcohol-related cancerm | |||||||||
| Deaths N = 901 | 9 | 37 | 41 | 19 | 248 | 282 | 224 | 41 | |
| HRi | 0.98 | 2.06 | 6.78 | REF | 1.20 | 1.53 | 2.83 | 5.68 | <0.001 |
| (95% CI) | (0.42; 2.30) | (1.13; 3.75) | (3.77; 12.2) | (0.73; 1.97) | (0.92; 2.53) | (1.70; 4.71) | (3.11; 10.4) | ||
| HRj | 0.90 | 1.77 | 5.02 | REF | 1.10 | 1.28 | 2.05 | 3.82 | <0.001 |
| (95% CI) | (0.38; 2.09) | (0.98; 3.20) | (2.80; 8.99) | (0.67; 1.81) | (0.78; 2.11) | (1.23; 3.40) | (2.09; 6.97) | ||
| Other neoplasmsn | |||||||||
| Deaths N = 3422 | 54 | 157 | 82 | 99 | 1168 | 1089 | 692 | 81 | |
| HRi | 1.24 | 1.64 | 2.63 | REF | 1.17 | 1.27 | 1.81 | 2.26 | <0.001 |
| (95% CI) | (0.88; 1.74) | (1.25; 2.15) | (1.93; 3.58) | (0.94; 1.45) | (1.02; 1.58) | (1.44; 2.27) | (1.65; 3.10) | ||
| HRj | 1.17 | 1.41 | 1.80 | REF | 1.10 | 1.09 | 1.32 | 1.49 | <0.001 |
| (95% CI) | (0.83; 1.64) | (1.08; 1.85) | (1.32; 2.45) | (0.88; 1.36) | (0.88; 1.36) | (1.05; 1.66) | (1.09; 2.04) | ||
| Respiratory causeo | |||||||||
| Deaths N = 517 | 13 | 33 | 24 | 40 | 183 | 131 | 83 | 10 | |
| HRi | 1.24 | 1.25 | 4.09 | REF | 0.84 | 0.95 | 1.44 | 2.21 | <0.001 |
| (95% CI) | (0.63; 2.42) | (0.74; 2.11) | (2.28; 7.33) | (0.56; 1.25) | (0.62; 1.44) | (0.91; 2.27) | (1.03; 4.76) | ||
| HRj | 1.04 | 0.86 | 2.35 | REF | 0.70 | 0.68 | 0.87 | 1.16 | 0.091 |
| (95% CI) | (0.54; 2.00) | (0.51; 1.45) | (1.30; 4.23) | (0.47; 1.05) | (0.45; 1.04) | (0.55; 1.39) | (0.53; 2.57) | ||
| Digestive causep | |||||||||
| Deaths N = 240 | 6 | 17 | 11 | 11 | 69 | 62 | 58 | 6 | |
| HRi | 1.26 | 1.89 | 3.93 | REF | 0.76 | 0.89 | 1.95 | 2.34 | <0.001 |
| (95% CI) | (0.46; 3.47) | (0.81; 4.42) | (1.61; 9.60) | (0.39; 1.50) | (0.44; 1.79) | (0.96; 3.95) | (0.81; 6.71) | ||
| HRj | 1.07 | 1.52 | 2.74 | REF | 0.71 | 0.73 | 1.37 | 1.51 | 0.001 |
| (95% CI) | (0.39; 2.95) | (0.66; 3.48) | (1.10; 6.80) | (0.36; 1.37) | (0.37; 1.47) | (0.68; 2.79) | (0.53; 4.30) | ||
| External causeq | |||||||||
| Deaths N = 500 | 14 | 23 | 11 | 17 | 128 | 160 | 135 | 12 | |
| HRi | 1.91 | 1.48 | 1.68 | REF | 0.70 | 0.93 | 1.69 | 1.36 | <0.001 |
| (95% CI) | (0.94; 3.91) | (0.76; 2.89) | (0.76; 3.74) | (0.41; 1.20) | (0.54; 1.58) | (0.98; 2.91) | (0.63; 2.94) | ||
| HRj | 1.85 | 1.36 | 1.42 | REF | 0.69 | 0.89 | 1.53 | 1.19 | <0.001 |
| (95% CI) | (0.91; 3.76) | (0.69; 2.68) | (0.63; 3.21) | (0.41; 1.19) | (0.52; 1.53) | (0.88; 2.67) | (0.55; 2.59) | ||
| Other causer | |||||||||
| Deaths N = 888 | 19 | 59 | 29 | 43 | 237 | 255 | 211 | 35 | |
| HRi | 1.98 | 2.72 | 4.03 | REF | 0.74 | 1.00 | 2.05 | 4.98 | <0.001 |
| (95% CI) | (1.10; 3.54) | (1.73; 4.27) | (2.40; 6.76) | (0.52; 1.06) | (0.70; 1.44) | (1.41; 2.97) | (3.02; 8.21) | ||
| HRj | 1.80 | 2.41 | 3.12 | REF | 0.73 | 0.93 | 1.60 | 3.54 | <0.001 |
| (95% CI) | (1.00; 3.24) | (1.53; 3.80) | (1.84; 5.30) | (0.51; 1.05) | (0.64; 1.34) | (1.09; 2.35) | (2.13; 5.88) | ||
CHD, coronary heart disease; CVD, cardiovascular disease; HR, hazard rate ratio; REF, reference; rec., recommended.
a Report of no use of alcohol at all points in time (age 20, 30, 40, 50 and at enrolment).
b Report of alcohol use in the past but not at the time of enrolment.
c Report of use of alcohol at past points in time and at the time of enrolment.
d ≤60 g/d.
e >60 g/d.
f ≤2 g/d.
g ≤24 g/d (American Heart Association; World Cancer Research Fund/American Institute for Cancer Research; National Institute of Alcoholism and Alcohol Abuse).
h P for trend across HR of death in lifetime alcohol users employing median alcohol use at the time of enrolment
i Joint Cox proportional hazard model, stratified by age and centre.
j Joint Cox proportional hazard model, as i and adjusted for body mass index, height, waist circumference, intake of fruits, vegetables, red meat and meat products, dietary fibre, physical activity, education and smoking.
k I20–I25.
l I00–I99 excl. I20–I25.
m C01–C06, C09–C15, C18–22; C32.
n C00–D48 excl. C01–C06, C09–C15, C18–22; C32.
o J00-J99.
p K00-K93, excl. K292, K70, K860.
q S00-T98, V01-Y98.
r All causes excl. I00–I99; C00–D48; J00-J99; K00-K93, excl. K292, K70, K860; S00-T98, V01-Y98; and unknown causes.
In contrast, men whose lifetime alcohol consumption remained below the recommended limit or light to moderate had a 28% and 24% lower risk of death from CVD, respectively. No association was seen among always or occasionally heavy users.
Similarly to men, women who were heavy alcohol users throughout their lifetime had higher risks of death from alcohol-related cancer (about 2-fold) respiratory (3.5-fold), digestive (about 10-fold) or other causes (about 3-fold) compared with lifetime light users. Also similarly to men, lower relative risks of death from cardiovascular events were observed in women who used alcohol always below the recommended limit, never-heavy or occasionally heavy (34%, 38% and 46%, respectively) compared with lifetime light users. It should be noted that the lower relative risk of death among occasionally heavy users was stronger and statistically significant only after multiple adjustments. The numbers of deaths among former heavy users was small for all causes; however, former heavy users had a higher risk of death from respiratory diseases and external causes (about 4–5-fold increased risk), digestive diseases (about 15-fold) and other causes (about 3-fold), compared with always light alcohol users (Table 4).
Table 4.
Hazard rate ratios of cause specific deaths across categories of lifetime pattern of alcohol-use among women participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study
| Exclusive categories of pattern of alcohol use | Formerb |
Lifetimec |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cause of death | Nevera | Former light to moderated | Former heavye | Lightf | Below rec. limitg | Light to moderated | Occasionally heavye | Heavye | P for trendh |
| Person-years | 361 973 | 161 992 | 7700 | 440 937 | 1 295 299 | 855 524 | 340 591 | 12 618 | |
| CHDk | |||||||||
| Deaths N = 832 | 111 | 84 | 2 | 191 | 269 | 127 | 48 | 0 | |
| HRi | 1.02 | 1.25 | 1.02 | REF | 0.70 | 0.68 | 0.76 | 0.132 | |
| (95% CI) | (0.78; 1.35) | (0.93; 1.68) | (0.25; 4.18) | (0.57; 0.86) | (0.53; 0.86) | (0.55; 1.07) | |||
| HRj | 0.94 | 1.07 | 0.68 | REF | 0.66 | 0.62 | 0.54 | 0.001 | |
| (95% CI) | (0.71; 1.24) | (0.79; 1.44) | (0.17; 2.82) | (0.54; 0.82) | (0.49; 0.80) | (0.37; 0.78) | |||
| CVDl | |||||||||
| Deaths N = 1816 | 272 | 166 | 8 | 313 | 571 | 352 | 134 | 0 | |
| HRi | 1.40 | 1.58 | 2.05 | REF | 0.90 | 1.05 | 1.14 | 0.060 | |
| (95% CI) | (1.16; 1.68) | (1.28; 1.95) | (1.02; 4.11) | (0.77; 1.04) | (0.89; 1.24) | (0.92; 1.42) | |||
| HRj | 1.26 | 1.37 | 1.50 | REF | 0.88 | 1.01 | 0.85 | 0.658 | |
| (95% CI) | (1.04; 1.54) | (1.09; 1.71) | (0.71; 3.17) | (0.75; 1.03) | (0.85; 1.21) | (0.66; 1.10) | |||
| Alcohol = related cancerm | |||||||||
| Deaths N = 1,863 | 181 | 107 | 8 | 243 | 686 | 425 | 203 | 10 | |
| HRi | 1.20 | 1.27 | 2.08 | REF | 1.02 | 1.07 | 1.31 | 2.41 | <0.001 |
| (95% CI) | (0.97; 1.49) | (0.99; 1.62) | (1.03; 4.23) | (0.87; 1.19) | (0.91; 1.26) | (1.07; 1.59) | (1.28; 4.54) | ||
| HRj | 1.15 | 1.20 | 1.89 | REF | 0.98 | 1.02 | 1.16 | 2.20 | 0.013 |
| (95% CI) | (0.92; 1.44) | (0.93; 1.55) | (0.93; 3.83) | (0.83; 1.15) | (0.85; 1.21) | (0.94; 1.43) | (1.16; 4.18) | ||
| Other neoplasmsn | |||||||||
| Deaths N = 4,367 | 460 | 271 | 16 | 557 | 1557 | 1011 | 484 | 11 | |
| HRi | 1.19 | 1.36 | 1.83 | REF | 0.99 | 1.06 | 1.27 | 1.24 | <0.001 |
| (95% CI) | (1.04; 1.36) | (1.16; 1.59) | (1.10; 3.03) | (0.89; 1.09) | (0.95; 1.18) | (1.12; 1.45) | (0.68; 2.27) | ||
| HRj | 1.21 | 1.29 | 1.15 | REF | 0.97 | 1.02 | 1.12 | 1.08 | 0.065 |
| (95% CI) | (1.04; 1.40) | (1.09; 1.54) | (0.64; 2.08) | (0.86; 1.09) | (0.90; 1.15) | (0.97; 1.30) | (0.57; 2.03) | ||
| Respiratory causeo | |||||||||
| Deaths N = 608 | 63 | 48 | 7 | 102 | 228 | 107 | 50 | 3 | |
| HRi | 1.43 | 1.91 | 7.40 | REF | 1.08 | 0.96 | 1.17 | 4.37 | 0.350 |
| (95% CI) | (1.01; 2.04) | (1.28; 2.83) | (3.27; 16.74) | (0.84; 1.40) | (0.72; 1.28) | (0.82; 1.68) | (1.32; 14.47) | ||
| HRj | 1.49 | 1.53 | 4.61 | REF | 0.98 | 0.81 | 0.84 | 3.51 | 0.530 |
| (95% CI) | (1.01; 2.19) | (1.01; 2.32) | (1.94; 10.93) | (0.74; 1.30) | (0.60; 1.11) | (0.56; 1.25) | (1.09; 11.32) | ||
| Digestive causep | |||||||||
| Deaths N = 332 | 42 | 34 | 3 | 37 | 101 | 69 | 44 | 2 | |
| HRi | 2.43 | 3.43 | 11.62 | REF | 1.33 | 1.65 | 2.74 | 7.71 | <0.001 |
| (95% CI) | (1.49; 3.96) | (2.04; 5.78) | (3.57; 37.80) | (0.89; 1.98) | (1.08; 2.52) | (1.71; 4.40) | (2.06; 28.87) | ||
| HRj | 3.35 | 3.79 | 14.60 | REF | 1.34 | 1.65 | 2.59 | 9.57 | <0.001 |
| (95% CI) | (1.90; 5.92) | (2.10; 6.84) | (4.34; 49.14) | (0.85; 2.10) | (1.00; 2.70) | (1.48; 4.52) | (3.05; 30.0) | ||
| External causeq | |||||||||
| Deaths N = 472 | 67 | 33 | 5 | 64 | 156 | 101 | 45 | 1 | |
| HRi | 1.46 | 1.53 | 4.95 | REF | 0.97 | 0.97 | 1.09 | 0.77 | 0.601 |
| (95% CI) | (1.00; 2.11) | (0.98; 2.37) | (1.94; 12.68) | (0.72; 1.32) | (0.70; 1.35) | (0.73; 1.62) | (0.11; 5.47) | ||
| HRj | 1.42 | 1.58 | 4.11 | REF | 1.09 | 0.94 | 1.04 | 0.72 | 0.747 |
| (95% CI) | (0.90; 2.22) | (0.96; 2.60) | (1.49; 11.37) | (0.76; 1.56) | (0.63; 1.42) | (0.65; 1.68) | (0.10; 5.20) | ||
| Other causer | |||||||||
| Deaths N = 1516 | 170 | 100 | 8 | 239 | 496 | 316 | 180 | 7 | |
| HRi | 1.19 | 1.54 | 3.22 | REF | 0.94 | 0.96 | 1.38 | 2.78 | <0.001 |
| (95% CI) | (0.96; 1.49) | (1.20; 1.99) | (1.57; 6.59) | (0.80; 1.11) | (0.80; 1.14) | (1.12; 1.69) | (1.30; 5.91) | ||
| HRj | 1.00 | 1.36 | 2.70 | REF | 0.89 | 0.91 | 1.33 | 2.58 | 0.001 |
| (95% CI) | (0.75; 1.33) | (1.00; 1.84) | (1.15; 6.30) | (0.72; 1.09) | (0.73; 1.15) | (1.03; 1.73) | (1.06; 6.27) | ||
CHD, coronary heart disease; CVD, cardiovascular disease; HR, hazard rate ratio; REF, reference; rec., recommended.
a Report of no use of alcohol at all points in time (age 20, 30, 40, 50 and at enrolment).
b Report of alcohol use in the past but not at the time of enrolment.
c Report of use of alcohol at past points in time and at the time of enrolment.
d ≤30 g/d.
e >30 g/d.
f ≤1 g/d.
g ≤12 g/d (American Heart Association; World Cancer Research Fund/American Institute for Cancer Research, National Institute on Alcohol Abuse and Alcoholism).
h P for trend across HR of death in lifetime alcohol-usersemploying median alcohol use at the time of enrolment.
i Joint Cox proportional hazard model, stratified by age and centre.
j Joint Cox proportional hazard model, as i and adjusted for body mass index, height, waist circumference, intake of fruits, vegetables, red meat and meat products, dietary fibre, physical activity, education, smoking, menopausal hormone use, number of live births and menopausal status.
k I20–I25.
l I00–I99 excl. I20–I25.
m C01–C06, C09–C15, C18–22; C32; C50.
n C00–D48 excl. C01–C06, C09–C15, C18–22; C32; C50.
o J00-J99.
p K00-K93, excl. K292, K70, K860.
q S00-T98, V01-Y98.
r All causes excl. I00–I99; C00–D48; J00-J99; K00-K93, excl. K292, K70, K860; S00-T98, V01-Y98; and unknown causes.
When analyses for broad causes of death (CVD, cancer and other causes) were stratified by disease status at enrolment, the lower risks of death from CVD in men and women were only evident amongst those who had not developed diseases before enrolment (Supplementary Tables 1 and 2, available as Supplementary data at IJE online). In women, but not in men, the shape of the relationship differed according to disease status (P for interaction = 0.013), although the risk estimates were similar between men and women, with a 50% lower risk of death from CVD among alcohol users without a chronic disease at enrolment and a 33% higher risk among never alcohol users who had developed a chronic disease at enrolment, compared with lifetime light alcohol users.
Further sensitivity analyses showed that excluding deaths that occurred within the first 3 years of follow-up, or participants who were aged ≤50 years at the time of enrolment, did not alter the results. However, some of the associations were more evident either in northern or in southern countries such as: death from CVD (excluding CHD) among men, where a lower risk of death among those who used alcohol within the recommended limit existed only in northern countries (P for interaction = 0.043); and death from other neoplasms in women, where the higher risks among never and former alcohol users were only seen in southern countries (P for interaction = 0.0049).
Relative risk curves for alcohol consumption at enrolment
The risk relationship between alcohol consumption during midlife and cause of death was non-linear in men for CVD, respiratory, digestive, external and other causes, and for CHD, other neoplasms, respiratory and other causes in women (Figures 3 and 4). Although the gradients and the nadirs of the relative risk curves differed considerably, most of the associations between alcohol use and cause of death were statistically significant. In men, two patterns of curves were observed for alcohol consumption at the time of enrolment. One was associated with a higher relative risk of death from alcohol-related cancers, digestive and external causes, other neoplasms and other causes, only for consumption higher than 24g/day (Figure 3). However, this pattern was not observed for death from CHD, other CVD or respiratory diseases where the risk curve did not increase with higher alcohol consumption. In women, the risk relationships for death from almost all causes were similar to those seen in men except for external causes, alcohol-related cancers, and diseases of the digestive system, where with increasing alcohol consumption the relative risk curves of death from digestive causes increased more steeply and those from external causes and alcohol-related cancers attenuated apparently (Figure 4).
Figure 3.
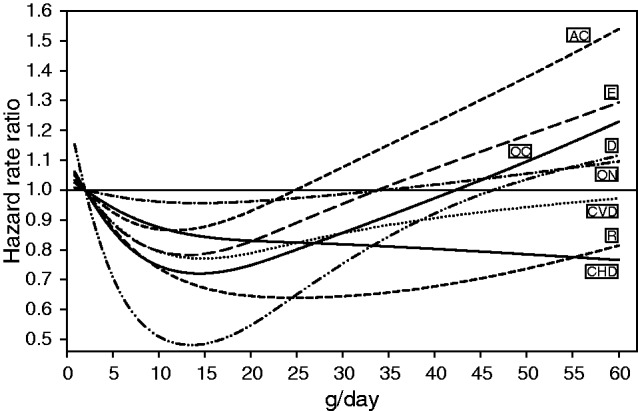
Spline regression of the association of g/day alcohol use at enrolment and causes of death among men participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study, whose alcohol use was >0 at that time, stratifying by centre and age, and adjusting for body mass index, height, waist circumference, intake of fruits, vegetables, red meat and meat products, dietary fibre, physical activity, education, smoking and prevalent disease (self-reported cancer, myocardial infarction, stroke, diabetes mellitus or hypertension). P–values for linearity and for general effect respectively were for CHD = 0.151 and 0.004, CVD = 0.001 and 0.001, alcohol-related cancers = 0.059 and <0.001, other neoplasms = 0.172 and <0.001, respiratory causes = <0.001 and <0.001, digestive causes = <0.001 and <0.001, external causes = 0.044 and <0.001 and other causes = <0.001 and <0.001. CHD, coronary heart disease; CVD, cardiovascular disease other than CHD; AC, alcohol-related cancer; ON, other neoplasms; R, respiratory system; D, digestive system; E, external causes; OC, other causes
Figure 4.
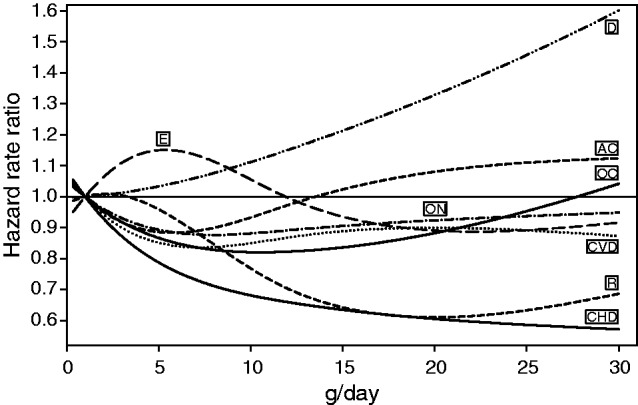
Spline regression of the association of g/d alcohol use at enrolment and causes of death among women participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study, whose alcohol use was >0 at that time; stratifying by centre and age and adjusting for body mass index, height, waist circumference, intake of fruits, vegetables, red meat and meat products, dietary fibre, physical activity, education, smoking, menopausal hormone use, number of live births, menopausal status and prevalent disease (self-reported cancer, myocardial infarction, stroke, diabetes mellitus or hypertension). P-values for linearity and for general effect respectively were for CHD = 0.028 and <0.001, CVD = 0.089 and 0.061, alcohol-related cancers = 0.076 and 0.028, other neoplasms = 0.013 and 0.033, respiratory causes = 0.001 and 0.003, digestive causes = 0.950 and 0.002, external causes = 0.399 and 0.602 and other causes = 0.001 and <0.001. CHD, coronary heart disease; CVD, cardiovascular disease other than CHD; AC, alcohol-related cancer; ON, other neoplasms; R, respiratory system; D, digestive system; E, external causes; OC, other causes
Sensitivity analyses investigated the shapes of the curves following exclusion of participants with a self-reported prevalent disease and following adjustment for prevalent disease. The shapes of the risk relationship for alcohol consumption and death changed slightly in men and obviously in women for diseases of the digestive system, and slightly for alcohol-related cancer and other causes in women (Supplementary Figures 3 and 4, available as Supplementary data at IJE online). Excluding women with prevalent diseases (instead controlling for disease status at enrolment) attenuated the relative risk of death from alcohol-related cancers and increased the relative risk of death from a disease of the digestive system even further at a level of alcohol use higher than 12 g/day.
Alcohol consumption at enrolment and cause of death taking past use into account
Compared with participants who used alcohol within recommended limits throughout their lifetime, those who had used it more heavily in the past (but not at enrolment) had higher risks of death from CVD [women: HR 1.21; 95% confidence interval (CI) 1.02, 1.43], alcohol-related cancer (men: HR 1.32; 95% CI 1.10, 1.60), disease of the respiratory system (men: HR 1.28; 95% CI 1.01, 1.62), external cause (men: HR 1.51; 95% CI 1.18, 1.93) or other causes (men: HR 1.49; 95% CI 1.24, 1.79; women: HR 1.27; 95% CI 1.03, 1.58). Compared with men who had a lifetime alcohol use below the recommended limit, there was a higher risk of death from alcohol-related cancer in men who used more than the recommended amount at enrolment, regardless of their past consumption habits (HR 1.36; 95% CI 1.05, 1.76 if their whole past consumption was below the limit or HR 1.57; 95% CI 1.33, 1.87 if their past consumption was always or occasionally above the limit). Higher risks were also seen with a lifetime alcohol use above the recommended limit for death from digestive (HR 1.42; 95% CI 1.02, 1.96), external (HR 1.57; 95% CI 1.24, 1.99) and other causes (HR 1.54; 95% CI 1.29, 1.84). In women however, compared with those who used alcohol below the recommended limit throughout their lifetime, a lower relative risk of death from CHD was seen among those who used alcohol heavily at enrolment although having using alcohol in the past below the recommended limit (HR 0.57; 95% CI 0.41, 0,78).
A total of 266 participants died from a cause directly related to alcohol use (e.g. alcohol-induced intoxication, dependence, certain external causes), of whom 197 were men. Compared with men who consistently used alcohol below the recommended limit (i.e. at enrolment and in the past), men who had previously used it above the limit (but whose consumption was lower at enrolment) had a risk of death from alcohol-related causes of 7.12 (95% CI 3.79, 13.37); those who had used more than the recommended limit, but not in the past, had a corresponding risk of 5.08 (95% CI 2.48, 10.52); men who used more than the recommended limit over their lifetime (i.e. in the past and at enrolment) had a risk of death from a cause directly related to alcohol of 9.22 (95% CI 5.11, 16.63). The number of women who died from a cause directly related to alcohol was too small for reasonable analysis.
Competing risks across causes
The statistical tests for both models of lifetime alcohol use and alcohol consumption at enrolment showed different relationships for many causes in men and in women (Supplementary Tables 3 and 4, available as Supplementary data at IJE online). Distinct relationships were observed for death from alcohol-related cancer as compared with CHD or CVD in men and in women (all P-values for difference were statistically significant). Further contrasts between risk associations of lifetime pattern or alcohol use at enrolment emerged for external and other causes in men and for diseases of the digestive system in women when compared with all other groups of causes.
All-cause mortality and rate advancement period
Compared with men and women who used alcohol lightly throughout their lifetime, those who used it in restricted amounts (i.e. below the recommended limits throughout their lifetime) had the lowest relative risk of overall death, whereas those who used alcohol heavily, either at enrolment or in the past, had the highest relative risk of death (Table 5). Women who were never alcohol users also had a greater risk of death than lifetime light users. Expressing the relative risks as advancement periods, compared with lifetime light alcohol users of the same age at enrolment, former light to moderate users had died, on average, 1.5–years earlier; former heavy users died, on average, 5.2 years earlier. The corresponding figures for women were 1.5 and 3.7 years, respectively. Lifetime heavy alcohol users died on average 3.2 (men) and 2.0 (women) years earlier than lifetime light users. Men who never used alcohol died 5.1 years earlier than lifetime light users when a prevalent disease was present at enrolment, whereas those without a disease had a death rate similar to that of lifetime light alcohol users. Further, former heavy alcohol users with a prevalent disease died about 17.4-years earlier than lifetime light users with a prevalent disease; among those without prevalent disease, former heavy users died only 3.4 years earlier than lifetime light users.
Table 5.
Hazard rate ratios of death from all causes and risk advancement periods across categories of lifetime pattern of alcohol use among men and women participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study
| Exclusive categories of pattern of alcohol use | Formerb |
Lifetimec |
||||||
|---|---|---|---|---|---|---|---|---|
| All causes | Nevera | Former light to moderated | Former heavye | Lightf | Below rec. limitsg | Light to moderated | Occasionally heavye | Heavye |
| Men | ||||||||
| Person-years/N deaths | 21 087/240 | 41 918/698 | 16 108/320 | 39 168/441 | 490 252/4 061 | 477 358/3486 | 212 821/2235 | 24 645/257 |
| HR (95% CI)h | 1.17 (0.99; 1.38) | 1.29 (1.14; 1.47) | 2.05 (1.76; 2.40) | REF | 0.90 (0.81; 1.00) | 0.94 (0.84; 1.04) | 1.22 (1.09; 1.37) | 1.52 (1.29; 1.80) |
| HR (95% CI)i | 1.16 (0.98; 1.36) | 1.21 (1.06; 1.38) | 1.94 (1.66; 2.27) | REF | 0.90 (0.81; 1.00) | 0.93 (0.83; 1.04) | 1.21 (1.07; 1.35) | 1.51 (1.28; 1.78) |
| RAPi | 1.1 (0.0; 2.2) | 1.5 (1.0; 2.1) | 5.2 (5.1; 5.3) | REF | −0.8 (−2.2;−0.6) | −0.6 (−3.5; 2.4) | 1.5 (0.9; 2.0) | 3.2 (3.0; 3.4) |
| Person-years/N deaths | 5213/101 | 13 606/327 | 6541/174 | 8642/187 | 109 567/1701 | 117 887/1488 | 63 380/1028 | 7 529/101 |
| Prevalent diseasej | ||||||||
| HR (95% CI)h | 1.22 (0.94; 1.59) | 1.18 (0.97; 1.45) | 1.98 (1.57; 2.49) | REF | 0.97 (0.81; 1.14) | 0.98 (0.82; 1.17) | 1.25 (1.04; 1.50) | 1.38 (1.06; 1.80) |
| RAPh | 5.1 (4.5; 5.6) | 4.3 (3.7; 4.9) | 17.4 (17.3; 17.5) | REF | −1.0 (−5.9; 3.9) | 0.7 (−10.7; 9.3) | 5.6 (5.2; 6.1) | 8.2 (7.8; 8.5) |
| Person-years/N deaths | 15 874/139 | 28 312/371 | 9567/146 | 30526/254 | 380 685/2360 | 359 471/1998 | 149 440/1207 | 17 117/156 |
| No prevalent disease | ||||||||
| HR (95% CI)h | 1.15 (0.92; 1.43) | 1.32 (1.11; 1.57) | 2.00 (1.61; 2.50) | REF | 0.86 (0.74; 0.99) | 0.89 (0.77; 1.03) | 1.16 (0.99; 1.35) | 1.57 (1.26; 1.96) |
| RAPh | 0.7 (−1.8; 3.1) | 1.4 (0.8; 1.9) | 3.4 (3.2; 3.5) | REF | −0.7 (−2.1; 0.6) | −0.6 (−3.0; 1.9) | 0.7 (−0.9; 2.3) | 2.2 (1.9; 2.5) |
| Women | ||||||||
| Person-years/N deaths | 361 973/1693 | 161 982/1136 | 7700/65 | 440 937/1,992 | 1 295 299/5123 | 855 524/3103 | 340 591/1522 | 12 618/39 |
| HR (95% CI)h | 1.19 (1.10; 1.29) | 1.30 (1.19; 1.42) | 1.81 (1.39; 2.37) | REF | 0.92 (0.86; 0.98) | 0.94 (0.88; 1.01) | 1.04 (0.96; 1.13) | 1.37 (0.98; 1.91) |
| HR (95% CI)i | 1.17 (1.08; 1.27) | 1.25 (1.14; 1.36) | 1.77 (1.35; 2.31) | REF | 0.93 (0.87; 0.99) | 0.94 (0.88; 1.01) | 1.04 (0.96; 1.14) | 1.38 (0.99; 1.93) |
| RAPi | 1.1 (0.4; 1.7) | 1.5 (1.1; 1.9) | 3.7 (3.6; 3.9) | REF | −0.5 (−2.4; 1.4) | −0.4 (−3.3; 2.4) | 0.3 (−6.8; 7.4) | 2.0 (1.4; 2.6) |
| Person-years/N deaths | 98 118/828 | 49 351/572 | 2340/28 | 99 995/862 | 290 004/2068 | 171 747/1193 | 72 157/568 | 2035/11 |
| Prevalent diseasej | ||||||||
| HR (95% CI)h | 1.19 (1.05; 1.35) | 1.30 (1.14; 1.48) | 1.73 (1.14; 2.60) | REF | 0.95 (0.86; 1.05) | 0.93 (0.83; 1.03) | 1.04 (0.91; 1.20) | 1.15 (0.58; 2.25) |
| RAPh | 1.6 (0.8; 2.3) | 2.3 (1.9; 2.8) | 4.4 (4.1; 4.7) | REF | −0.5 (−4.2; 3.1) | −0.7 (−3.0; 1.6) | 0.3 (−10.8; 11.4) | 1.1 (−4.4; 6.5) |
| Person-years/N deaths | 263 855/865 | 112 631/564 | 5360/37 | 340 942/1130 | 1 005 295/3055 | 683 777/1910 | 268 434/954 | 10 583/28 |
| No prevalent disease | ||||||||
| HR (95% CI)h | 1.16 (1.04; 1.30) | 1.23 (1.09; 1.39) | 1.74 (1.22; 2.48) | REF | 0.91 (0.84; 0.99) | 0.95 (0.87; 1.04) | 1.04 (0.93; 1.16) | 1.46 (0.99; 2.15) |
| RAPh | 0.9 (−0.0; 1.9) | 1.2 (0.6; 1.9) | 3.2 (3.0; 3.5) | REF | −0.5 (−2.5; 1.4) | −0.3 (−5.8; 5.2) | 0.2 (−12.7; 13.2) | 2.0 (1.3; 2.6) |
HRR, hazard rate ratio; REF, reference; RAP, risk advancement periods; rec., recommended.
a Report of no use of alcohol at all points in time (age 20, 30, 40, 50 and at enrolment).
b Report of alcohol use in the past but not at the time of enrolment
c Report of use of alcohol at past points in time and at the time of enrolment.
d ≤60 g/d (men); ≤30 g/d (women).
e >60 g/d (men) >30 g/d (women).
f ≤2 g/d (men); ≤1 g/d (women).
g ≤24 g/d (men); ≤12 g/d (women) (American Heart Association; World Cancer Research Fund/American Institute for Cancer Research, National Institute on Alcohol Abuse and Alcoholism).
h Stratified by age and centre, and adjusted for body mass index, height, waist circumference, intake of fruits, vegetables, red meat and meat products, dietary fibre, physical activity, education, smoking, women additionally for menopausal hormone use, number of live births, menopausal status.
i Stratified and adjusted as h, and additionally for prevalent disease.
j Prevalent disease defined as self-reported cancer, myocardial infarction, stroke, diabetes mellitus or hypertension.
Discussion
In this large European cohort study, cause of death was differentially associated with the pattern of lifetime alcohol use, with light to moderate alcohol consumption throughout their lifetime showing a lower risk of CHD (CVD in men) and heavy use increasing the risk of alcohol-related cancer, other cancers, digestive diseases and other causes. Compared with users who consumed low amounts of alcohol throughout their lifetime, former alcohol users had a higher risk of death from CHD (CVD in men), which is in line with a previous meta-analysis.4 Conversely, those whose lifetime alcohol consumption was below the recommended limits of two (men) or one (women) drinks per day (16–18) had the lowest risk of death from CVD, although this was only among those without a prevalent disease at time of enrolment (i.e. hypertension, diabetes, myocardial infarction, stroke or cancer). Women who used light to moderate amounts, as well as those who were occasional heavy alcohol users, had a 30–40% lower risk of death from CHD compared with lifetime light users. These apparent beneficial effects were again seen only in women without a prevalent disease at the time of enrolment. Relative risks in men and women were higher for death from alcohol-related cancer, digestive, respiratory or external cause among those who used alcohol below the recommended limit at enrolment but had used it above this limit in the past, suggesting that they may have reduced their alcohol consumption due to illness. These figures suggest that risk associations between alcohol use in middle to later age and death, as also observed in many previous studies, reflect an underlying pattern of lifetime alcohol use where moderate use of up to 30 g/day alcohol may actually be a sign of good physical and mental health and not necessarily a cause of it. Previous studies leading to the causal understanding may be also biased by self-selection of healthy survivors. This speculation is supported by the findings in our study that former moderate users still had a higher riskof dying than ‘current’ moderate users. By removing former alcohol users from a sample, one is likely to intrinsically select the healthy. This also may be an explanation for the fact that women who were categorised as ‘occasionally heavy’ alcohol users had a >40% lower risk of death from CHD. Another possible explanation can be found based on competing risks because the number of deaths due to CHD was low among women using alcohol occasionally heavily, and no CHD death was seen among the heavy users, very likely due to most or all of them dying of causes other than CHD. This is supported by the competing risks analysis that clearly showed an opposite association of alcohol use and death from CHD vs alcohol-related cancers and most of the other causes. In the Nurses’ Health Study, a lower risk of death among consumers of alcohol was found not specifically for death from CHD, but also for non-cancer causes,25 suggesting that there may be competing risks rather than a real benefit of alcohol consumption for CHD prevention.
Many studies including our own showed that moderate alcohol consumption at enrolment was related to lower CHD or other CVD mortality.3,26 Our study and others also showed evidence that heavy alcohol use increases the risk of several cancers27 and various causes of death,2 even among heavy users who had reduced alcohol consumption to below the recommended limit at the time of enrolment.
Women who never used alcohol had a higher risk of death from CVD (but not CHD), compared with those whose lifetime alcohol use was light. Never alcohol users had also higher risks of death from non-alcohol related cancers and diseases of the respiratory and digestive systems. This suggests that the higher mortality rate is unlikely to be due to the lack of alcohol per se, but rather due to other characteristics that differ between never users and users of alcohol.28 This is further supported by a study that found that the higher risk of death among women who abstained for a longer period was attenuated when characteristics such as income, employment status, ethnicity, religiosity and social isolation were taken into consideration.29 Other studies have also shown that never users have different health behaviours compared with users of alcohol.7
It is also possible that a certain proportion of alcohol users were misclassified as never users (e.g. due to social desirability bias or due to infrequent alcohol users retrospectively defining themselves as a never user).30,31 However, extensive misclassification seems unlikely because <1% of alcohol-related deaths (such as alcoholic liver disease, alcohol misuse or alcohol-induced chronic pancreatitis) occurred in never alcohol users. Conversely, the highest proportion of alcohol-related deaths (60%) occurred in heavy alcohol users. Furthermore, in terms of the amount of alcohol used in the past, trends of lifetime alcohol use in the EPIC study corresponded to the trends of the per capita consumption in the European countries across age cohorts, suggesting that our exposure measure is valid.32
Similarly to other observational studies, uncontrolled selection biases limit the interpretation of our findings and experimental study designs to investigate the effect of alcohol on cause of death are not feasible. Analysis was also limited by the fact that there was no information available on: the age of onset of alcohol use; potential periods of abstaining throughout life; or prospective information on alcohol use after enrolment until death. The effect of alcohol use during follow-up is unlikely to have as big an effect as the previous alcohol consumption during lifetime until enrolment. We did not have information on the number of drinks used on one occasion (binge drinking), the frequency of consumption or whether alcoholic beverages were consumed with meals, all of which may influence disease risk.33–36 The stratification of our analysis by northern versus southern European countries, where the pattern of alcohol use is known to be different,36 indicated that men from the north (Denmark, Germany, The Netherlands and the UK) who used alcohol below the recommended limit had a lower risk of death from CVD; this association was not observed in southern countries (Greece, Spain and Italy). Conversely, only women who never used alcohol and were from southern countries had a higher risk of death from cancers not related to alcohol consumption. This suggests the presence of some other relevant factors we could not account for, or that these differences could be due to chance. Further, the small numbers of heavy alcohol users, particularly in women, impedes the reliable estimation of the risk of death in women beyond those using >30 g/day alcohol.
We did not present results of lifetime alcohol use on the risk of death after adjustment for prevalent diseases because diseases occurring before enrolment into the study may have been related to alcohol consumption. Controlling for lifestyle and socio-demographic factors (e.g. diet, cigarette smoking, physical activity or educational attainment) at the time of enrolment revealed possible confounding of the associations of alcohol use with cause of death since relative risk estimates for categories of lifetime light to moderate consumption were only statistically significantly in the fully adjusted models. Moreover, relative risk estimates were generally attenuated particularly in the categories of heavy alcohol consumption, after full adjustment. Residual confounding, therefore, cannot be ruled out.
We chose lifetime light alcohol users of ≤2 g/day and ≤1 g/day as the reference because these men and women, although they are physically able to use alcohol, consume alcohol at a level that is so low and infrequent that it presumably could not affect disease risk.6
Only a few prospective studies (mainly on CVD mortality) have been able to distinguish between never and former alcohol users,3,26 and to our knowledge no previous study has information on lifetime variation of alcohol consumptions at different times throughout life. A further strength of our study is the application of one model for all leading causes. Most previous studies or meta-analyses have focused on single causes3,27 or on former users,4 or have needed to pool different studies to look at multiple endpoints.2
In conclusion, limiting alcohol consumption throughout lifetime to, on average, one drink per day in women and two drinks per day in men (as recommended by the American Heart Association, the World Cancer Research Fund/American Institute for Cancer Research and the National Institute of Alcoholism and Alcohol Abuse) is associated with an apparent lower risk of death. However it is difficult to determine whether this association is causal, due to the possibility of selection bias, confounding and competing risks.
Supplementary Data
Supplementary data is available at IJE online.
Funding
The work for the EPIC study on which this analysis is based was financially supported by the Europe Against Cancer Program of the European Commission (SANCO); Danish Cancer Society; German Institute of Human Nutrition, German Cancer Aid; German Cancer Research Center; German Federal Ministry of Education and Research; Dutch Ministry of Public Health, Welfare and Sports; National Cancer Registry and the Regional Cancer Registries Amsterdam, East and Maastricht of The Netherlands; Health Research Fund (FIS) of the Spanish Ministry of Health; Greek Ministry of Health; Greek Ministry of Education; Italian Association for Research on Cancer; Health Research Fund (FIS) of the Spanish Ministry of Health; the ISCIII Network RCESP (C03/09) and RETICC C03/10, Spanish Regional Governments of Andalusia, Asturias, Basque Country, Granada, Murcia and Navarra and the Catalan Institute of Oncology; Cancer Research UK; Medical Research Council, UK; Stroke Association, UK; British Heart Foundation; Department of Health, UK; Food Standards Agency, UK; Wellcome Trust, UK; Ligue Nationale contre le Cancer, 3M Company, INSERM. This work was also conducted on behalf of the mortality working group for the EPIC-elderly Nah project (contract no. 2004 126) that aims to understand specific health problems of the elderly population in Europe. Part of this work was also supported by la Direction Générale de la Santé (French Ministry of Health) with contract (GR-IARC-2003-09-12-01).
Supplementary Material
Acknowledgement
Wolfgang Bernigau, who conducted all statistical analyses for this study, deserves particular thanks.
Conflict of interest: None declared.
KEY MESSAGES.
The role of alcohol in relation to premature cause-specific death was determined by estimating the use of alcohol throughout life.
Compared with men and women who used low amounts of alcohol throughout their lifetime, heavy alcohol use at any time during life was associated with a higher risk of death from alcohol-related cancer, external causes and ‘other causes’ but not from coronary heart disease and other cardiovascular diseases (except among men who were former heavy users).
Upper limits for alcohol use of one and two drinks per day for women and men, respectively, were recommended by the American Heart Association, the World Cancer Research Fund/American Institute for Cancer Research and the National Institute of Alcoholism and Alcohol Abuse. In this study, the relative risk of overall death and cardiovascular death was lower among those with an average lifetime use of one drink per day compared with one-half drink per week for women, and two drinks per day compared with one drink per week for men. However, these lower relative risks applied only to those study participants considered free of disease at time of enrolment into the study.
The apparent health benefit of low to moderate alcohol use found in observational studies could therefore in large part be due to various selection biases and competing risks, which are related to both lifetime alcohol use and risk of disease, usually occurring later in life.
Individual data on lifetime alcohol use, in contrast to information about alcohol use at enrolment only, adds relevant information because it allows investigation of the effect of past alcohol use on health.
References
- 1.Rehm J, Baliunas D, Borges GL, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105:817–43. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–19. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roerecke M, Rehm J. Ischemic Heart Disease Mortality and Morbidity Rates in Former Drinkers: A Meta-Analysis. Am J Epidemiol. 2011;173:245–58. doi: 10.1093/aje/kwq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet. 1988;2:1267–73. doi: 10.1016/s0140-6736(88)92890-5. [DOI] [PubMed] [Google Scholar]
- 6.Fillmore KM, Kerr WC, Stockwell T, Chikritzhs T, Bostrom A. Moderate alcohol use and reduced mortality risk: Systematic error in prospective studies. Add Res Theory. 2006;14:101–32. doi: 10.1016/j.annepidem.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Green CA, Polen MR. The health and health behaviors of people who do not drink alcohol. Am J Prev Med. 2001;21:298–305. doi: 10.1016/s0749-3797(01)00365-8. [DOI] [PubMed] [Google Scholar]
- 8.Lipton RI. The effect of moderate alcohol use on the relationship between stress and depression. Am J Public Health. 1994;84:1913–17. doi: 10.2105/ajph.84.12.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gronbaek M. Confounders of the Relation between Type of Alcohol and Cardiovascular Disease. Ann Epidemiol. 2007;17:S13–S15. [Google Scholar]
- 10.Ferrence R, Kozlowski LT. Moderate drinking and health: being confounded by confounders. Addiction. 1995;90:485–88. doi: 10.1111/j.1360-0443.1995.tb02180.x. discussion 493–98. [DOI] [PubMed] [Google Scholar]
- 11.Saarni SI, Joutsenniemi K, Koskinen S, et al. Alcohol consumption, abstaining, health utility, and quality of life – a general population survey in Finland. Alcohol Alcohol. 2008;43:376–86. doi: 10.1093/alcalc/agn003. [DOI] [PubMed] [Google Scholar]
- 12.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 13.Lemmens PH. The alcohol content of self-report and ‘standard' drinks. Addiction. 1994;89:593–601. doi: 10.1111/j.1360-0443.1994.tb03336.x. [DOI] [PubMed] [Google Scholar]
- 14.Slimani N, Deharveng G, Charrondiere RU, et al. Structure of the standardized computerized 24-h diet recall interview used as reference method in the 22 centers participating in the EPIC project. European Prospective Investigation into Cancer and Nutrition. Comput Methods Programs Biomed. 1999;58:251–66. doi: 10.1016/s0169-2607(98)00088-1. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Global Status Report on Alcohol 2004. World Geneva: WHO; 2004. [Google Scholar]
- 16.American Heart Association. Alcohol, Wine and Cardiovascular Disease. http://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/Alcohol-Wine-and-Cardiovascular-Disease_UCM_305864_Article.jsp (15 January 2013, date last accessed) [Google Scholar]
- 17.World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. http://www.dietandcancerreport.org/expert_report/recommendations/recommendation_alcoholic_drinks.php (15 January 2013, date last accessed) [Google Scholar]
- 18.National Institute of Alcoholism and Alcohol Abuse. Rethinking Drinking. Alcohol and Your Health. http://pubs.niaaa.nih.gov/publications/RethinkingDrinking/Rethinking_Drinking.pdf (15 January 2013, date last accessed) [Google Scholar]
- 19.Haftenberger M, Lahmann PH, Panico S, et al. Overweight, obesity and fat distribution in 50- to 64-year-old participants in the European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2002;5:1147–62. doi: 10.1079/PHN2002396. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari P, Kaaks R, Fahey MT, et al. Within- and Between-Cohort Variation in Measured Macronutrient Intakes, Taking Account of Measurement Errors, in the European Prospective Investigation into Cancer and Nutrition Study. Am J Epidemiol. 2004;160:814–22. doi: 10.1093/aje/kwh280. [DOI] [PubMed] [Google Scholar]
- 21.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–32. [PubMed] [Google Scholar]
- 22.Brenner H, Gefeller O, Greenland S. Risk and rate advancement periods as measures of exposure impact on the occurrence of chronic diseases. Epidemiology. 1993;4:229–36. doi: 10.1097/00001648-199305000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Lieber CS. Gender differences in alcohol metabolism and susceptibility. In: Wilsnack RW, Wilsnack SC, editors. Gender and Alcohol. New Brunswick, NJ: Rutgers Center of Alcohol Studies; [Google Scholar]
- 24.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 25.Baer HJ, Glynn RJ, Hu FB, et al. Risk Factors for Mortality in the Nurses' Health Study: A Competing Risk Analysis. Am J Epidemiol. 2011;173:319–29. doi: 10.1093/aje/kwq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patra J, Taylor B, Irving H, et al. Alcohol consumption and the risk of morbidity and mortality for different stroke types – a systematic review and meta-analysis. BMC Public Health. 2010;10:258. doi: 10.1186/1471-2458-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelucchi C, Tramacere I, Boffetta P, Negri E, Vecchia CL. Alcohol Consumption and Cancer Risk. Nutr Cancer. 2011;63:983–90. doi: 10.1080/01635581.2011.596642. [DOI] [PubMed] [Google Scholar]
- 28.Ng Fat L, Shelton N. Associations between self-reported illness and non-drinking in young adults. Addiction. 2012;107:1612–20. doi: 10.1111/j.1360-0443.2012.03878.x. [DOI] [PubMed] [Google Scholar]
- 29.Fillmore KM, Golding JM, Graves KL, et al. Alcohol consumption and mortality. III: Studies of female populations. Addiction. 1998;93:219–29. doi: 10.1046/j.1360-0443.1998.9322196.x. [DOI] [PubMed] [Google Scholar]
- 30.Rehm J, Irving H, Ye Y, Kerr WC, Bond J, Greenfield TK. Are Lifetime Abstainers the Best Control Group in Alcohol Epidemiology? On the Stability and Validity of Reported Lifetime Abstention. Am J Epidemiol. 2008;168:866–71. doi: 10.1093/aje/kwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldwell TM, Rodgers B, Power C, Clark C, Stansfeld SA. Drinking histories of self-identified lifetime abstainers and occasional drinkers: findings from the 1958 British Birth Cohort Study. Alcohol Alcohol. 2006;41:650–54. doi: 10.1093/alcalc/agl088. [DOI] [PubMed] [Google Scholar]
- 32.Klipstein-Grobusch K, Slimani N, Krogh V, et al. Trends in self-reported past alcoholic beverage consumption and ethanol intake from 1950 to 1995 observed in eight European countries participating in the European Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2002;5:1297–310. doi: 10.1079/PHN2002406. [DOI] [PubMed] [Google Scholar]
- 33.Laatikainen T, Manninen L, Poikolainen K, Vartiainen E. Increased mortality related to heavy alcohol intake pattern. J Epidemiol Community Health. 2003;57:379–84. doi: 10.1136/jech.57.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hupkens CL, Knibbe RA, Drop MJ. Alcohol consumption in the European community: uniformity and diversity in drinking patterns. Addiction. 1993;88:1391–404. doi: 10.1111/j.1360-0443.1993.tb02026.x. [DOI] [PubMed] [Google Scholar]
- 35.Murray RP, Connett JE, Tyas SL, et al. Alcohol Volume, Drinking Pattern, and Cardiovascular Disease Morbidity and Mortality: Is There a U-shaped Function? Am J Epidemiol. 2002;155:242–48. doi: 10.1093/aje/155.3.242. [DOI] [PubMed] [Google Scholar]
- 36.Sieri S, Agudo A, Kesse E, et al. Patterns of alcohol consumption in 10 European countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) project. Public Health Nutr. 2002;5:1287–96. doi: 10.1079/PHN2002405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.