Abstract
Sphingosine-1-phosphate (S1P), a biologically active lysophospholipid that is enriched in blood, controls the trafficking of osteoclast precursors between the circulation and bone marrow cavities via G protein-coupled receptors, S1PRs. While S1PR1 mediates chemoattraction toward S1P in bone marrow, where S1P concentration is low, S1PR2 mediates chemorepulsion in blood, where the S1P concentration is high. The regulation of precursor recruitment may represent a novel therapeutic strategy for controlling osteoclast-dependent bone remodeling. Through intravital multiphoton imaging of bone tissues, we reveal that the bidirectional function of S1P temporospatially regulates the migration of osteoclast precursors within intact bone tissues. Imaging technologies have enabled in situ visualization of the behaviors of several players in intact tissues. In addition, intravital microscopy has the potential to be more widely applied to functional analysis and intervention.
Keywords: cell dynamics, chemokine, chemotaxis, lipid mediator, live imaging
INTRODUCTION
Bone is a highly dynamic organ that is continuously turned over during growth, even in adults. During bone remodeling, homeostasis is regulated by the balance between bone formation by osteoblasts and bone resorption by osteoclasts (Harada et al., 2003; Teitelbaum et al., 2003). However, in pathological conditions such as osteoporosis, osteopetrosis, arthritic joint destruction, and bone metastasis, this equilibrium is disrupted. Since osteoclasts are excessively activated in osteolytic diseases, the inhibition of osteoclast function has been a major therapeutic strategy. Bisphosphonates, the most widely used group of antiosteoporosis drugs, bind to hydroxyapatite, enter osteoclasts via endocytosis, and induce osteoclast apoptosis (Russell et al., 2007). Recently, the inactivation of osteoclasts, as opposed to their elimination, has generated interest as an alternative treatment strategy (Deal, 2009; Yasuda et al., 2005). One promising regulation point is the recruitment of osteoclast precursors. In addition to several chemokines that are known regulators of migration, including CXCL12 (Yu et al., 2003) and CX3CL1 (Koizumi et al., 2009), we have shown that sphingosine 1- phosphate (S1P), a lysophospholipid abundant in the plasma, plays an important role as both a chemoattractant and a chemorepellent (Ishii et al., 2009; 2010). In this review, we summarize the bidirectional regulation of osteoclast precursor migration by S1P and briefly describe intravital bone imaging in living animals.
S1P and its receptors
S1P is a bioactive sphingolipid metabolite that regulates diverse biological functions including cell proliferation, motility, and survival (Cyster, 2005; Rivera et al., 2008; Rosen et al., 2005; 2007). Sphingolipids are essential plasma membrane constituents composed of a serine head group and one or two fatty acid tails. They are easily metabolized and converted to sphingosines, which are ATP-dependently phosphorylated by sphingosine kinases 1 and 2 (SPHK1 and SPHK2) in most cells, yielding S1P (Hannun et al., 2008). SPHKs, which are regulated by a variety of growth factors, hormones, and cytokines, control S1P’s acute reactive generation and homeostasis in the circulation (Hannun et al., 2008). Immediately after its synthesis, free S1P is irreversibly degraded by intracellular S1P lyase or dephosphorylated by S1P phosphatases. As a result, the levels of S1P in most tissues, including bone marrow, are relatively low. In contrast, large amounts of S1P are continuously produced in the plasma, especially by erythrocytes, and the serum concentration of S1P is extremely high (several hundred nanomolar to low-micromolar range). Most S1P in the circulation is bound to high-density lipoprotein (HDL) and albumin, which serve as stable reservoirs and efficiently deliver S1P to epithelial cell-surface receptors (Argraves et al., 2008). In addition, because S1P is an amphiphilic molecule that cannot easily cross membranes, an S1P gradient between the blood and tissues is maintained.
S1P signals via five 7-transmembrane receptors or G proteincoupled receptors (GPCRs), S1PR1 to S1PR5, previously referred to as endothelial differentiation gene (Edg) receptors (Rivera et al., 2008; Rosen et al., 2007). Because of the different distribution of these receptors and their different coupling to signal-transducing G proteins, S1P shows a broad range of bioactivities (Table 1). S1PR1 is ubiquitously expressed and primarily coupled to PTX-sensitive Gi/o proteins, whereas S1PR2 and S1PR3, whose distributions are more limited, are coupled to G12/13 as well as Gq, Gs, and Gi. The expression of S1PR4 and S1PR5 is much lower than that of S1PR1, S1PR2, and S1PR3, and their functions remain to be elucidated. However, it has been reported that they are coupled to Gi/o and G12/13.
Table 1.
S1P receptors and phenotypes of their genetic deletion
| S1P Receptors | S1PR1 | S1PR2 | S1PR3 | S1PR4 | S1PR5 |
|---|---|---|---|---|---|
| Coupling G proteins | Gi/O | Gi | Gi | Gi | Gi/o |
| Gq | Gq | G12/13 | G12/13 | ||
| Gs | Gs | ||||
| G12/13 | G12/13 | ||||
| Distribution | Ubiquitous | Ubiquitous | Spleen, heart, lung, thymus, kidney, testis, brain, skeletal muscle | Thymus, spleen, lung, peripheral leukocytes | Brain, spleen, peripheral leukocytes |
| Highest expressed in embryonic brain | |||||
| Expressed high in adult heart and lung | |||||
| Phenotypes of gene deletion (mouse) | Embryonic lethal (e12.5-e14.5) | Vestibular defects | Disruption of alveolar epithelial junctions | Ddisorder of megakaryocyte differentiation | Reduced number of NK cells |
| Hearing loss | |||||
| Seizures (C57BL/6 only) | |||||
| Perinatal lethal (reduce litter size) | |||||
| Survivours show no phonotype | |||||
| Biological function | Rac activation | Rho activation | Cardioprotection by HDL | ||
| Vasoconstriction angiogenesis | |||||
| Wound healing | |||||
| References | Liu et al. (2000) | Kono et al. (2007) | Nofer et al. (2004) | Golfier et al. (2010) | Walzer et al. (2007) |
| Matloubian et al. (2004) | Serriere-Lanneau et al. (2007) | Gon et al. (2005) | |||
S1P receptors have key roles in the regulation of cellular motility. S1PR1 activates Rac through Gi and promotes cell migration and intercellular connection, whereas S1PR2 activates Rho signaling via G12/13, thereby counteracting the effects of S1PR1 and inhibiting Rac activity (Takuwa, 2002). These differences account for the different biological functions of S1PR1 and S1PR2, which produce opposite effects on migration toward/ against S1P gradients in vitro (Okamoto et al., 2000).
Osteoclast precursors and S1P
Osteoclasts are derived from macrophage/monocyte-lineage cells that express both S1PR1 and S1PR2 (Ishii et al., 2009). As described above, S1PR1 and S1PR2 have opposite effects on the migration of osteoclast precursors. Osteoclast precursors are chemoattracted to S1P in vitro, a response that is blocked by PTX. In addition, treatment with S1P increases osteoclast precursor levels of the active form of Rac (GTP-Rac), suggesting that Rac and Gi are involved in S1PR1 chemotactic signaling in osteoclast precursors. On the other hand, S1PR2 requires a higher concentration of S1P for activation and induces negative chemotactic responses, “chemorepulsion,” to S1P gradients. S1PR2 activation causes cells to move from the bloodstream into bone marrow cavities (Ishii et al., 2010). As in leukocytes, the migration of osteoclast precursors is regulated by chemokines. Like the S1PRs, chemokine receptors are GPCRs and signal via Gi components. One of the best-known chemoattractants for osteoclast precursors is CXCL12 (also known as stromal derived factor-1), a CXCR4 ligand (Yu et al., 2003). CXCL12 is constitutively expressed at high levels by osteoblastic stromal cells and vascular endothelial cells in bone, whereas CXCR4 is expressed on a wide variety of cells types, including circulating monocytes and osteoclast precursors. CXCL12 has chemotactic effects on osteoclast precursors, which express large amounts of CXCR4.
Recently, another chemokine, CX3CL1 (also known as fractalkine), which functions as a membrane-bound adhesion molecule, was shown to act as a chemoattractant after its cleavage by ADAM10 and ADM7. Expressed by osteoblastic stromal cells, it was reported to be involved in both the recruitment and attachment of osteoclast precursors (Koizumi et al., 2009). Expression of both chemokine receptors and S1PRs is reduced by RANKL stimulation, dependent on NF-κB, but not on NF-AT. Presumably, after cells mature and arrive at their ultimate destinations these chemoattractants are no longer needed.
Application of intravital imaging to the analysis of cell behavior in bone
To study the behavior of osteoclasts and their precursors in vivo, we developed a new intravital two-photon imaging system for use in the analysis of bone tissues (Fig. 1) (Ishii et al., 2009; 2010). Recent advances in microscope, laser, and fluorophore technology have made it possible to visualize living cells in intact organs and to analyze their mobility and interactions in a quantitative manner.
Fig. 1. Bone marrow structure visualized by intravital two-photon imaging. Murine skull bone tissues of heterozygous Cx3CR1-EGFP knock-in mice. Collagen fibers in bone are detected by secondharmonic generation (in blue), and the microvasculature are visualized by intravenous injection of 70 kDa dextran-conjugated Texas Red. CX3CR1-EGFP positive cells appear green in bone marrow cavity.
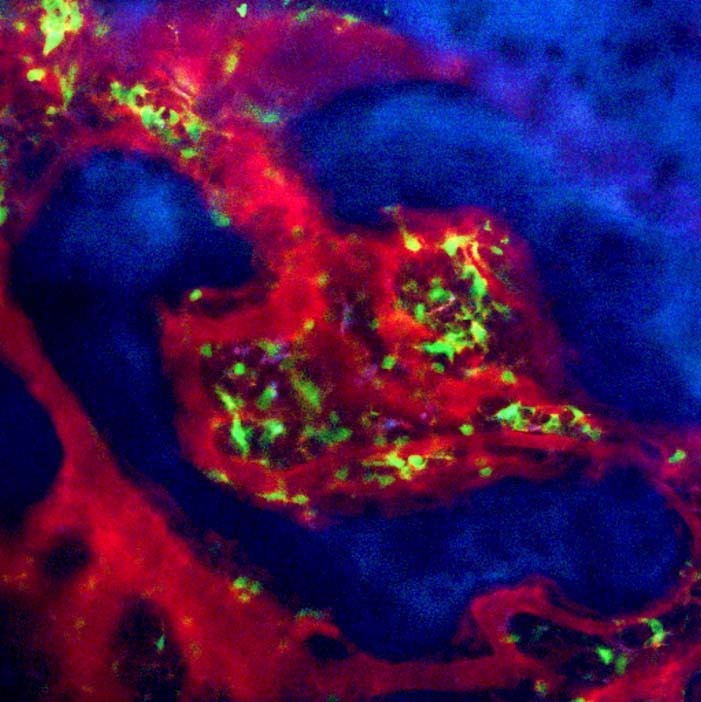
As calcium phosphate, the main structural component of the bone matrix, can scatter laser beams, it was difficult to access the deep interior of bone tissues, even using a near-infrared laser. We decided to use parietal bone in which the distance from the bone surface to the bone marrow cavity is 80-120 μm (within the appropriate range for two-photon microscopy). We modified the method used in a pilot study, which revealed that central memory CD8+ T cells were preferentially recruited to, and accumulated in, the bone marrow cavity and interacted with mature circulating dendritic cells (Cavanagh et al., 2005; Mazo et al., 2005).
Using this new intravital two-photon imaging method, we showed that S1P controls the migratory behavior of osteoclast precursors, dynamically regulating bone mineral homeostasis, and we identified a critical control point in osteoclastogenesis. While monocytoid cells containing osteoclast precursors (CSF1REGFP- positive or CX3CR1-EGFP-positive cells) were stationary at the steady state, osteoclast precursors were stimulated and moved into vessels when a potent S1PR1-specific agonist, SEW2871 (Wei et al., 2005), was injected intravenously.
To clarify the physiological significance of S1P-directed chemotaxis of osteoclast precursors in bone homeostasis, we examined osteoclast/monocyte-specific S1PR1-deficient (S1PR1-/-) mice. [Global S1PR1 deficiency causes embryonic lethally at e12.5 to e14.5 due to defective blood vessel development (Liu et al., 2000)]. The attachment of osteoclast precursors to bone surfaces was significantly enhanced in S1PR1-/- animals compared with controls. S1PR1-/- osteoclasts precursors on bone surfaces subsequently develop into mature osteoclasts and absorb bone tissues. S1P-mediated chemotaxis of osteoclast precursors would thus be expected to contribute to their redistribution from bone tissues to blood vessels.
We also performed intravital two-photon imaging of bone tissues to define the role of S1PR2 in vivo (Ishii et al., 2010). We showed that certain osteoclast precursors (CX3CR1-EGFPpositive cells) moved into the bloodstream when a potent S1PR2 antagonist, JTE013 (Osada et al., 2002), was injected intravenously. The effect of JTE013 was less pronounced than that of the S1PR1 agonist SEW2871. Furthermore, to clarify the physiological significance of S1P-/- chemotaxis of osteoclast precursors in bone homeostasis, we examined S1PR2-deficient (S1PR2-/-) mice. Although S1PR2-/- mice suffer auditory impairment due to vessel defects in the inner ear, they survive and reproduce (Kono et al., 2007). Although bone resorption of osteoclasts was significantly lower in S1PR2-/- animals than in controls, in vitro osteoclast formation was not significantly affected. In a high-S1P environment such as the bloodstream, S1PR1 is activated and rapidly internalized, allowing S1PR2 to predominate. Osteoclast precursors enter the bone marrow as a result of chemorepulsion mediated by S1PR2, and other chemokines attract them to bone surfaces. After they enter a low-S1P environment such as bone marrow, S1PR1 is transported back to the cell surface, and osteoclast precursors return from bone tissues to blood vessels as a result of chemotaxis to an S1P gradient.
The number of osteoclast precursors on bone surfaces is determined by the balance between the trafficking of osteoclast precursors to and from the circulation. These data provide evidence that S1P controls the migratory behavior of osteoclast precursors, dynamically regulating bone mineral homeostasis, and identify a critical control point in osteoclastogenesis. Based on our findings, we propose that regulation of the migratory behavior of osteoclast precursors controls osteoclast differentiation. This control mechanism is summarized in Fig. 2. This critical control point in osteoclastogenesis may represent an attractive target for new treatments for osteoporosis. We previously showed that treatment with FTY720, which is metabolized by SPHK2 to a compound that acts as an agonist for four of the five S1P receptors (not S1PR2) (Cyster, 2005; Matloubian et al., 2004), relieved ovariectomy-induced osteoporosis in mice by reducing the number of mature osteoclasts attached to bone surfaces (Ishii et al., 2009). The mechanism of action of S1P is completely different from that of conventional treatments such as bisphosphonates, which suppress mature osteoclasts. We anticipate that the regulation of osteoclast precursor migration may be a useful clinical strategy in the near future.
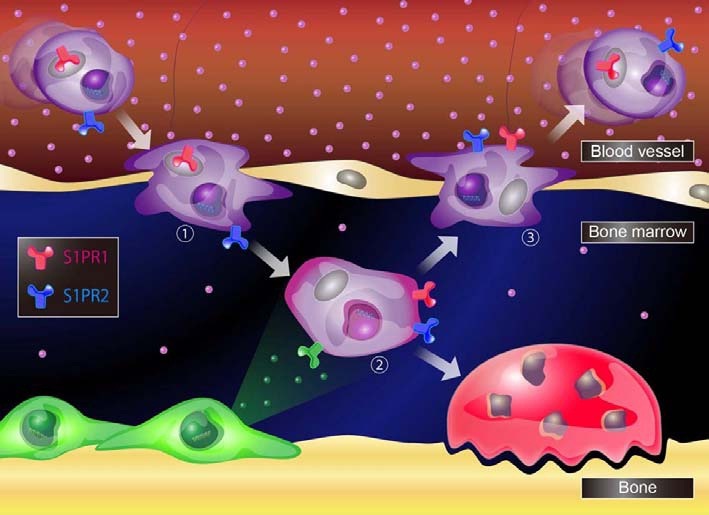
Fig. 2. A schematic model for S1P-mediated osteoclast precursors localization. The entry of osteoclast precursors from blood vessels where S1P is at high concentration, is initiated by chemorepulsion through S1PR2 (1). Once enter in bone marrow, osteoclast precursors migrate toward chemokines enriched in bone marrow cavity (2). On the other hand, their recirculation toward blood vessels is regulated by chemoattraction through S1PR1 (3).
FTY720 is a reversible immunosuppressive agent approved as a treatment for multiple sclerosis in the United States. It induces lymphopenia by confining lymphocytes to lymphoid organs (Mandala et al., 2002). The precise mechanisms behind this phenomenon remain controversial, and it is necessary to determine how FTY720 produces the opposite effect on monocyte- macrophage cells in bone marrow (which are expelled into the circulation by FTY720).
Future directions for two-photon microscopy
Two-photon intravital imaging has revealed, and continues to reveal, dynamic features of physiological and pathological process. Its greatest strength is its ability to provide spatiotemporal information in living organisms, which cannot be achieved using other methods. However, current two-photon microscopy imaging techniques have several limitations. First, we cannot see everything in the visual fields in two-photon microscopy. Although fluorescence labeling and second-harmonic generation enable us to observe target cells and organs, the lack of a signal does never reflect an open field, as diverse structures and cellular components should be present. To avoid misinterpretation, we must interpret our observations with caution. Second, although two-photon microscopy has greater penetration depth than conventional confocal microscopy, its penetration depth is only 800-1000 μm in soft tissues (e.g., brain cortex) and 200 μm in hard tissues (e.g., bone). Because of these resolution limitations, it may only be applied to small animals, such as mice and rats. Moreover, due to the wide scattering of light by the skin, it is necessary that target organs should be exteriorized. It is possible that the necessary operative invasion and changes in oxygen concentration and humidity may influence cellular behavior. To resolve these problems, technical innovations in fluorochrome and optical systems, including improvements in light emission and amelioration of resolution problems (Ntziachristos, 2010), are needed.
Intravital microscopy has begun to be applied not only to observational studies, but also to functional analysis and interventions. Recently, several new fluorescence tools have been developed. These include cell-cycle indicators (Sakaue-Sawano et al., 2008) and light-sensing devices such as photoactivatable fluorescent proteins (Victora et al., 2010) and light-induced activators of G protein-coupled receptors (Airan et al., 2009).
CONCLUSION
As the recruitment of osteoclast precursors during osteoclastogenesis is dynamic and dependent on the microenvironment of the bone marrow cavity, temporospatial information is very important. Intravital imaging has made a huge contribution to improving our understanding of these processes. It enables us to visualize, temporospatially, complicated systems in living organisms. This new technique has revealed that S1P acts in concert with several chemoattractants to shepherd osteoclast precursors to appropriate sites. Controlling the recruitment and migration of osteoclast precursors represents a promising new therapeutic strategy for combating bone diseases. Although their limitations remain to be resolved, the range of applications for in vivo imaging techniques continues to expand.
References
- 1.Airan R.D., Thompson K.R., Fenno L.E., Bernstein H., Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. (2009);458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 2.Argraves K.M., Gazzolo P.J., Groh E.M., Wilkerson B.A., Matsuura B.S., Twai W.O., Hammad S.M., Argraves W.S. High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J. Biol. Chem. (2008);283:25074–25081. doi: 10.1074/jbc.M801214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavanagh L.L., Bonasio R., Mazo I.B., Halin C., Cheng G., van der Velden A.W., Cariappa A., Chase C., Russell P., Starnbach M.N., et al. Activation of bone marrow-resident memory T cells by circulating antigen-bearing dendritic cells. Nat. Immunol. (2005);6:1029–1037. doi: 10.1038/ni1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cyster J.G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. (2005);23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 5.Deal C. Future therapeutic targets in osteoporosis. Curr. Opin. Rheumatol. (2009);4:380–385. doi: 10.1097/BOR.0b013e32832cbc2a. [DOI] [PubMed] [Google Scholar]
- 6.Golfier S., Kondo S., Schulze T., Takeuchi T., Vassileva G., Achtman A.H., Gräler M.H., Abbondanzo S.J., Wiekowski M., Kremmer E., et al. Shaping of terminal megakaryocyte differentiation and proplatelet development by sphingosine-1- phosphate receptor S1P4. FASEB J. (2010);24:4701–4710. doi: 10.1096/fj.09-141473. [DOI] [PubMed] [Google Scholar]
- 7.Gon Y., Wood M.R., Kiosses W.B., Jo E., Sanna M.G., Chun J., Rosen H. S1P3 receptro-induced reorganization of epithelial tight junctions compromises lung barrier integritiy and is potentiated by TNF. Proc. Natl. Acad. Sci. USA. (2005);102:9270–9275. doi: 10.1073/pnas.0501997102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Hannun Y.A., Obeid L.M. Principles of bioactive lipid signaling: lessons from sphingolipids. Nat. Rev. Mol. Cell. Biol. (2008);9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 9.Harada S., Rodan G.A. Control of osteoblast function and regulation of bone mass. Nature. (2003);423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 10.Ishii M., Egen J.G., Klauschen F., Meier-Schellersheim M., Saeki Y., Vacher J., Proia R.L., Germain R.N. Sphingosine- 1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. (2009);458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii M., Kikuta J., Shimazu Y., Meier-Schellersheim M., Germain R.N. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J. Exp. Med. (2010);207:2793–2798. doi: 10.1084/jem.20101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koizumi M., Saitoh Y., Minami T., Takeno N., Tsuneyama K., Miyahara T., Nakayama T., Sakurai H., Takano Y., Nishimura M., et al. Role of CX3CL1/fractalkine in osteoclast differentiation and bone resorption. J. Immunol. (2009);183:7825–7831. doi: 10.4049/jimmunol.0803627. [DOI] [PubMed] [Google Scholar]
- 13.Kono M., Belyantseva I.A., Skoura A., Frolenkov G.I., Starost M.F., Dreier J.L., Lidngton D., Bolz S.S., Friedman T.B., Hla T., et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. J. Biol. Chem. (2007);282:10690–10696. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Wada R., Yamashita T., Mi Y., Deng C.X., Hobson J.P., Rosenfeldt H.M., Nava V.E., Chae S.S., Lee M.J., et al. Edg-1, the G-protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. (2000);106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandala S., Hajdu R., Bergstrom J., Quackenbush E., Xie J., Milligan J., Thornton R., Shei G.J., Card D., Keohane C. Alteration of lymphocyte trafficking by sphingosine-1- phosphate receptor agonists. Science. (2002);296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 16.Matloubian M., Lo C.G., Cinamon G., Lesneski M.J., Xu Y., Brinkmann V., Allende M.L., Proia R.L., Cyster J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. (2004);6972:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 17.Mazo I.B., Honczarenko M., Leung H., Cavanagh L.L., Bonaisio R., Weninger W., Engelke K., Xia L., McEver R.P., Koni P.A., et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8 T cells. Immunity. (2005);22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Nofer J.R., van der Giet M., Tolle M., Wolinska I., von Wnuck Lipinski K., Baba H.A., Tietge U.J., Godecke A., Ishii I., Kleuser B., et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Invest. (2004);113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat. Methods. (2010);7:603–614. doi: 10.1038/nmeth.1483. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto H., Takuwa N., Yokomizo T., Sugimoto N., Sakurada S., Shigematsu H., Takuwa Y. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol. Cell. Biol. (2000);20:9247–9261. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osada M., Yatomi Y., Ohmori T., Ikeda H., Ozaki Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem. Biophys. Res. Commun. (2002);299:483–487. doi: 10.1016/s0006-291x(02)02671-2. [DOI] [PubMed] [Google Scholar]
- 22.Rivera J., Proia R.L., Olivera A. The alliance of sphingosine- 1-phosphate and its receptors in immunity. Nat. Rev. Immunol. (2008);8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen H., Goetzl E.J. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. (2005);5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 24.Rosen H., Sanna M.G., Cahalan S.M., Gonzalez-Cabrera P.J. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. (2007);28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Russell R.G.G., Xia Z., Dunford J.E., Oppernann U., Kwaasi A., Hulley P.A., Kavanagh K.L., Triffitt J.T., Lundy M.W., Phipps R.J., et al. Bisphosphonates. An update on mechanisms of action and how these relate to clinical efiicacy. Ann. N Y Acad. (2007);1117:209–257. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 26.Sakaue-Sawano A., Kurokawa H., Morimura T., Hanyu A., Hama H., Osawa H., Kashiwagi S., Fukami K, Miyata T., Miyoshi H., et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. (2008);132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 27.Serrier-Lanneau V., Teixeira-Clerc F., Li L., Schippers M., de Wris W., Julien B., Tran-Van-Nhieu J., Manin S., Pelstra K., Chun J. et al. The sphingosine 1-phosphate receptor S1P2 triggers hepatic wound healing. FASEB J. (2007);21:2005–2013. doi: 10.1096/fj.06-6889com. [DOI] [PubMed] [Google Scholar]
- 28.Takuwa Y. Subtype-specific differential regulation of Rho family G proteins and cell migration by the Edg family sphingosine- 1-phosphate receptors. Biochem. Biophys. Acta. (2002);1682:112–120. doi: 10.1016/s1388-1981(02)00145-2. [DOI] [PubMed] [Google Scholar]
- 29.Teitelbaum S.L., Ross F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genetic. (2003);4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 30.Victora G.D., Schwichkert T.A., Fooksman D.R., Kamphorst A.O., Meyer-Hermann M., Dustin M.L., Nussenzweig M.C. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. (2010);143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei S.H., Rosen H., Matheu M.P., Sanna M.G., Wang S.K., Jo E., Wong C.H., Parker I., Cahalan M.D. Sphingosine 1-phospate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat. Immunol. (2005);12:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- 32.Yasuda Y., Kaleta J., Bromme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv. Drug. Deliv. Rev. (2005);57:973–993. doi: 10.1016/j.addr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Yu X., Huang Y., Collin-Osdoby P., Osdoby P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) acrivity, and collagen transmigration. J. Bone Miner. Res. (2003);18:1404–1418. doi: 10.1359/jbmr.2003.18.8.1404. [DOI] [PubMed] [Google Scholar]


