Abstract
Systematic searches using the complete genome sequence of rice (Oryza sativa) identified OsSUS7, a new member of the rice sucrose synthase (OsSUS) gene family, which shows only nine single nucleotide substitutions in the OsSUS5 coding sequence. Comparative genomic analysis revealed that the synteny between OsSUS5 and OsSUS7 is conserved, and that significant numbers of transposable elements are scattered at both loci. In particular, a 17.6-kb genomic region containing transposable elements was identified in the 5′ upstream sequence of the OsSUS7 gene. GFP fusion experiments indicated that Os- SUS5 and OsSUS7 are largely associated with the plasma membrane and partly with the cytosol in maize mesophyll protoplasts. RT-PCR analysis and transient expression assays revealed that OsSUS5 and OsSUS7 exhibit similar expression patterns in rice tissues, with the highest expression evident in roots. These results suggest that two redundant genes, OsSUS5 and OsSUS7, evolved via duplication of a chromosome region and through the transposition of transposable elements.
Keywords: duplication, localization, rice, sucrose synthase, transposable element
INTRODUCTION
Sucrose is the principal product of photosynthesis in the source tissues of higher plants and is also the major form by which carbon is transported from source leaves to various sink tissues via the phloem. Sucrose utilization in these tissues is initiated by its hydrolysis, catalyzed by two different classes of enzymes, invertase (INV) and sucrose synthase (SUS). Unlike INV, which catalyzes the irreversible hydrolysis of sucrose to glucose and fructose, SUS catalyzes the reversible conversion of sucrose and UDP to UDP-glucose and fructose (Sturm, 1999; Sturm and Tang, 1999). In higher plants, INV and SUS are both present as multiple isoforms. The INVs can be classified into three distinct types, cell-wall invertase (CIN), vacuolar invertase (VIN) and cytosolic neutral or alkaline invertase (NIN). SUSs exist as either free molecules in the cytosol or in association with various subcellular compartments, including the plasma membrane, actin filaments, mitochondria and vacuolar membrane (Duncan and Huber, 2007; Etxeberria and Gonzalez, 2003; Subbaiah et al., 2006; Winter et al., 1997; 1998).
The sucrose that is unloaded in sink tissues is hydrolyzed to monosaccharide sugars to serve as a storage material, carbohydrate backbone, and energy source in many higher plants. In this regard, SUS activities in sink tissues appeared to be essential for plant growth and development. Important roles of the SUS enzymes have now been demonstrated in the sink organs of many plant species, including potato tuber (Zrenner et al., 1995), tomato fruit (D’Aoust et al., 1999), cotton fiber (Ruan et al., 2003), maize endosperm (Chourey et al., 1998), watermelon seed (Kim et al., 2002) and the Arabidopsis silique and seed (Fallahi et al., 2008). In these previous reports, SUS activities correlated well with the sink strength of the storage tissues. For instance, the reduction of SUS activity in tomato fruit and potato tuber resulted in the reduction of the starch content and sucrose unloading capacity, coupled with a decrease in the fruit setting or tuber dry weight (D’Aoust et al., 1999; Zrenner et al., 1995).
SUS genes have been found in a number of plant species, including maize (Carlson et al., 2002), rice (Harada et al., 2005; Hirose et al., 2008; Huang et al., 1996; Wang et al., 1992), Arabidopsis (Barratt et al., 2001; 2009; Bieniawska et al., 2007), pea (Barratt et al., 2001), potato (Fu and Park, 1995), tomato (Chengappa et al., 1999), carrot (Sturm et al., 1999) and lotus (Horst et al., 2007). Through systematic searches of the complete genome sequence, it has been reported that the Arabidopsis and rice genomes both encode a small multigene family consisting of six SUS genes (Barratt et al., 2001; Bieniawska et al., 2007; Harada et al., 2005; Hirose et al., 2008). Phylogenetic analyses have further shown that SUS genes can be divided into three different groups, SUSI, SUSII, and SUSIII, each likely having distinct functions (Bieniawska et al., 2007). Of note, the SUSIII group has been found only in Arabidopsis and rice and its function remains unclear. Hence, further analysis of this third group of genes is necessary to more fully understand the function of the entire SUS gene family.
In our present study, we isolated a new member of the OsSUS gene family, OsSUS7, through analysis of the complete genome sequence of rice. OsSUS7 is nearly identical to OsSUS5 except for nine single nucleotide differences. We first investigated the evolutionary relationship between OsSUS7 and the previously reported SUS genes including OsSUS5. We then compared the expression and subcellular localization of OsSUS7 with that of OsSUS5 and other OsSUS genes by RTPCR analysis and transient expression assays using promoterluciferase (LUC) fusion and GFP fusion constructs. Finally, we discuss the duplication of OsSUS5 and OsSUS7 genes and their function in rice.
MATERIALS AND METHODS
Plant materials
Rice seeds (Oryza sativa L. cultivar Jinmi) provided by the National Institute of Crop Science (Korea) were used in all experiments, except for the seed coat- and endosperm- specific cDNA libraries (Jun et al., 2004) which were constructed using the cultivar Dongjin. Plants were grown until maturity in a greenhouse at 30℃ during the day and at 20℃ at night in a light/dark photoperiod of 16/8 h. To examine the expression pattern of OsSUS genes, all rice samples were harvested as described by Cho et al. (2005; 2006), frozen in liquid nitrogen and kept at -80℃ until needed.
Cloning of the OsSUS5 and OsSUS7 genes
Full-length cDNAs of the OsSUS5 and OsSUS7 genes were isolated by RT-PCR, using gene-specific primers designed to encompass the translation start and stop codons as listed in the rice sequence database (Supplementary Table 1). cDNAs synthesized using the total mRNAs isolated from the root were used in the PCR experiments. All cDNAs were cloned into the pENTR/D-TOPO vector (Invitrogen) and sequenced automatically. To avoid PCR errors, at least five independent cDNA clones for each gene were analyzed. These cDNA sequences have been submitted to the NCBI database and the accession numbers are HQ895723 and HQ895725 for OsSUS5 and OsSUS7, respectively.
Comparative analysis of genomic structures
The genomic structure of each OsSUS gene was determined by aligning the cDNA and genomic sequences of BAC clones obtained from the NCBI database and the Rice Genome Annotation Project Database (http://rice.plantbiology.msu.edu/). The accession numbers (BAC clone numbers) of the clones used to analyze the genomic structure are as follows: OsSUS1, AC- 084380 (OSJNBa0090P23); OsSUS2, AP004280 (P0648E08); OsSUS3, AP004988(B1056G08); OsSUS4, AC131175 (OSJNBa0030D15); OsSUS5, AL662942 (OsJNBa0033H08) and AL731596 (OsJNBa0024J22); OsSUS6, AP004082 (OJ1149_ C12); and OsSUS7, AL663002 (OsJNBb0026I12).
Sequence comparisons and phylogenetic analysis
The deduced amino acid sequences of the translated OsSUS genes were aligned with those of previously reported genes using the Clustal W program (Thompson et al., 1994). Phylogenetic analyses were conducted using MEGA version 4 (Tamura et al., 2007) and the neighbor-joining method. The accession numbers of the sequences used to construct the phylogenetic tree are as follows: AtSUS1-AtSUS6 from Arabidopsis, At5g20830, At5g49190, At4g02280, At3g43190, At5g- 37180, and At1g73370, respectively (Baud et al., 2004); AgSUS from Alnus glutinosa, X92378 (van Ghelue et al., 1996); BoSUS1-BoSUS4 from Bambusa oldhamii, AF412036, AF- 412038, AF412037, and AF412039, respectively; BvSBSS1 and BvSBSS2 from sugar beet, AY457173 and EF660856, respectively (Hesse and Willmitzer, 1996; Klotz and Haagenson, 2008); ClSUS from Citrullus lanatus, AB018561; Cpss1 and Cpss2 from Craterostigma plantagineum, AJ131999 and AJ132000, respectively (Kleines et al., 1999); CrSUS from Chenopodium rubrum, X82504 (Godt et al., 1995); CitSUS1 and CitSUSA from citrus, AB022092 and AB022091 (Komatsu et al., 2002); DcSUS1 and DcSUS2 from carrot, X75332 and Y16091 (Sebkova et al., 1995; Sturm et al., 1999); GhSUS from Gossypium hirsutum, U73588; GmSUS from soybean, AF030231; HvSUS1 and HvSUS2 from barley, X65871 and X69931 (Sánchez de la Hoz et al., 1992); LeSUS1 and LeSUS2 from tomato, L19762 and AJ011319 (Chengappa et al., 1999; Wang et al., 1993); MsSUS from Medicago sativa, AF049487; MtSUS1 from Medicago truncatula, AJ131943 (Hohnjec et al., 1999); MySUS from the tropical epiphytic CAM orchid Mokara Yellow, AF530568 (Li et al., 2004); OgSUS from the tropical epiphytic orchid Oncidium goldiana, AF530567; OsSUS1-OsSUS7 from rice, LOC_Os03g28330, LOC_Os06g 09450, LOC_Os07g42490, LOC_Os03g22120, LOC_Os04g 24430, LOC_Os02g58480, and LOC_Os04g17650, respectively (Hirose et al., 2008; Huang et al., 1996; Wang et al., 1992); PdSUS1 and PdSUS2 from Potamogeton distinctus, AB193515 and AB193516; PtSUS from Populus tremuloides, AY341026; PvSS from bean, AF315375 (Silvente et al., 2003); PsSUS1-PsSUS3 from pea, AJ012080, AJ001071, and AJ311496, respectively (Barratt et al., 2001; Craig et al., 1999); SoSusy2 from Saccharum officinarum, AY118266; StSUS2-StSUS4 from potato, AY205302, U24088, and U24087, respectively (Fu and Park, 1995; Salanoubat and Belliard, 1987); TaSUS1 and TaSUS2 from Triticum aestivum, AJ001117 and AJ000153; TgSUS1 and TgSUS2 from tulip, X96938 and X96939 (Balk and de Boer, 1999); VfSUS from fava bean, X69773; Vrvss1 from mung bean, D10266 (Arai et al., 1992); and ZmSH1, ZmSUS1 and ZmSUS3 from maize, X02400, L22296 and AY124703, respectively (Werr et al., 1985).
Semi-quantitative RT-PCR analysis
Total RNA was prepared from various organs using Trizol reagent (Invitrogen), treated with DNaseI (Ambion) and then reverse-transcribed using an oligo-dT primer and a First-Strand cDNA Synthesis kit (Roche). Endosperm and seed coat cDNAs were prepared from seeds harvested 6-10 days after fertilization (DAF) in accordance with the method of Cho et al. (2005; 2006). To prevent genomic DNA contaminations, primers were designed to amplify a region encompassing at least one intron for each gene (Supplementary Table 2). The rice actin1 gene OsAct1 was used as a PCR control (Cho et al., 2006). These experiments were repeated at least three times. To distinguish between the expression patterns of OsSUS5 and OsSUS7, the RT-PCR products of both genes were further digested with the restriction enzyme DraIII, which specifically cuts the amplified product of OsSUS5 only to yield 315- and 236-bp fragments, whilst the 551-bp OsSUS7 amplicon remains intact.
Subcellular localization analysis
Full-length cDNAs of the OsSUS genes were amplified by PCR using proofreading DNA polymerase (SolGent, Korea) and the appropriate primer pairs (Supplementary Table 1). PCR products were inserted into the pENTR/D-TOPO vector (Invitrogen) and sequenced. Validated cDNAs were subcloned into p2FGW7 using LR clonase (Invitrogen) to produce N-terminal GFP fusion products (Karimi et al., 2002). The resulting GFPOsSUS fusion constructs driven by the CaMV35S promoter were delivered into maize mesophyll protoplasts using a polyethylene glycol (PEG)-calcium mediated method followed by 12-24 h incubation to allow transient expression (Cho et al., 2009; Hwang and Sheen, 2001). Chlorophyll autofluorescence was used as a chloroplast marker. Expression of these fusion constructs was monitored using a confocal microscope (LSM 510 META, Carl Zeiss, Germany).
Transient expression assay
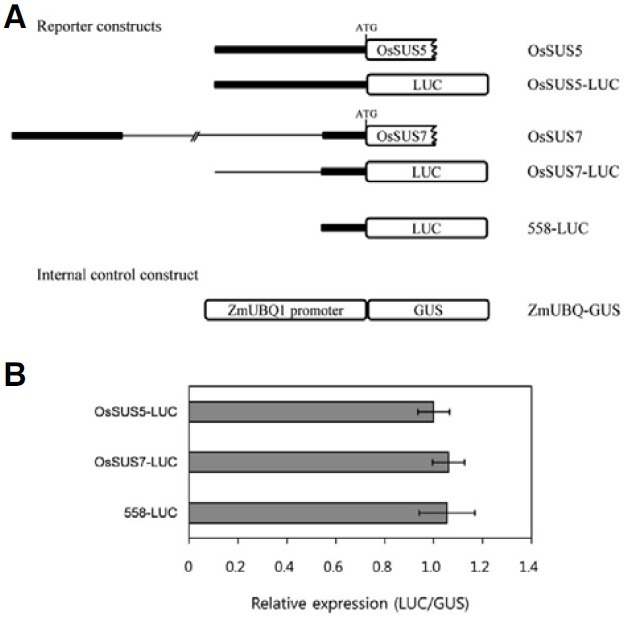
To construct reporter vectors, the native 2.19-kb OsSUS5 promoter and 2.46-kb OsSUS7 promoter, and the conserved 558-bp 5′ regulatory region of OsSUS5 and OsSUS7, were amplified by PCR using the appropriate primer pairs (Supplementary Table 1). The amplified PCR products were then inserted into the pENTR/D-TOPO vector (Invitrogen) and fused to the LUC gene of JJ2749 derived from pHGWL7 (Karimi et al., 2007) using LR clonase (Invitrogen). Three OsSUS promoter-LUC fusion constructs, OsSUS5-LUC, OsSUS7-LUC and 558-LUC, were used in this study. The ZmUBQ1-GUS (β-glucuronidase) fusion construct (Cho et al., 2009) was used as an internal control. Maize mesophyll protoplasts (1-2 × 105 cells/200 μl) isolated from the second leaves of etiolated plants were cotransfected with reporter constructs and an internal control ZmUBQ1-GUS using a PEG-calcium mediated method (Cho et al., 2009; Hwang and Sheen, 2001). Transformed protoplasts were incubated for 6 h and then harvested. The harvested protoplasts were resuspended in lysis buffer and used for LUC and GUS assays. LUC and GUS assays were performed using previously described methods (Cho et al., 2009; Jefferson et al., 1987). The LUC and GUS fluorescence activity was measured using the VICTOR2 1420 multilabel counter (PerkinElmer Life Sciences). All transient expression experiments were repeated three times and produced similar results.
RESULTS
Identification and characterization of the OsSUS7 genomic region
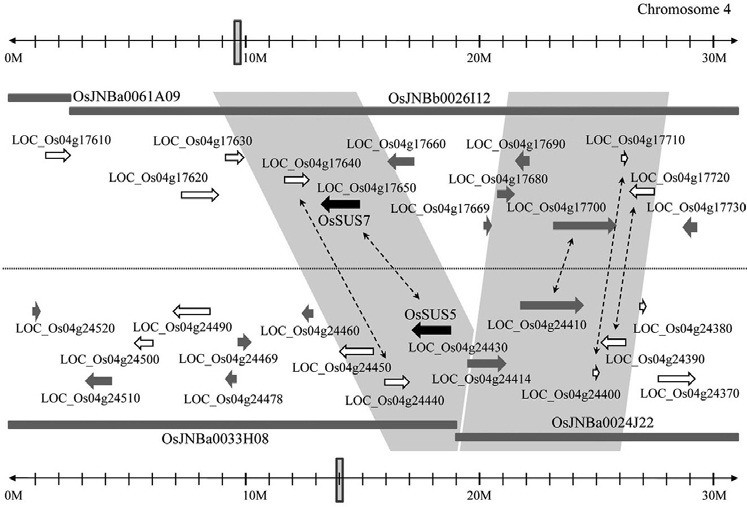
Systemic blast searches of the rice genome, using nucleotide and amino acid sequences of previously reported rice SUS genes (Harada et al., 2005; Hirose et al., 2008; Huang et al., 1996; Wang et al., 1992), identified seven genes encoding sucrose synthase enzymes. Thus, our analysis enabled the detection of a previously uncharacterized sucrose synthase gene, named OsSUS7. An in-depth examination further found that the genomic sequence of OsSUS7 is nearly identical to that of OsSUS5, which are located near to each other on chromosome 4. Comparative analysis of both genomic regions revealed an insertion region of a 17.6 kb in the promoter of OsSUS7, which was absent in the corresponding region of OsSUS5 (Fig. 1). Further analysis of the OsSUS5 and OsSUS7 genomic regions using BAC clones provided an interesting insight into the evolutionary history of both genes (Fig. 2). We found that several genes located at the periphery of OsSUS5 and OsSUS7 were nearly identical to each other. LOC_Os04g17640 and LOC_Os04g24440, LOC_Os04g17700 and LOC_ Os04g24410, LOC_Os04g17710 and LOC_Os04g24400, and LOC_Os04g17720 and LOC_Os04g24390, respectively, showed near identity indicating a conserved synteny between both loci. In addition, we found that a number of transposable elements are scattered among the OsSUS5 and OsSUS7 genes (Fig. 2). These data suggest that the OsSUS7 chromosomal region might have been duplicated from the OsSUS5 genomic region, which was followed by transposition events involving transposable elements. Alternatively, the OsSUS5 genomic segment may have been duplicated from that of OsSUS7 via an excision of the 17.6-kb insertion region.
Fig. 1. Comparative analysis of the 5′ upstream regions of OsSUS5 and OsSUS7. An insertion region of 17.6-kb is present in the promoter of OsSUS7, but absent in the corresponding region of OsSUS5. The accession numbers of the BAC clones used to analyze 5′ upstream regions of OsSUS5 and OsSUS7 are listed in “Materials and Methods”.
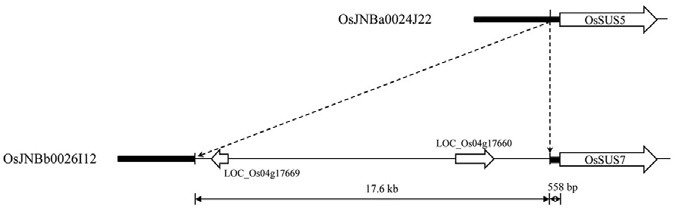
Fig. 2. Comparative analysis of the chromosomal regions encompassing OsSUS5 and OsSUS7 using BAC clones. Black arrows indicate OsSUS5 and OsSUS7 and open arrows indicate transposable elements. The accession numbers of the BAC clones used to identify the genomic structure of chromosomal region containing OsSUS5 and OsSUS7 are listed in “Materials and Methods”.
To determine their coding regions, we cloned both the OsSUS5 and OsSUS7 cDNAs by RT-PCR. Alignment of the cDNA and deduced amino acid sequences of these two genes indicated that they harbored only nine single nucleotide substitutions within their coding regions, resulting in a change of six amino acids (Supplementary Table 3).
Phylogenetic relationship between OsSUS7 and other SUS genes
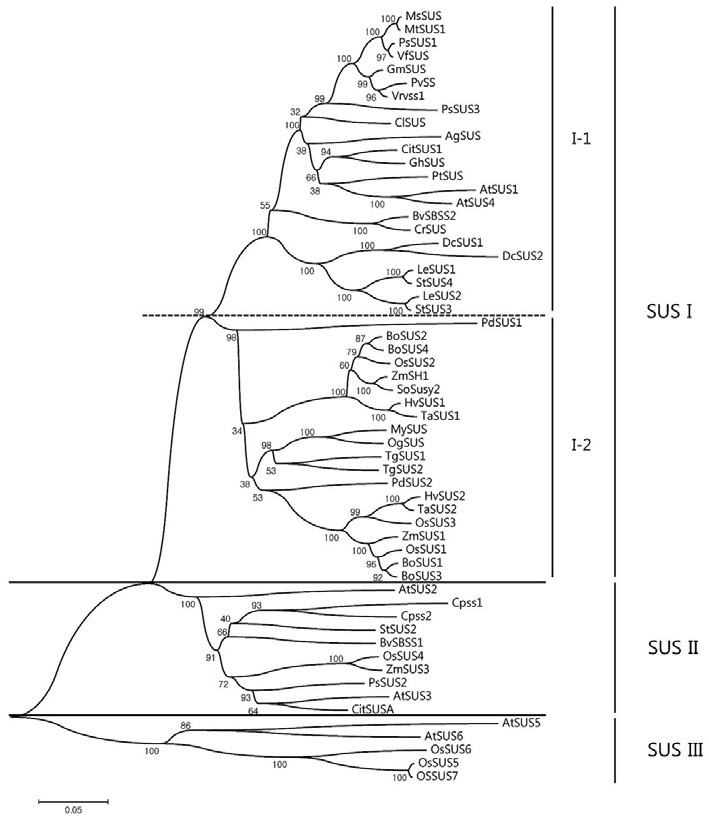
Further alignment of the deduced amino acid sequences of all OsSUS cDNAs including OsSUS7 confirmed the presence of two separate conserved domains in each case, a sucrose synthase domain and a glycosyl-transferase domain. These are common to SUS proteins (Baud et al., 2004; data not shown). To classify these plant SUS enzymes, we constructed a rooted phylogenetic tree using the neighbor-joining method (Fig. 3). Three major groups were thereby identified: SUS group I, SUS group II (also referred to as SUSA), and SUS group III. Group I was further subdivided into group I-1 consisting of dicot SUSs and group I-2 containing monocot SUSs. The rice OsSUS1, OsSUS2, and OsSUS3 genes belong to the monocot group I-2 along with the maize sucrose synthases, ZmSH1 and ZmSUS1, whereas the Arabidopsis genes AtSUS1 a nd AtSUS4 were classified into the dicot group I-1. This result suggests that SUS group I was subdivided into the dicot group I-1 and monocot group I-2 prior to the monocot-dicot divergence. The SUS group II gene, OsSUS4, could be classified with the maize SUS, ZmSUS3, and two Arabidopsis SUS genes, AtSUS2 and AtSUS3. Interestingly, OsSUS5, OsSUS6, and OsSUS7 belong to SUS group III, the members of which possess unique carboxy-terminal extensions relative to the other groups, as is the case for AtSUS5 and AtSUS6 (Baud et al., 2004).
Fig. 3. Phylogenetic tree of the sucrose synthase (SUS) genes constructed using the neighbor-joining method with MEGA version 4 software. The scale bar corresponds to a distance of 5 changes per 100 amino acids. Bootstrap analysis was performed with 1000 replicates and numbers in the branches indicate bootstrap values as percentages. Accession numbers are listed in “Materials and Methods”.
Genomic structure of the rice SUS genes
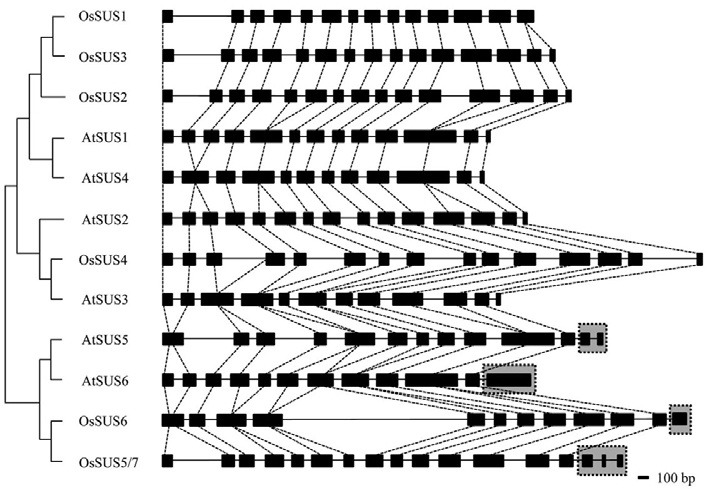
The inferred genomic structures of seven OsSUS genes were determined by aligning the cDNA sequences and genomic sequences of the corresponding BAC/PAC clones obtained from the NCBI database. The Arabidopsis Information Resource (TAIR) (http://arabidopsis.org/) database was used to additionally determine the genomic structures of the six Arabidopsis SUS genes. The exon/intron structures of these SUS genes were analyzed and compared using the coding region between the translation start and stop codons, except for the non-coding leader exon 1 found in some SUS genes (Fig. 4 and Supplementary Figs. 1 and 2). The results indicate that the exon sizes in the rice SUS genes are highly conserved except for the unique 3′ extension regions of the group III genes. Among the four rice SUS genes belonging to the groups I-2 and II, OsSUS1 is composed of 14 exons due to the presence of a 14th 187-bp unsplit exon, whereas the other three OsSUS genes comprised 15 exons. In the SUS group II gene OsSUS4, the number and sizes of the exons were quite similar to the three OsSUS genes belonging to the SUS group I-2. However, OsSUS4 is the largest SUS gene in rice due to four relatively large introns of 400-600 bp. Three OsSUS genes belonging to group III, OsSUS5, OsSUS6 and OsSUS7, possess unique 3′ extension regions, similar to AtSUS5 and AtSUS6 (Fig. 4 and Supplementary Figs. 1 and 2).
Fig. 4. Genomic structures of Arabidopsis SUS (AtSUS) genes and rice SUS (OsSUS) genes. Exons are indicated by black rectangles and introns by lines. The unique C-terminal regions of the group III genes are denoted by gray boxes with a dotted line.
Comparative expression analysis of OsSUS5 and OsSUS7
The 17.6-kb fragment located 558-bp upstream of the translation start site of OsSUS7 (Fig. 1) could inhibit gene expression. To test this possibility, we generated three promoter-LUC fusion constructs driven by the native 2.19-kb OsSUS5 promoter and 2.46-kb OsSUS7 promoter, and the conserved 558-bp 5′ regulatory region of OsSUS5 and OsSUS7, respectively (Fig. 5A). In transient expression analysis using maize mesophyll protoplasts, the relative expression levels of these three constructs were not significantly different, indicating that the 558-bp upstream region of OsSUS7 is sufficient for its endogenous expression, and that the 17.6-kb insertion may not have a large influence on the expression of OsSUS7, at least in mesophyll protoplasts (Fig. 5).
Fig. 5. Comparative expression analysis of the upstream regulatory regions of OsSUS5 and OsSUS7 using maize protoplasts. (A) Schematic diagram of the OsSUS5 and OsSUS7 genomic structure. Promoter regions are indicated by the thick line and the 17.6 kb region present in the promoter of OsSUS7 is highlighted by the thin line. Vectors used in transient expression experiments; OsSUS5- LUC, OsSUS7-LUC and 558-LUC. The ZmUBQ1-GUS construct was used as an internal control. (B) Transient expression assays of the three reporter constructs, OsSUS5-LUC, OsSUS7-LUC and 558-LUC, in maize mesophyll protoplasts. The bar indicates standard errors.
Subcellular localization of the OsSUS5 and OsSUS7 proteins
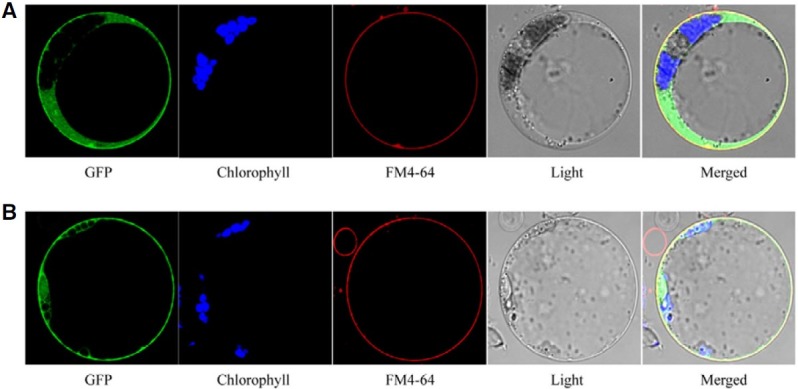
The subcellular localization of SUS proteins was an important key to elucidating their intracellular function. To determine the subcellular localization of OsSUS5 and OsSUS7, we generated GFP-OsSUS fusion constructs under the control of the CaMV35S promoter. In transient expression assays using maize mesophyll protoplasts, signals of GFP-OsSUS5 and GFP-OsSUS7 were found to be strongly associated with the plasma membrane and detectable in part in the cytosol (Figs. 6A and 6B). To confirm these plasma membrane-associated GFP signals, protoplasts were stained with a lipophilic styryl dye, FM4-64, which primarily stains the plasma membrane (Cho et al., 2010; Ueda et al., 2001; Uemura et al., 2004). The GFP signals were indeed well merged with those of FM4-64 in the plasma membrane (Figs. 6A and 6B), indicating that OsSUS5 and OsSUS7 are predominantly associated with this structure.
Fig. 6. Subcellular localization of GFP-OsSUS5 and GFP-OsSUS7 fusion proteins in transfected mesophyll protoplasts of maize. (A) GFPOsSUS5, (B) GFP-OsSUS7. Chlorophyll autofluorescence and FM4-64 were used as chloroplast and plasma membrane markers, respectively. The GFP signals are indicated in green, and plasma membranes stained with FM4-64 are shown in red. A false color (blue) was used to monitor chlorophyll autofluorescence to distinguish it from GFP (green) and FM4-64 (red).
Expression profile analysis of OsSUS genes during rice seed development
The spatiotemporal expression pattern of the OsSUS genes was examined by RT-PCR (Supplementary Fig. 3). Whilst only two genes, OsSUS2 and OsSUS4, were found to be expressed at considerable levels in the leaf tissues, transcripts of many of OsSUS genes were more abundantly detectable in sink tissues such as roots, flowers, and immature seeds, suggesting that these SUS enzymes may have functions in these sink organs. Interestingly, we also found that OsSUS5 and OsSUS7 are highly expressed in roots with similar expression patterns in all tested rice tissues, which is consistent with the results of the previous transient expression assay.
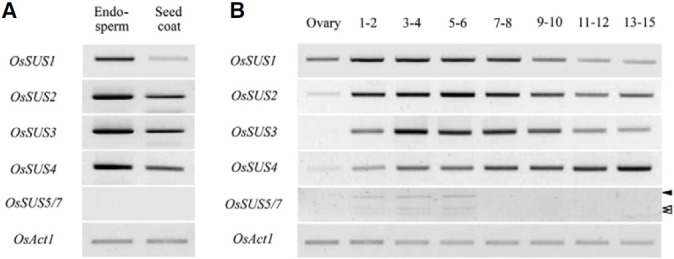
To elucidate the roles of OsSUS genes, particularly during rice seed development, we examined their expression in the endosperm and seed-coats of a series of seeds collected at different developmental stages (Cho et al., 2005). OsSUS1 to OsSUS4 are expressed abundantly in immature seeds and were found to be highly expressed in the endosperm rather than the seed coat prepared from 6 to 10 days after fertilization (DAF), whereas OsSUS5/OsSUS7 transcripts were not detected in these samples (Fig. 7A). During different developmental stages in immature seeds, OsSUS1 to OsSUS4 were found to be expressed throughout the pre-storage phase (1-8 DAF) and up to the starch-filling phase (9-15 DAF). In contrast, OsSUS5 and OsSUS7 transcripts were detectable only in 1-6 DAF immature seeds (Fig. 7B), which is consistent with the finding that OsSUS5 and OsSUS7 are barely expressed in 6-10 DAF immature seeds (Fig. 7A). These expression analysis data suggest that the OsSUS gene products have a role in rice seed development.
Fig. 7. Expression profiles of OsSUS genes during rice seed development. (A) Expression patterns of OsSUS genes in the endosperm and seed coat. (B) Changes in the gene expression levels of the OsSUS genes during rice seed development. Rice seeds were harvested over a time course up until 15 DAF. OsAct1 is a PCR control. The two open arrowheads and single closed arrowhead indicate the DraIII-digested OsSUS5 and DraIII-resistant OsSUS7 RT-PCR products, respectively.
DISCUSSION
OsSUS5 and OsSUS7 were formed by duplication and transposition events
In the present study, we identified a new rice SUS member, OsSUS7, which is a duplicate of the OsSUS5 gene. Our data indicate that OsSUS5 and OsSUS7 are almost identical at the nucleotide and amino acid sequence, genomic structure, expression pattern, and subcellular localization. We also found synteny between the OsSUS5 and OsSUS7 loci and identified a number of transposable elements widely scattered at the periphery of these loci. In addition, a 17.6-kb genomic region located 5′ upstream region of OsSUS7 consists of transposable elements. We thus concluded that OsSUS5 and OsSUS7 were formed via duplication, and by the transposition of transposable elements.
We previously reported three pairs of rice cell-wall invertases, OsCIN2 and OsCIN3, OsCIN5 and OsCIN6, and OsCIN7 and OsCIN8, each located in pairs, and a number of transposable elements that could be observed at the periphery of these OsCIN genes (Cho et al., 2005). In addition, OsCIN8, which is quite similar to OsCIN7, harbors a transposable element similar to the maize transposon MuDR within its second intron which causes the inactivation of the OsCIN8 gene (Cho et al., 2005). We therefore speculate that these OsCIN genes have also been formed via duplication and transposition events and that the surrounding regions of these genes are the preferred target loci of transposable elements.
Gene duplication and diversification events are important evolutionary processes. Duplication plays a central role in creating complex genetic systems by creating new loci such as the formation of resistance (R) gene cluster (Ronald, 1998). Transposition contributes to allelic diversity. Published sequence data have revealed that the rice genome contains more than 40% repetitive sequences related to transposable elements (Feng et al., 2002; Goff et al., 2002; Sasaki et al., 2002; Yu et al., 2002). Hence, retrotransposons are the most frequent transposable elements in rice and occupy approximately 15% of the rice genome (Jiang et al., 2004). Although most of the transposable elements are inactive in higher eukaryotes, some are active under normal conditions or can be activated in tissue cultures, such as Tos17 or Karma which become active in rice culture cells in response to DNA hypomethylation (Hirochika et al., 1996; Komatsu et al., 2003; Kwon et al., 2009). It is therefore likely that duplication and the transposition of transposable elements has occurred in the OsSUS5 and OsSUS7 genes.
OsSUS5 and OsSUS7 are predominantly associated with the plasma membrane
It is known that SUS proteins exist both freely in the cytosol and in association with various subcellular compartments including the plasma membrane (Duncan and Huber, 2007; Etxeberria and Gonzalez, 2003; Subbaiah et al., 2006; Winter et al., 1997; 1998). Interestingly, in our subcellular localization analysis of the OsSUS proteins, we found that OsSUS5 and OsSUS7, belonging to SUS group III, are associated predominantly with the plasma membrane and exist in part in the cytosol in mesophyll cells. In this regard, the in vitro phosphorylation of the ZmSUS1 protein in maize, belonging to group I-2, was found to cause the release of SUS from the membrane fraction (Winter et al., 1997). In the developing tomato fruit, the subcellular localization of SUS isoforms has also been found to be developmentally controlled, the membrane form being specifically detected in actively growing fruits, depending on the phosphorylation of SUS proteins (Anguenot et al., 2006). It will thus be interesting to determine whether the phosphorylation/dephosphorylation of OsSUS5 and OsSUS7 alters their subcellular localization. Notably, signals for the C-terminal GFP fusion constructs, OsSUS5-GFP and OsSUS7-GFP, were barely detectable in maize mesophyll protoplasts (data not shown). This may imply that the unique carboxy-terminal extensions of OsSUS5 and OsSUS7 are indispensible for the membrane association of these SUS proteins.
Generally, it is known that SUS activity correlates with the sink strength of various carbohydrate-storage or consuming organs (Hirose et al., 2008; Zrenner et al., 1995). In our current expression profile analysis, OsSUS transcripts, including those of OsSUS5 and OsSUS7 were abundantly detected in sink tissues such as roots, flowers, and immature seeds (Fig. 7 and Supplementary Fig. 3). Therefore, our results further support the contention that SUS enzymes play a major role in sink organs in the provision of carbon sources for sink metabolism. The SUS group III genes have recently been identified only in Arabidopsis and rice. The roles of these SUS isoforms are therefore still the subject of uncertainty and debate. Analysis of transgenic rice plants and loss-of function mutants for the novel group III genes, OsSUS5 and OsSUS7, will be invaluable for our enhanced understanding of the in vivo function of these rice isoforms.
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
Acknowledgments
This work was supported, in part, by grants from the Mid-Career Researcher Program (2010-0026679) and the World Class University program (R33-2008-000-10168-0) of the Korean Ministry of Education, Science and Technology.
References
- 1.Anguenot R., Nguyen-Quoc B., Yelle S., Michaud D. Protein phosphorylation and membrane association of sucrose synthase in developing tomato fruit. Plant Physiol. Biochem. (2006);44:294–300. doi: 10.1016/j.plaphy.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Arai M., Mori H., Imaseki H. Expression of the gene for sucrose synthase during growth of mung bean seedlings. Plant Cell Physiol. (1992);33:503–506. [Google Scholar]
- 3.Balk P.A., de Boer A.D. Rapid stalk elongation in tulip (Tulipa gesneriana L. cv. Apeldoorn) and the combined action of cold-induced invertase and the water-channel protein gammaTIP. Planta. (1999);209:346–354. doi: 10.1007/s004250050642. [DOI] [PubMed] [Google Scholar]
- 4.Barratt D.H., Barber L., Kruger N.J., Smith A.M., Wang T.L., Martin C. Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiol. (2001);127:655–664. [PMC free article] [PubMed] [Google Scholar]
- 5.Barratt D.H., Derbyshire P., Findlay K., Pike M., Wellner N., Lunn J., Feil R., Simpson C., Maule A.J., Smith A.M. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc. Natl. Acad. Sci. USA. (2009);106:13124–13129. doi: 10.1073/pnas.0900689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baud S., Vaultier M.N., Rochat C. Structure and expression profile of the sucrose synthase multigene family in Arabidopsis. J. Exp. Bot. (2004);55:397–409. doi: 10.1093/jxb/erh047. [DOI] [PubMed] [Google Scholar]
- 7.Bieniawska Z., Paul Barratt D.H., Garlick A.P., Thole V., Kruger N.J., Martin C., Zrenner R., Smith A.M. Analysis of the sucrose synthase gene family in Arabidopsis. Plant J. (2007);49:810–828. doi: 10.1111/j.1365-313X.2006.03011.x. [DOI] [PubMed] [Google Scholar]
- 8.Carlson S.J., Chourey P.S., Helentjaris T., Datta R. Gene expression studies on developing kernels of maize sucrose synthase (SuSy) mutants show evidence for a third SuSy gene. Plant Mol. Biol. (2002);49:15–29. doi: 10.1023/a:1014457901992. [DOI] [PubMed] [Google Scholar]
- 9.Chengappa S., Guilleroux M., Phillips W., Shields R. Transgenic tomato plants with decreased sucrose synthase are unaltered in starch and sugar accumulation in the fruit. Plant Mol. Biol. (1999);40:213–221. doi: 10.1023/a:1006136524725. [DOI] [PubMed] [Google Scholar]
- 10.Cho J.I., Lee S.K., Ko S., Kim H.K., Jun S.H., Lee Y.H., Bhoo S.H., Lee K.W., An G., Hahn T.R., et al. Molecular cloning and expression analysis of the cell-wall invertase gene family in rice (Oryza sativa L.). Plant Cell Rep. (2005);24:225–236. doi: 10.1007/s00299-004-0910-z. [DOI] [PubMed] [Google Scholar]
- 11.Cho J.I., Ryoo N., Ko S., Lee S.K., Lee J., Jung K.H., Lee Y.H., Bhoo S.H., Winderickx J., An G., et al. Structure, expression, and functional analysis of the hexokinase gene family in rice (Oryza sativa L.). Planta. (2006);224:598–611. doi: 10.1007/s00425-006-0251-y. [DOI] [PubMed] [Google Scholar]
- 12.Cho J.I., Ryoo N., Eom J.S., Lee D.W., Kim H.B., Jeong S.W., Lee Y.H., Kwon Y.K., Cho M.H., Bhoo S.H., et al. Role of the rice hexokinases OsHXK5 and OsHXK6 as glucose sensors. Plant Physiol. (2009);149:745–759. doi: 10.1104/pp.108.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho J.I., Burla B., Lee D.W., Ryoo N., Hong S.K., Kim H.B., Eom J.S., Choi S.B., Cho M.H., Bhoo S.H., et al. Expression analysis and functional characterization of the monosaccharide transporters, OsTMTs, involving vacuolar sugar transport in rice (Oryza sativa). New Phytol. (2010);186:657–668. doi: 10.1111/j.1469-8137.2010.03194.x. [DOI] [PubMed] [Google Scholar]
- 14.Chourey P.S., Taliercio E.W., Carlson S.J., Ruan Y.L. Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol. Gen. Genet. (1998);259:88–96. doi: 10.1007/s004380050792. [DOI] [PubMed] [Google Scholar]
- 15.Craig J., Barratt P., Tatge H., Dejardin A., Handley L., Gardner C.D., Barber L., Wang T., Hedley C., Martin C., et al. Mutations at the rug4 locus alter the carbon and nitrogen metabolism of pea plants through an effect on sucrose synthase. Plant J. (1999);17:353–362. [Google Scholar]
- 16.D’Aoust M.A., Yelle S., Nguyen-Quoc B. Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit. Plant Cell. (1999);11:2407–2418. doi: 10.1105/tpc.11.12.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan K.A., Huber S.C. Sucrose synthase oligomerization and F-actin association are regulated by sucrose concentration and phosphorylation. Plant Cell Physiol. (2007);48:1612–1623. doi: 10.1093/pcp/pcm133. [DOI] [PubMed] [Google Scholar]
- 18.Etxeberria E., Gonzalez P. Evidence for a tonoplastassociated form of sucrose synthase and its potential involvement in sucrose mobilization from the vacuole. J. Exp. Bot. (2003);54:1407–1414. doi: 10.1093/jxb/erg148. [DOI] [PubMed] [Google Scholar]
- 19.Fallahi H., Scofield G.N., Badger M.R., Chow W.S., Furbank R.T., Ruan Y.L. Localization of sucrose synthase in developing seed and siliques of Arabidopsis thaliana reveals diverse roles for SUS during development. J. Exp. Bot. (2008);59:3283–3295. doi: 10.1093/jxb/ern180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Q., Zhang Y., Hao P., Wang S., Fu G., Huang Y., Li Y., Zhu J., Liu Y., Hu X., et al. Sequence and analysis of rice chromosome 4. Nature. (2002);420:316–320. doi: 10.1038/nature01183. [DOI] [PubMed] [Google Scholar]
- 21.Fu H., Park W.D. Sink- and vascular-associated sucrose synthase functions are encoded by different gene classes in potato. Plant Cell. (1995);7:1369–1385. doi: 10.1105/tpc.7.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godt D.E., Riegel A., Roitsch T. Regulation of sucrose synthase expression in Chenopodium rubrum: characterization of sugar induced expression in photoautotrophic suspension cultures and sink tissue specific expression in plants. J. Plant Physiol. (1995);146:231–238. [Google Scholar]
- 23.Goff S.A., Ricke D., Lan T.H., Presting G., Wang R., Dunn M., Glazebrook J., Sessions A., Oeller P., Varma H., et al. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science. (2002);296:92–100. doi: 10.1126/science.1068275. [DOI] [PubMed] [Google Scholar]
- 24.Harada T., Satoh S., Yoshioka T., Ishizawa K. Expression of sucrose synthase genes involved in enhanced elongation of pondweed (Potamogeton distinctus) turions under anoxia. Ann. Bot. (Lond) (2005);96:683–692. doi: 10.1093/aob/mci220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hesse H., Willmitzer L. Expression analysis of a sucrose synthase gene from sugar beet (Beta vulgaris L.). Plant Mol. Biol. (1996);30:863–872. doi: 10.1007/BF00020799. [DOI] [PubMed] [Google Scholar]
- 26.Hirochika H., Sugimoto K., Otsuki Y., Tsugawa H., Kanda M. Retrotransposons of rice involved in mutations induced by tissue culture. Proc. Natl. Acad. Sci. USA. (1996);93:7783–7788. doi: 10.1073/pnas.93.15.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirose T., Scofield G.N., Terao T. An expression analysis profile for the entire sucrose synthase gene family in rice. Plant Sci. (2008);174:534–543. [Google Scholar]
- 28.Hohnjec N., Becker J.D., Puhler A., Perlick A.M., Kuster H. Genomic organization and expression properties of the MtSucS1 gene, which encodes a nodule-enhanced sucrose synthase in the model legume Medicago truncatula. Mol. Gen. Genet. (1999);261:514–522. doi: 10.1007/s004380050995. [DOI] [PubMed] [Google Scholar]
- 29.Horst I., Welham T., Kelly S., Kaneko T., Sato S., Tabata S., Parniske M., Wang T.L. TILLING mutants of Lotus japonicus reveal that nitrogen assimilation and fixation can occur in the absence of nodule-enhanced sucrose synthase. Plant Physiol. (2007);144:806–820. doi: 10.1104/pp.107.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J.W., Chen J.T., Yu W.P., Shyur L.F., Wang A.Y., Sung H.Y., Lee P.D., Su J.C. Complete structures of three rice sucrose synthase isogenes and differential regulation of their expressions. Biosci. Biotechnol. Biochem. (1996);60:233–239. doi: 10.1271/bbb.60.233. [DOI] [PubMed] [Google Scholar]
- 31.Hwang I., Sheen J. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature. (2001);413:383–389. doi: 10.1038/35096500. [DOI] [PubMed] [Google Scholar]
- 32.Jefferson R.A., Kavanagh T.A., Bevan M.W. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. (1987);6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang N., Feschotte C., Zhang X., Wessler S.R. Using rice to understand the origin and amplification of miniature inverted repeat transposable elements (MITEs). Curr. Opin. Plant Biol. (2004);7:115–119. doi: 10.1016/j.pbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Jun S.H., Han M.J., Lee S., Seo Y.S., Kim W.T., An G. OsEIN2 is a positive component in ethylene signaling in rice. Plant Cell. Physiol. (2004);45:281–289. doi: 10.1093/pcp/pch033. [DOI] [PubMed] [Google Scholar]
- 35.Karimi M., Inze D., Depicker A. Gateway vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. (2002);7:193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- 36.Karimi M., Bleys A., Vanderhaeghen R., Hilson P. Building blocks for plant gene assembly. Plant Physiol. (2007);145:1183–1191. doi: 10.1104/pp.107.110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J., Jun S.H., Kang H.G., Lee J., An G. Molecular characterization of a GA-inducible gene, Cvsus1, in developing watermelon seeds. Mol. Cells. (2002);14:255–260. [PubMed] [Google Scholar]
- 38.Kleines M., Elster R.C., Rodrigo M.J., Blervacq A.S., Salamini F., Bartels D. Isolation and expression analysis of two stress-responsive sucrose-synthase genes from the resurrection plant Craterostigma plantagineum (Hochst.). Planta. (1999);209:13–24. doi: 10.1007/s004250050602. [DOI] [PubMed] [Google Scholar]
- 39.Klotz K.L., Haagenson D.M. Wounding, anoxia and cold induce sugarbeet sucrose synthase transcriptional changes that are unrelated to protein expression and activity. J. Plant Physiol. (2008);165:423–434. doi: 10.1016/j.jplph.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Komatsu A., Moriguchi T., Koyama K., Omura M., Akihama T. Analysis of sucrose synthase genes in citrus suggests different roles and phylogenetic relationships. J. Exp. Bot. (2002);53:61–71. [PubMed] [Google Scholar]
- 41.Komatsu M., Shimamoto K., Kyozuka J. Two-step regulation and continuous retrotransposition of the rice LINEtype retrotransposon Karma. Plant Cell. (2003);15:1934–1944. doi: 10.1105/tpc.011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon S.J., Park K.C., Son J.H., Bureau T., Park C.H., Kim N.S. Sequence diversity of a domesticated transposase gene, MUG1, in Oryza species. Mol. Cells. (2009);30:459–465. doi: 10.1007/s10059-009-0061-8. [DOI] [PubMed] [Google Scholar]
- 43.Li C.R., Zhang X.B., Huang C.H., Hew C.S. Cloning, characterization and tissue specific expression of a sucrose synthase gene from tropical epiphytic CAM orchid Mokara Yellow. J. Plant Physiol. (2004);161:87–94. doi: 10.1078/0176-1617-01157. [DOI] [PubMed] [Google Scholar]
- 44.Ronald P.C. Resistance gene evolution. Curr. Opin. Plant Biol. (1998);1:294–298. doi: 10.1016/1369-5266(88)80049-9. [DOI] [PubMed] [Google Scholar]
- 45.Ruan Y.L., Llewellyn D.J., Furbank R.T. Suppression of sucrose synthase gene expression represses cotton fiber cell initiation, elongation, and seed development. Plant Cell. (2003);15:952–964. doi: 10.1105/tpc.010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salanoubat M., Belliard G. Molecular cloning and sequencing of sucrose synthase cDNA from potato (Solanum tuberosum L.): preliminary characterization of sucrose synthase mRNA distribution. Gene. (1987);60:47–56. doi: 10.1016/0378-1119(87)90212-5. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez de la Hoz P., Vicente-Carbajosa J., Mena M., Carbonero P. Homologous sucrose synthase genes in barley (Hordeum vulgare) are located in chromosomes 7H (syn. 1) and 2H. Evidence for a gene translocation? FEBS Lett. (1992);310:46–50. doi: 10.1016/0014-5793(92)81143-a. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki T., Matsumoto T., Yamamoto K., Sakata K., Baba T., Katayose Y., Wu J., Niimura Y., Cheng Z., Nagamura Y., et al. The genome sequence and structure of rice chromosome 1. Nature. (2002);420:312–316. doi: 10.1038/nature01184. [DOI] [PubMed] [Google Scholar]
- 49.Sebkova V., Unger C., Hardegger M., Sturm A. Biochemical, physiological, and molecular characterization of sucrose synthase from Daucus carota. Plant Physiol. (1995);108:75–83. doi: 10.1104/pp.108.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silvente S., Camas A., Lara M. Heterogeneity of sucrose synthase genes in bean (Phaseolus vulgaris L.): evidence for a nodule-enhanced sucrose synthase gene. J. Exp. Bot. (2003);54:749–755. doi: 10.1093/jxb/erg086. [DOI] [PubMed] [Google Scholar]
- 51.Sturm A. Invertase. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol. (1999);121:1–7. doi: 10.1104/pp.121.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sturm A., Tang G.Q. The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci. (1999);4:401–407. doi: 10.1016/s1360-1385(99)01470-3. [DOI] [PubMed] [Google Scholar]
- 53.Sturm A., Lienhard S., Schatt S., Hardegger M. Tissue-specific expression of two genes for sucrose synthase in carrot (Daucus carota L.). Plant Mol. Biol. (1999);39:349–360. doi: 10.1023/a:1006199003756. [DOI] [PubMed] [Google Scholar]
- 54.Subbaiah C.C., Palaniappan A., Duncan K., Rhoads D.M., Huber S.C., Sachs M.M. Mitochondrial localization and putative signaling function of sucrose synthase in maize. J. Biol. Chem. (2006);281:15625–15635. doi: 10.1074/jbc.M600355200. [DOI] [PubMed] [Google Scholar]
- 55.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. (2007);24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 56.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. (1994);22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ueda T., Yamaguchi M., Uchimiya H., Nakano A. Ara6, a plantunique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. EMBO J. (2001);20:4730–4741. doi: 10.1093/emboj/20.17.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uemura T., Ueda T., Ohniwa R.L., Nakano A., Takeyasu K., Sato M.H. Systematic analysis of SNARE molecules in Arabidopsis: dissection of the post-Golgi network in plant cells. Cell Struct. Funct. (2004);29:49–65. doi: 10.1247/csf.29.49. [DOI] [PubMed] [Google Scholar]
- 59.van Ghelue M., Ribeiro A., Solheim B., Akkermans A.D., Bisseling T., Pawlowski K. Sucrose synthase and enolase expression in actinorhizal nodules of Alnus glutinosa: comparison with legume nodules. Mol. Gen. Genet. (1996);250:437–446. doi: 10.1007/BF02174032. [DOI] [PubMed] [Google Scholar]
- 60.Wang A.Y., Yu W.P., Juang R.H., Huang J.W., Sung H.Y., Su J.C. Presence of three rice sucrose synthase genes as revealed by cloning and sequencing of cDNA. Plant Mol. Biol. (1992);18:1191–1194. doi: 10.1007/BF00047725. [DOI] [PubMed] [Google Scholar]
- 61.Wang F., Smith A.G., Brenner M.L. Isolation and sequencing of tomato fruit sucrose synthase cDNA. Plant Physiol. (1993);103:1463–1464. doi: 10.1104/pp.103.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Werr W., Frommer W.B., Maas C., Starlinger P. Structure of the sucrose synthase gene on chromosome 9 of Zea mays L. EMBO J. (1985);4:1373–1380. doi: 10.1002/j.1460-2075.1985.tb03789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winter H., Huber J.L., Huber S.C. Membrane association of sucrose synthase: changes during the graviresponse and possible control by protein phosphorylation. FEBS Lett. (1997);420:151–155. doi: 10.1016/s0014-5793(97)01506-8. [DOI] [PubMed] [Google Scholar]
- 64.Winter H., Huber J.L., Huber S.C. Identification of sucrose synthase as an actin-binding protein. FEBS Lett. (1998);430:205–208. doi: 10.1016/s0014-5793(98)00659-0. [DOI] [PubMed] [Google Scholar]
- 65.Yu J., Hu S., Wang J., Wong GK., Li S., Liu B., Deng Y., Dai L., Zhou Y., Zhang X., et al. A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science. (2002);296:79–92. doi: 10.1126/science.1068037. [DOI] [PubMed] [Google Scholar]
- 66.Zrenner R., Salanoubat M., Willmitzer L., Sonnewald U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J. (1995);7:97–107. doi: 10.1046/j.1365-313x.1995.07010097.x. [DOI] [PubMed] [Google Scholar]


