Abstract
Bone marrow-derived cells have been postulated as a source of multipotent mesenchymal stem cells (MSC). However, the whole fraction of MSC remains heterogeneous and the expansion of primitive subset of these cells is still not well established. Here, we optimized the protocol for propagating the low-adherent subfraction of MSC which results in long-term expansion of population characterized by CD45-CD14+CD34+ phenotype along with expression of common MSC markers. We established that the expanded MSC are capable of differentiating into endothelial cells highly expressing angiogenic markers and exhibiting functional properties of endothelium. Moreover, we found these cells to be multipotent and capable of giving rise into cells from neuronal lineages. Interestingly, the expanded MSC form characteristic cellular spheres in vitro indicating primitive features of these cells. In sum, we isolated the novel multipotent subpopulation of CD45-CD14+CD34+ bone marrow-derived cells that could be maintained in long-term culture without losing this potential.
Keywords: bone marrow, differentiation, endothelium, expansion, mesenchymal stem cells
INTRODUCTION
Bone marrow (BM) has been well described as a place harboring not only hematopoietic stem and progenitor cells (HSPC), but also heterogeneous fraction of non-hematopoietic stem/primitive cells able to differentiate into several cellular lineages (Grove et al., 2004). Apart from the HSPC, which are wellcharacterized and the most abundant stem cells residing in the bone marrow, the non-hematopoietic compartment contains also neuronal and endothelial progenitors (Kabos et al., 2002; Quirici et al., 2001) as well as primitive cells exhibiting multilineage differentiation potential such as mesenchymal stem/stromal cells (MSC), multipotent adult progenitors cells (MAPC), marrow- isolated adult multilineage inducible cells (MIAMI) and very small embryonic-like stem cells (VSEL) (D’Ippolito et al., 2004; Jiang et al., 2002; Kucia et al., 2006; 2008; Pittenger et al., 1999). Recently, potential therapeutic applications of bone marrow-derived stem/primitive cells are extensively investigated in animal models as well as in patients with several types of diseases (Abdel-Latif et al., 2007; Dawn et al., 2008; 2009; Hofstetter et al., 2002; Houlihan and Newsome, 2008; Karussis et al., 2008; Lim et al., 2006; Matoba et al., 2008; Martin-Rendon et al., 2008; Torrente and Polli, 2008).
Mesenchymal stem/stromal cells (MSC) belong to the adherent fraction of BM cells and have been characterized by their wide capacity for differentiation into osteogenic, chondrogenic, adipogenic, myogenic, neural, endothelial and other non-hematopoietic lineages (Jiang et al., 2002; Phinney and Prockop, 2007; Pittenger et al., 1999). This multilineage differentiation potential makes them interesting for regenerative applications. MSC have been described as cells relatively easy to expand in simple culture media, which may also be an advantage for their applications (Girdlestone et al., 2009). However, the expanded population of MSC remains heterogeneous and contains subpopulations of morphologically and antigenically distinct cells (Phinney and Prockop, 2007). Still little is known about the subpopulations of MSC which are able to differentiate into cells of specific lineages. Moreover, the culture conditions selectively propagating some fractions of MSC also have not been established.
The aim of the study was to identify MSC fraction that show broad plasticity and can be expanded in long-term culture. We found that low-adherent CD45-CD14+CD34+ subpopulatoion of MSC could be expanded for at least 10 passages without losing the primitive morphology and possessed the differentiation potential toward endothelial and neuronal phenotype.
MATERIALS AND METHODS
Bone marrow isolation and culture of adherent fractions
Bone marrow (BM) cells were collected from tibias and femurs of C57Bl/6 adult mice. The cavities of the bones were flushed with low glucose (LG) DMEM (PAA, Austria) with 20% serum (FBS GOLD, PAA, Austria). The bone marrow suspension was filtered through 40 μm strainer and the fraction of mononuclear cells was separated by centrifugation on Ficoll density gradient (1.077 g/ml, Sigma-Aldrich, USA). The mononuclear cells collected from one mouse were plated on 25 cm2 culture dish (Becton Dickinson, USA) and cultured in the DMEM LG medium supplemented with 20% serum, GlutaMAX (Gibco, Invitrogen, USA), 100 U/ml of penicillin (Sigma-Aldrich, USA), 0.1 mg/ml of streptomycin (Sigma-Aldrich, USA) and 10 ng/ml bFGF (Sigma-Aldrich, USA) in 37℃, 5% CO2, 95% air atmosphere. The medium was changed twice a week. The special protocol was applied during each passage to propagate only the low-adherent subpopulation. The cells were washed with 0.25% trypsin/EDTA (PAA, Austria) twice and only the easily detached fraction was passaged. The cells that were still adherent after this step were not scraped or further trypsinized and were left on dish. Each time the cells were seeded at the density of 1 × 104/cm2 and cultured up to 10 passages. The cells at passage 10 were further investigated for endothelial or neuronal differentiation as well as sphere/cluster formation (Fig. 1).
Fig. 1. The experimental scheme. Mononuclear BM-derived cells were isolated from C57Bl/6 adult mice following centrifugation on Ficoll gradient. The adherent fraction of MSC was initially expanded in in long-term culture and subsequently differentiated into endothelial and neuronal lineages. Additionally, the ability to sphere formation was evaluated.
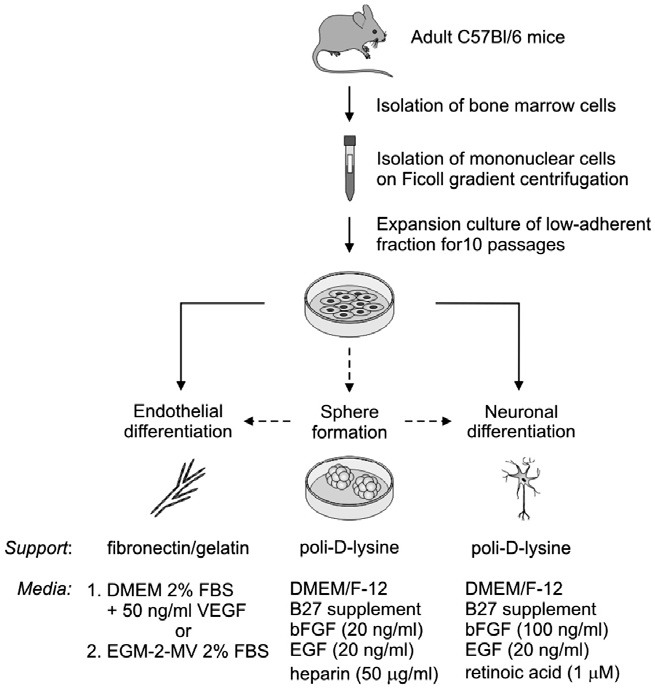
Endothelial differentiation
For endothelial differentiation the cells were detached with Accutase (PAA, Austria), washed twice with PBS and seeded at 1 × 104/cm2 on 6-well plate coated with a mixture of 20 μg/ml fibronectin derived from human plasma (Sigma-Aldrich, USA) and 0.25% gelatin type B from bovine skin (Sigma-Aldrich, USA) (Fig. 1). The cells were cultured according to two distinct protocols, either in DMEM medium with 2% serum and 50 ng/ml of VEGF (Sigma-Aldrich, USA) or in defined EGM-2-MV medium (Lonza, USA) with 2% serum. The medium was changed every two days and the cells were cultured in endothelial differentiation conditions for one week. The experiment was repeated three times.
When the endothelial differentiation was performed after the sphere formation step (Fig. 1), the clusters were initially dissociated by gently pipetting and the sphere-derived cells were seeded and cultured in the same conditions as described above.
TNFα stimulation, VCAM-1 labeling and flow cytometric analysis
The cells were culture without serum for 12 h and then stimulated with 10 ng/ml of TNFα (Sigma-Aldrich, USA) for 4 h. After the stimulation the cells were detached with Accutase and examined for VCAM-1 expression by flow cytometry. The cells were incubated with the unconjugated primary rat anti-mouse VCAM-1 antibody (1:200, BD Bioscience, USA) or matched isotype control rat IgG2ακ antibody (1:200 dilution, BD Bioscience, USA). Secondary goat anti-rat Ig antibody conjugated with FITC was used in the next step (1:200 dilution, BD Bioscience, USA). The labeled cells were analyzed on FACS LSR II flow cytometer (Becton Dickinson, USA) using FACS Diva (Becton Dickinson, USA) and FlowJo software (Tree Star, USA).
Uptake of AcLDL and Bandeiraea simplicifolia lectins binding
Non-differentiated MSC and differentiated with DMEM + VEGF and EGM-2-MV media for one week were labeled with Dillabeled AcLDL (Molecular Probes, USA) at 50 μg/ml for 24 h at 37℃. The AcLDL uptake was subsequently evaluated by fluorescence microscopy (Eclipse TS100, Nikon, Japan).
For lectin binding the cells were detached from culture plates with Accutase, washed twice with PBS, and incubated with biotinylated Bandeiraea simplicifolia isolectin B4 for 1.5 h 4℃ (1:100 dilution, Vector Laboratories, USA). After the incubation, cells were washed twice with 2% serum in PBS and incubated with FITC conjugated egg avidine (1:200 dilution, Sigma-Aldrich, USA). The sample without lectin incubation acted as a negative control. The samples were analyzed by flow cytometry as described above.
Tube formation on matrigel assay
The 40 μl of matrigel (BD Bioscience, USA) were put per well of a 96-well plate. After the polymerization, 7 × 103 cells in 100 μl of DMEM LG with 1% serum were seeded to each well and incubated for 24 h in 37℃, 5% CO2, 21% O2 atmosphere. Tube formation was investigated by phase-contrast microscopy (Eclipse TS100, Nikon, Japan).
Neuronal differentiation
The expanded MSC were detached from culture plates with Accutase, washed twice with PBS and subsequently seeded at 2 × 104/cm2 density on a 6-well plate (Becton Dickinson, USA) coated with 50 μg/ml of poli-D-lysine (Sigma-Aldrich, USA) (Fig.1). The cells were cultured in the DMEM/F12 medium (PAA, Austria) containing B27 supplement (PAA, Austria), 100 ng/ml of bFGF (Sigma-Aldrich, USA), 20 ng/ml EGF (Sigma- Aldrich, USA), 1 μM retinoic acid (Sigma-Aldrich, USA) for one week. The medium was changed every two days.
When the differentiation was performed after the sphere formation step, the spheres/clusters were dissociated by gently pipeting and the sphere-derived cells were seeded in the same conditions as above.
Sphere formation
The expanded MSC were detached with Accutase, washed twice with PBS and seeded in DMEM/F12 medium containing B27 supplement, 20 ng/ml of bFGF, 20 ng/ml of EGF, 50 μg/ml of heparin at 2 × 104/cm2 density on a 6-well plate pre-coated with 50 μg/ml of poli-D-lysine. Half of the medium was changed each three days by careful removing the old medium with a pipette to avoid collecting the spheres. After one week, the spheres-derived cells were further differentiated in endothelial or neuronal conditions as described above.
Immunohistochemistry staining
Cells cultured in chamber slides in non-differentiated conditions and neuronal differentiation conditions were fixed with 4% PFA, blocked with 0.25% glycine and 1% BSA and then incubated with anti-neurofilament heavy antibody at 4℃ overnight (dil. 1:1000, cat.: ab8135, Abcam, UK). The samples were incubated with secondary goat anti-rabbit antibody for 45 min. at room temperature (dil. 1:400, cat.: A11008, Molecular Probes, USA). The slides were analyzed with confocal microscopy (Leica, Germany).
RNA extraction, RT-PCR, real-time RT-PCR analysis
Total cellular RNA was extracted by using RNAgents® Total RNA Isolation System (Promega, USA) according to the manufactures instructions. Reverse transcription reaction was performed with M-MLV reverse transcriptase (Promega, USA). PCR reaction was conducted with Taq polymerase (Promega USA) using the following conditions: 95℃ for 5 min, 40 cycles of 95℃ for 30 s, annealing temperature for 30 s, 72℃ for 30 s and ended with 72℃ for 5 min. The PCR products electrophoresis was performed according to standard laboratory protocols. Specific primer sequences, annealing temperatures and product lengths are summarized in Table 1. Quantitative real-time RT-PCR was carried out using the StepOne Plus cycler (Applied Biosystems, UK) and SYBR® Green JumpStart™ Taq ReadyMix™ (Sigma-Aldrich, USA). Elongation factor 2 (EF-2) was used as housekeeping gene control. To show the mRNA fold changes the results were calculated as 2-ΔCt and the nondifferentiated cells were used as reference sample.
Table 1.
| Gene | Primer sequence (5′ → 3′) | Product length | Annealing temp. | |
|---|---|---|---|---|
| bIII tubulin | for | TGGAACCYGGAACCATGGACAGTG | 140 bp | 60℃ |
| rev | TCCACCAGCTCCGCSCCCTC | |||
| CD14 | for | AAGCCCGTGGAACCTGGAAGC | 242 bp | 58℃ |
| rev | GAAAGCGCTGGACCAATCTGGC | |||
| CD29 | for | GGACGCTTACTGCAGGAAAGAG | 241 bp | 56℃ |
| rev | ACAGTCACAKGCRCTGCCAGTG | |||
| CD31 | for | CACCRGGTGCTGTTCTATAAGG | 202 bp | 54℃ |
| rev | CCAGTGTCACCYTGGGMTTGG | |||
| CD34 | for | GGAGCCACCAGAGCTAYTCC | 172 bp | 56℃ |
| rev | CCTGGCCTCCACCRTTCTCC | |||
| CD44 | for | CCTCGTCACGTCCAACACCTCC | 383 bp | 58℃ |
| rev | TCGATGGTGGAGCCGCTGC | |||
| CD45 | for | GCATCCATCCTCGTCCACTGC | 180 bp | 54℃ |
| rev | GATAGATGCTGGCGATGATGTC | |||
| CD105 | for | CGCTTCAGCTTCCTCCTCCG | 281 bp | 59℃ |
| rev | CACCACGGGCTCCCGCTTG | |||
| CD140b | for | TACGTGCCCATGYTGGACATG | 175 bp | 54℃ |
| rev | TGGTAGCTGAAGCCCACGAG | |||
| CD166 | for | TGGCGGCTTCAACGACCATCAC | 136 bp | 60℃ |
| rev | TCCACACCACAGTCGCGTTCCT | |||
| EF2 | for | GCGGTCAGCACAATGGCATA | 218 bp | 60℃ |
| rev | GACATCACCAAGGGTGTGCAG | |||
| GFAP | for | ATC ACC AAG GGT GTG CAG | 150 bp | 54℃ |
| rev | ACCACGATGTTCCTCTTGAGG | |||
| KDR | for | CCTCACCTGTTTCCTGTATGGAG | 301 bp | 60℃ |
| rev | GAKGCCACAGACTCCCTGC | |||
| Nestin | for | GAAAAGTTCCAGCTGGCTGTG | 112 bp | 54℃ |
| rev | AGGGACATCTTGAGGTGYGC | |||
| Neuro filament H | for | CTGCTCAAYGTCAAGATGGC | 130 bp | 60℃ |
| rev | GAGGGAATTTTKGGGAGTCCTTC | |||
| Neuroglycan C | for | CCACCATTGCCGAGGGCTCTC | 150 bp | 60℃ |
| rev | CCTGGTCACCTTTGCTGCCACCCTC | |||
| Tau | for | TGGCCAAGCAGGGTTTGTGATC | 205 bp | 60℃ |
| rev | CCCATCACTGATTTTGAAGTCCCG | |||
| Tenascin R | for | GAACTGCCACCGGACCAAC | 114 bp | 60℃ |
| rev | CTTCATTTCYACAAAGGGGATG | |||
| vWF | for | CCCCATCAGCCACACTTGATGC | 180 bp | 60℃ |
| rev | GCACCAGCACAGGGTTCAGC | |||
Statistical analysis
Data are reported as means ± SEM. The data were analyzed with non-paired Student’s t-test. A one-way ANOVA with posthoc Tukey test was applied when more then two groups were compared. The graphs design and statistical analysis were performed using GraphPad Prism 4 software (GraphPad Software, USA)
RESULTS
Long-term propagation of low-adherent fraction of BM-derived cells does not reduce their primitive characteristics and multipotentiality
In early stages of long-term cultures of BM-derived adherent cells, we observed their morphological heterogeneity persisting during few initial passages. As shown in Fig. 2A, several types of cells exhibiting different morphological characteristics could be distinguished in such cultures in early stages. Moreover, the existence of two cell fractions that differ with dish adherence was noticed. The subpopulation that was still adherent after short trypsinization (see “Materials and Methods”) was not capable of being further propagated in long-term culture. Oppositely, due to prolonged propagation of the fraction of cells with low adhesive capacity, we were able to obtain more homogenous population that exhibited broad plasticity. At passage 10, this population of cells presented more homogenous morphology and retained the spindle-like shape (Fig. 2A), typical for MSC.
Fig. 2. Phenotype of expanded low-adherent MSC during culture. (A) Morphology of expanded cells under phase-contrast microscope (magnification 100×). At passage 2 adherent fraction exhibits significant heterogeneity and contains different types of cells, while at passage 10 cells are more homogenous and uniform in their morphology. (B) The expression of markers characterizing MSC (CD29, CD44, CD105, CD166), neuronal progenitors (nestin, βIII-tubulin, GFAP), hematopoietic cells (CD14, CD45) and primitive cells (CD34) in expanded low-adherent BM-derived cells. All the listed genes were expressed on both passage 2 (P2) and passage 10 (P10), except for CD45 that was present only on P2, and GFAP that was expressed only on P10. EF-2 - housekeeping gene; Negative - a sample without any template served as negative control. (C) Adipocyte morphology of low-adherent population at passage 10 in prolonged confluent cultures. The adipocyte differentiation was confirmed by high expression of PPARγ in cultures with cells showing the adipocyte morphology compared to initial BM cultures where the adipocyte cells were not observed.
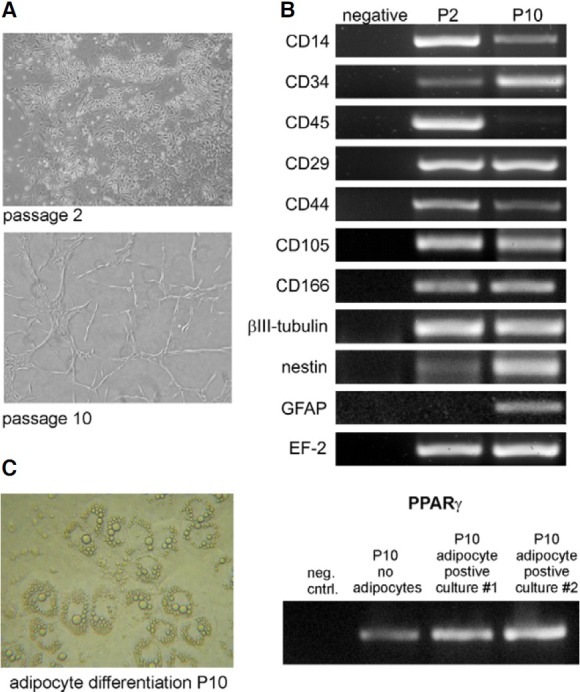
The cultured cells were investigated for expression of several markers characteristic for MSC at passages 2 and 10. We checked the transcription of CD14, CD34 and CD45, which have been indicated to be absent on MSC as well as CD29, CD44, CD105 and CD166 which are typical markers expressed by MSC (Phinney and Prockop, 2007; Pittenger et al., 1999). Additionally, the presence of some neuronal (nestin, βIII-tubulin) and glial marker (glial fibrillary acidic protein; GFAP) was also investigated.
The cultured adherent cells expressed markers typical for MSC such as CD29, CD44, CD105 and CD166 at both passage 2 and passage 10. Moreover, the cells expressed neuronal and glial markers when examined at these two time points (Fig. 2B). The adherent cells at passage 2 were enriched in a fraction of cells expressing CD14 and CD45 antigens indicating the presence of hematopoietic cells from monocytic/macrophages lineage in such culture (Fig. 2B). Importantly, the cultured cells at passage 10 hardly expressed the hematopoietic marker CD45 and exhibited increased expression of CD34 antigen suggesting their primitive characteristics (Fig. 2B).
The expression of the markers CD14 and CD34 did not fulfill the mentioned general MSC criteria. Nevertheless, spontaneous adipocyte differentiation of the isolated population was observed what proofed its mesenchymal precursor character. The adipocyte cells possessing the typical morphology with fat droplets were detected in high numbers among the lowadherent population on P10, when cultured in confluence for prolonged time (Fig. 2C). In these cultures the expression of PPARγ was checked as it is critical molecular sign used to characterize adipocyte differentiation (Chawla et al., 1994). We confirmed high expression of PPARγ in the low-adherent population on P10 where adipocytes were observed, in contrast to low expression in initial culture of bone-marrow derived cells where adipocyte differentiation was not observed (Fig. 2C).
Therefore, we were able to establish the simple pre-plating culture protocol resulting in long-term propagation of BMderived MSC with primitive features characterized by CD45-CD14+CD34+ phenotype.
Expanded low-adherent BM-derived MSC exhibit endothelial differentiation capacity in vitro
In the next step, we investigated the differentiation capacity of the expanded low-adherent MSC by testing their endothelial potential. For this purpose the cells were propagated up to passage 10 and subsequently exposed to growth factors stimulating endothelial differentiation. Two different strategies of endothelial differentiation were used. The first strategy relies on use of defined endothelial EGM-2-MV medium with low serum content (2%). The second strategy, originally described by Oswald and co-workers (Oswald et al., 2004) employs culture in DMEM medium with low serum content (2%) and addition of VEGF (50 ng/ml). As a result of both applied differentiation protocols, MSC lost their fibroblast morphology and acquired a cobblestone shape. However, the cells cultured in defined EGM-2-MV medium occurred to be smaller and more rounded than cells cultured in the presence of VEGF, and resembled more endothelial- like phenotype (Fig. 3A).
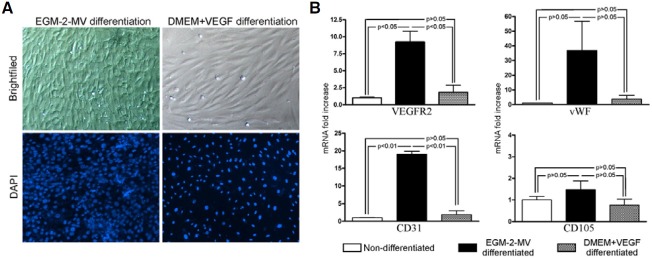
Fig. 3. Endothelial differentiation of expanded MSC. (A) Cell morphology after endothelial differentiation (magnification 200×). Additionally, DAPI staining of confluent cultures (magnification 100×) evidenced that EGM-2-MV differentiated cells were much smaller and condensed in the culture comparing to the cells differentiated with DMEM+VEGF. (B) Expression of endothelial markers evaluated by real-time RT-PCR following the differentiation of expanded MSC in defined EGM-2-MV medium or DMEM with VEGF, when compared to non-differentiated MSC. The level of expression of endothelial markers was evaluated in two independent representative experiments. Results are presented as mean ± SEM.
The MSC differentiated in two distinct media were subsequently examined for the expression of markers characteristic for endothelial cells by employing multiple methods.
MSC express endothelial markers
The expanded and differentiated low-adherent MSC expressed mRNA for VEGFR2, CD31, vWF and CD105 indicating their ability to give rise into endothelial phenotype (Fig. 3B). Moreover, MSC cultured in EGM-2-MV media exhibited greater concentration of mRNA for VEGFR2, CD31 and vWF when compared to MSC cultured in the presence of only the VEGF or to non-differentiated cells (Fig. 3B). Importantly, mRNA levels for VEGFR2, CD31 and vWF highly increased after endothelial differentiation in EGM-2-MV media (9.22 ± 1.59, 19.02 ± 0.88 and 36.82 ± 19.95 fold difference, respectively), while the expression of CD105 was not elevated (1.48 ± 0.41 fold difference, respectively) (Fig. 3B). On the other hand, the above mentioned markers were hardly up-regulated when the differentiation with DMEM and VEGF was applied (Fig. 3B).
MSC express VCAM-1 after TNFα stimulation
The expression of VCAM-1 after stimulation with TNFα has been proposed as one of the feature that can help to distinguish endothelial progenitor cells from other cell types (Fernandez Pujol et al., 2000; Ingram et al., 2004).
In the present study, flow cytometric analysis showed basal expression of VCAM-1 on the cells before and after differentiation with both media. The 4 h long incubation with TNFα was enough to enhance expression of VCAM-1 not only in differentiated MSC but also in non-differentiated cells (Fig. 4A). There were no significant differences between cells in either tested conditions.
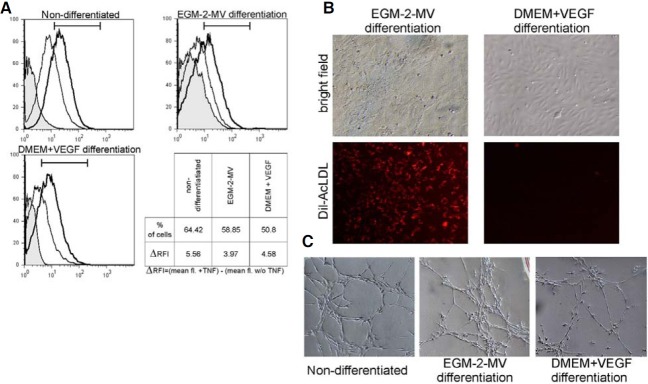
Fig. 4. The endothelial characteristic of non-differentiated and differentiated cells. (A) Flow cytometry analysis of VCAM-1 expression before and after stimulation with TNFα. Curves: gray - isotype control; thin line - VCAM-1 expression without TNFα stimulation; bold line - VCAM-1 expression after TNFα stimulation. (B) Expanded MSC differentiated with EGM-2-MV medium incorporated Dil-AcLDL, in opposite to cells differentiated with DMEM+VEGF (magnification 100×). (C) Both the non-differentiated and differentiated cells efficiently formed tubes on matrigel support (magnification 100×).
MSC uptake AcLDL and bind Bandeiraea simplicifolia lectins
The phenomenon of the uptake of acetylated low density lipoproteins (AcLDL) has been described for endothelial cells and was successfully applied for their isolation (Pitas et al., 1985). Binding of various lectins to the cellular surface has been also described as another endothelial attribute (Fei et al., 1990).
In this study, the uptake of Dil-labeled AcLDL by expanded low-adherent MSC was investigated before and after culture in differentiating conditions. As shown in Fig. 4B, only MSC differentiated in EGM-2-MV medium were capable to uptake AcLDL exhibiting endothelial properties. Moreover, MSC differentiated accordingly to both culture protocols, in opposite to nondifferentiated cells, bound Bandeiraea simplicifolia lectins when examined by flow cytometry (data not shown).
MSC form tubes in matrigel assay
The tube formation test employing the culture of cells on matrigel matrix has been proposed for studying the angiogenic potential of various cells. In this study, both differentiated and non-differentiated MSC showed the capacity of tube formation proving their functional angiogenic potential (Fig. 4C).
In sum, the data confirmed by employing various assays testing the endothelial phenotype and function, indicate that lowadherent MSC expanded in long-term culture preserves their angiogenic capacity in vitro (Table 2).
Table 2.
| Non-differentiated | EGM-2-MV differentiation | DMEM+ VEGF differentiation | |
|---|---|---|---|
| VEGFR2 | +/- | ++ | +/- |
| vWF | +/- | ++ | +/- |
| CD31 | +/- | ++ | +/- |
| CD105 | + | + | + |
| VCAM after TNFa stimulation | + | + | + |
| acLDL incorporation | - | + | - |
| B.s lectin binding | - | + | + |
| Tube formation on Matrigel | + | + | + |
Expanded low-adherent BM-derived MSC exhibit neuronal differentiation potential in vitro
The expression of neuronal markers, such as neurofilament heavy, tau, neuroglycan C and tenascin-R, changed during neuronal differentiation of analyzed MSC. The tested genes were significantly up-regulated after differentiation proving the neuronal potential of expanded MSC (Fig. 5A). The mRNA levels for neurofilament heavy and tau protein highly increased after differentiation (27.4 ± 6.8 and 111.2 ± 21.7 fold difference, respectively), while the expression of neuroglycan C and tenascin- R was also elevated, but to a lower extent (3.0 ± 0.4 and 5.7 ± 0.1 fold difference, respectively) (Fig. 5A).
Fig. 5. Neuronal differentiation of expanded MSC. (A) Expression of neuroglycan C, neurofilament heavy, tau and tenascin-R evaluated in expanded MSC after neuronal differentiation by real-time RTPCR, when compared to non-differentiated MSC. The expression levels of neuronal markers were evaluated in two independent representative differentiation experiments. Results are presented as mean ± SEM. (B) Morphology of MSC undergoing neuronal differentiation (magnification 100×). The morphology of cells changed during culture resulting in elongated, needle-like phenotype of cells after one week of culture in neuronal conditions. (C) Confocal microscopy analysis confirmed the neurofilament heavy protein expression in cells acquiring the neuron-like morphology after neuronal differentiation.
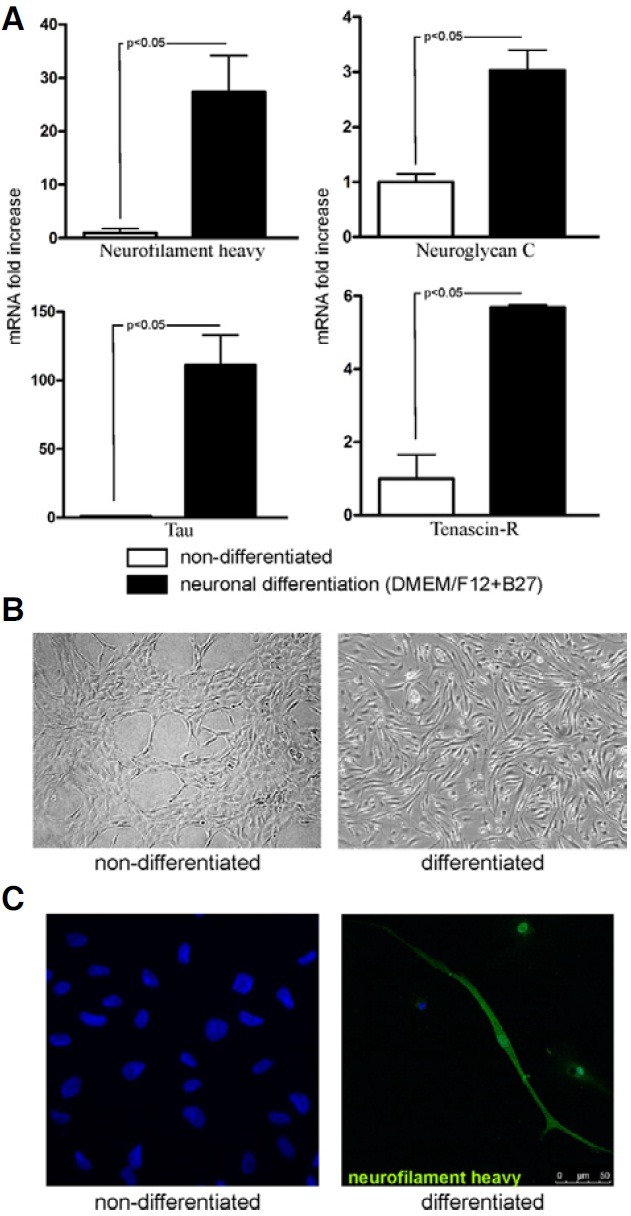
At the same time, the morphological changes of the cells after the neuronal differentiation accompanied the changes in expression of above mentioned genes, as evidenced by more elongated and needle-like cells resembling neurons (Fig. 5B). Moreover, in these cells the neurofilament heavy protein was present as demonstrated by immunohistochemical staining in contrast to non-differentiated cells that were negative for this antigen (Fig. 5C).
These observations suggest that the low-adherent fraction of MSC expanded in our culture system exhibits multipotent differentiation capacity.
The low-adherent BM-derived MSC form spheres/clusters in vitro
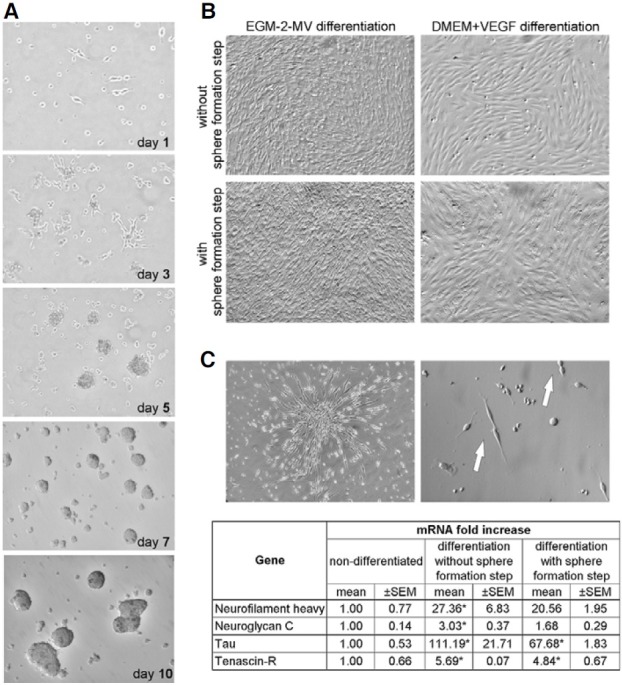
Interestingly, low-adherent MSC, expanded in the long-term culture exhibited capacity to form spherical structures in vitro, when seeded on poli-D-lysine coated dish in the medium designated for neurosphere formation. The process of sphere formation was initiated very early (24 h after initial seeding), when the loosely attached cells started to migrate to form few-cell aggregates (Fig. 6A, day 1). The cells rapidly proliferated and formed larger clusters during next 3-4 days (Fig. 6A, day 3 and 5). Eventually, after about one week, the cultures predominantly contained large round cellular clusters/spheres (Fig. 6A, day 7 and 10) which often detached from the bottom of the plate, floated in the culture medium and fused together during prolonged incubations. Such clusters contained normal living cells capable of forming secondary spheres when dissociated to a single-cell suspension and re-seeded on a new plate. Interestingly, all expanded low-adherent MSC cultured under the sphere formation conditions, uniformly participated in the phenomenon of the clusters formation. The experiment was repeated several times, always leading the MSC to the sphere formation.
Fig. 6. Sphere formation and differenttiation of sphere-derived cells. (A) Process of sphere/cluster formation. At 24 h after seeding few cells adhered to the poli-D-lysine coated dish (day 1). The cells migrated during next 3-4 days forming cellular aggregates initiating spheres (day 3). Rapid proliferation of these cells resulted in increased size of clusters (day 5). Finally, around one week after seeding some of the large clusters spontaneously detached from the culture dish and grew into floating round spheres (day 7). When the culture was prolonged the fusion of floating spheres was observed (day 10) (magnification 100×). (B) Morphology of expanded MSC without or with sphere formation step following endothelial differentiation (magnification 100×). The cells were culture in EGM- 2-MV defined medium or DMEM supplemented with VEGF. (C) The neuronal differentiation of MSC with and without the sphere formation step. The cells acquired the elongated, needle-like bipolar morphology (magnification 100×). Proliferating cells are indicated by arrows (magnification 200×). Real-time RTPCR analysis showed the changes in expression of neuronal markers on mRNA level in differentiated MSC (without and with sphere formation step) when compared to non-differentiated cells. The expression levels of indicated markers were evaluated in two representative differentiation experiments. Results are presented as mean ± SEM. (*) p < 0.05 vs. non-differentiated cells.
Sphere-derived cells preserve the multipotent differentiation capacity
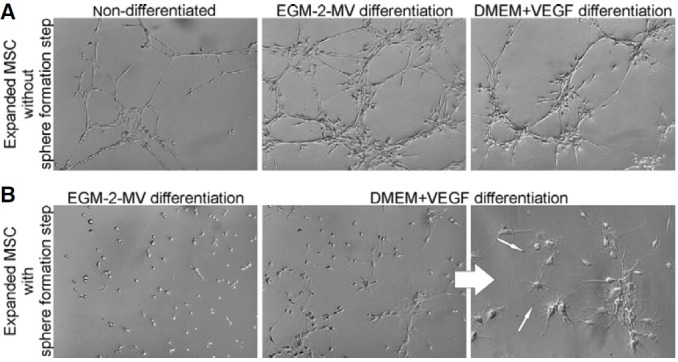
In order to investigate the endothelial differentiation capacity, the cells isolated from spheres/clusters were cultured either in defined EGM-2-MV medium or DMEM supplemented with VEGF as described before. Independently of the applied endothelial differentiation media, the cells derived from spheres became more heterogeneous when compared to the initially expanded population of low-adherent MSC with uniform morphology (Fig. 6B). Moreover, despite the initial differentiation of cells in the endothelial medium, we observed a great decrease in the tube formation ability by the MSC derived from spheres (Fig. 7) when compared to the expanded MSC which did not undergo the sphere formation step (Fig. 7). Interestingly, the MSC derived from spheres/clusters formed on matrigel unorganized groups of cells which morphologically resembled oligodendrocytes (Fig. 7). These observations indicate diminished endothelial differentiation potential of MSC which were cultured through the sphere formation stage.
Fig. 7. Tube formation on Matrigel. (A) Both non-differentiated and differentiated MSC (in EGM-2MV medium or DMEM with VEGF) efficiently formed tubes without the sphere formation step (magnification 100×). (B) Tube formation by MSC which undergo differentiation in endothelial media following sphere formation step, was not observed (magnification 100×). Interestingly, they acquired more neuronal-like morphology indicted by arrows on magnified image (magnification 200×).
Parallelly, the neuronal differentiation capacity of MSC derived from spheres was investigated. The cells isolated from such clusters and re-seeded in the culture medium promoting neuronal differentiation changed their morphology to bipolar needle-like shape, resembling neuronal phenotype (Fig. 6C). Moreover, expressions of mRNA for neuronal markers such as neuroglycan C, tenascin-R, neurofilament heavy and tau were increased in the differentiated sphere-derived MSC in comparison to non-differentiated cells (Fig. 6C). However, the upregulation of neuronal markers was less pronounced in MSC derived from spheres, than in cells which did not undergo the step of sphere formation (Fig. 6C). Thus, it appears that lowadherent MSC cultured through the sphere formation step preserve neuronal capacity, however at a lower level than the expanded MSC propagated without this culture step.
DISCUSSION
In this study, we isolated murine bone-marrow derived primitive cell population that retains mesenchymal stem cells. Importantly, this fraction of BM cells exhibited low-adhesive properties and express CD34 marker and monocytic antigen CD14. However, taking into consideration our results and other literature reports, we proposed to classify these cells as subpopulation of MSC, despite the confusing expression of CD14 and CD34. Firstly, they express several MSC positive markers CD29, CD44, CD105, CD166, showed plastic adherence and fibroblast-like morphology. It is also possible that this CD14+ CD34+ subpopulation is initially much more infrequent among the major CD14-CD34- MSC population and could be expanded only in long-term culture with the application of the novel protocol. Moreover, there are reports indicating the CD14+ population as origin of the MSC (Kuwana et al., 2003), as well as, describing CD34+ cells as precursors of human (Kopher et al., 2010) and murine (Kaiser et al., 2007) MSC. Interestingly, the CD34 expression in long-term MSC culture was observed (Li et al., 2008), in addition to report demonstrating higher angiogenic potential of CD34+ subpopulation of MSC (Copland et al., 2008), what is altogether consistent with our study. Finally, the spontaneous differentiation to adipocytes confirmed the potential to differentiate also toward mesenchymal lineages, what is intrinsic MSC feature (Pittenger et al., 1999).
The study shows that at initial stages of in vitro culture, the adherent fraction derived from BM mononuclear cells contained two distinct subpopulations according to their adhesiveness. The low-adherent subpopulation which was eventually longterm expanded during our study and showed fibroblastic appearance typical for MSC. Importantly, endothelial and neuronal differentiation potential was not only preserved in low-adherent expanded MSC, but was exclusively exhibited by this long-term cultured population with fibroblastic shape and proliferative capacity. It was not possible to expand in long term-culture the cells the showed strong adherence characteristic. Moreover, we did not observe any endothelial or neuronal differentiation in these cultures (data not shown). Thus, highly adherent fraction did not fulfill the experiment criteria being unable to long-term propagation, showed no plasticity and therefore it was excluded from further analysis.
The existence of murine bone marrow cells with different adherence to culture plate was also described by Zhang and colleagues (Zhang et al., 2007). Moreover, the same authors found a CD14+ subpopulation of adherent cells able to differentiate into endothelial cells, but only in early stages of culture suggesting their monocytic origin (Zhang et al., 2007).
The obtained results indicate that the expression of CD14 by subpopulation of low-adherent MSC expanded in our study is not only related to the presence of transient, non-proliferating monocytes that mimic endothelial cells and this population is distinct from the cells described by Zhang et al. (2007). The examined low-adherent CD14+CD34+ MSC population were capable to differentiate into endothelial phenotype as demonstrated at mRNA and functional levels. Simultaneously, the upregulation of neuronal markers was also observed what indicates that the presented population is not only restricted to endothelial phenotype, but posses broader plasticity. Interestingly, Romagnani and co-workers established the presence of similar double-positive (CD14+CD34+) fraction of cells, capable to differentiate into both endothelial as well as neuronal cells, in human bone marrow and blood (Romagnani et al., 2005). The cells isolated in this study, with comparable differentiation plasticity and expression of CD14 along with CD34 may potentially represent murine population that corresponds to this described by Romagnani et al. in humans (Romagnani et al., 2005). Moreover, the population of murine cells could be expanded in long-term culture.
The potential of MSC to differentiate toward endothelial cells was previously shown by Oswald and co-workers (Oswald et al., 2004) and confirmed by other groups (Alviano et al., 2007; Gang et al., 2006), although some papers speculate about MSC endothelial differentiation potential (Wang et al., 2008; Zhang et al., 2007). The current study indicates the existence of CD14+CD34+ subfraction of MSC, that is capable to differentiate to endothelial cells and additionally shows neuronal differentiation.
Different isolation approaches and culture conditions applied by different investigators may explain the discrepancies between the results coming from different studies. We employed the culture strategy propagating only the low-adherent fraction of MSC in medium with high serum content, whereas majority of other protocols expands whole fraction of adherent BM cells (Beyer Nardi and da Silva Meirelles, 2006; Brunt et al., 2007; Gnecchi and Melo, 2009). This fact may significantly change characteristics of the output population eventually obtained due to prolonged culture.
The effective endothelial differentiation of expanded MSC was observed when EGM-2-MV medium containing several growth factors such as VEGF, bFGF, IGF-1, hydrocortisone, heparin and ascorbic acid, was used in culture. EGM-2-MV medium occurred to be significantly more efficient in promoting endothelial differentiation of expanded low-adherent CD14+ CD34+ MSC than DMEM medium supplemented with VEGF that is widely used culture medium (Alviano et al., 2007; Chen et al., 2009; Oswald et al., 2004). Additionally to morphological changes, the up-regulation of typical endothelial markers such as vWF, VEGFR2 and CD31 occurred only in the MSC which were differentiated in EGM-2-MV medium, but not in DMEM with VEGF. The endothelial potential of expanded low-adherent and differentiated MSC was finally confirmed by the up-regulation of VCAM-1 expression upon TNFα stimulation, incorporation of Dil-labeled AcLDL and binding of Bandeiraea simplicifolia lectins that may distinguish the functional endothelial progenitor cells from other cells (Fei et al., 1990; Ingram et al., 2004; Pitas et al., 1985).
Possibly, because of the presence of many growth factors in EGM-2-MV medium, it activates endothelial differentiation more efficiently via molecular signaling pathways involved in endothelial development in vivo (Cleaver and Melton, 2003; Jain, 2003).
Interestingly, the low-adherent CD14+CD34+ fraction of MSC was capable to form spheres/cellular clusters in the specific culture conditions. The sphere formation has been initially described as a feature of neural stem cells (Reynolds and Weiss, 1992), but other reports indicated this phenomenon as appropriate method for isolation of multipotent stem cells from a multitude of sources and differentiating them to various cell lineages (Shiota et al., 2007). Since the CD14+CD34+ MSC exhibited multipotentiality by differentiating into both endothelial and neuronal phenotype, we believe that the sphere formation may be related to the primitive characteristics of these cells.
Recent study by Shiota and co-workers showed the impact of sphere formation on following differentiation of murine MSC towards several lineages, but not into endothelium (Shiota et al., 2007). Similarly, significant change in differentiation potential of low-adherent MSC after sphere formation was observed in the current study, which, however, included also endothelial differentiation. MSC differentiated towards endothelial cells after sphere formation step lost their ability to form tubes on Matrigel assay, indicating that the sphere formation step decreases the angiogenic differentiation capacity of these cells. Depending on the endothelial differentiation medium, these cells either did not grow at all on Matrigel or acquired the oligodendrocyte-like morphology. However, the neuronal differentiation of MSC in a specific medium was also diminished due to the sphere formation stage as shown by decreased level of expression of neuronal markers. Taking these observations together, the sphere formation step was not beneficial for endothelial and neuronal differentiation of MSC.
Importantly, this work confirmed possible usage of dish adherence criterion to obtain more homogenous and primitive population from initial heterogeneous culture. However, we did not aimed to sort directly the double positive CD14+CD34+ cells to ensure completely pure population, we observed all the expanded low-adhesiveness restricted cells uniformly react to applied differentiation conditions.
In summary, we isolated a novel population of MSC with a unique capacity. To our best knowledge, this is the first study showing the isolation and long-term expansion culture conditions for multipotent bone marrow-derived primitive cells that are non-hematopoietic (CD45-) and characterized by the presence of typical markers of MSC as well as the expression of CD14 and CD34 antigens. This population possesses endothelial and neuronal differentiation potential in vitro which may be decreased by sphere formation.
Such a relatively easily accessible and expandable population of BM-derived cells with the functional endothelial differentiation capacity may be applied for cell-based therapies of vascular diseases in the future. Furthermore, if next studies confirm that the neuronal potential of these cells can be used to obtained truly functional neuronal cells, the possibility for cellbased therapies of neurological disorders can be also considered.
Acknowledgments
The study was supported by the grants N301 08032/3156 and N301 3148/37 from the Polish Ministry of Science and Higher Education awarded to JD and by the European Union structural funds, Innovative Economy Operational Programme, grant No. POIG 01.01.02-00-109/99 ‘Innovative methods of stem cell applications in medicine’ and No. POIG 01.01.02-00-069/99 “Vascular endothelium in civilization diseases”. AJ is a recipient of the Wellcome Trust Senior Research Fellowship in Basic Biomedical Science. EZS is the recipient of the “Homing” grant (HOM/2008/15B) from the Foundation for Polish Science and grants from Ministry of Polish Science (N N301 422738; N N302 177338) Department of Medical Biotechnology participates in the COST CM0602 Action (ANGIOKEM) and TD0901 “Hypoxia sensing, signaling and adaptation”. The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of the structural funds from European Union (grant No: POIG.02.01.00-12-064/08 and 02.02.00-00- 014/08).
References
- 1.Abdel-Latif A., Bolli R., Tleyjeh I.M., Montori V.M., Perin E.C., Hornung C.A., Zuba-Surma E.K., Al-Mallah M., Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch. Intern. Med. (2007);167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 2.Alviano F., Fossati V., Marchionni C., Arpinati M., Bonsi L., Franchina M., Lanzoni G., Cantoni S., Cavallini C., Bianchi F., et al. Term Amniotic membrane is a high throughput source for multipotent Mesenchymal Stem Cells with the ability to differentiate into endothelial cells in vitro. BMC Dev. Biol. (2007);7:11. doi: 10.1186/1471-213X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyer Nardi N., da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb. Exp. Pharmacol. (2006);174:249–282. [PubMed] [Google Scholar]
- 4.Brunt K.R., Hall S.R.R., Ward C.A., Melo L.G. Endothelial progenitor cell and mesenchymal stem cell isolation, characterization, viral transduction. Methods Mol. Med. (2007);139:197–210. doi: 10.1007/978-1-59745-571-8_12. [DOI] [PubMed] [Google Scholar]
- 5.Chawla A., Schwarz E.J., Dimaculangan D.D., Lazar M.A. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. (1994);135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- 6.Chen M., Lie P., Li Z., Wei X. Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp. Hematol. (2009);37:629–640. doi: 10.1016/j.exphem.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Cleaver O., Melton D.A. Endothelial signaling during development. Nat. Med. (2003);9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- 8.Copland I., Sharma K., Lejeune L., Eliopoulos N., Stewart D., Liu P., Lachapelle K., Galipeau J. CD34 expression on murine marrow-derived mesenchymal stromal cells: impact on neovascularization. Exp. Hematol. (2008);36:93–103. doi: 10.1016/j.exphem.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 9.Dawn B., Tiwari S., Kucia M.J., Zuba-Surma E.K., Guo Y., Sanganalmath S.K., Abdel-Latif A., Hunt G., Vincent R.J., Taher H., et al. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. (2008);26:1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawn B., Abdel-Latif A., Sanganalmath S.K., Flaherty M.P., Zuba- Surma E.K. Cardiac repair with adult bone marrowderived cells: the clinical evidence. Antioxid. Redox Signal. (2009);11:1865–1882. doi: 10.1089/ars.2009.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Ippolito G., Diabira S., Howard G.A., Menei P., Roos B.A., Schiller P.C. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J. Cell Sci. (2004);117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 12.Fei R.G., Penn P.E., Wolf N.S. A method to establish pure fibroblast and endothelial cell colony cultures from murine bone marrow. Exp. Hematol. (1990);18:953–957. [PubMed] [Google Scholar]
- 13.Fernandez Pujol B., Lucibello F.C., Gehling U.M., Lindemann K., Weidner N., Zuzarte M.L., Adamkiewicz J., Elsässer H.P., Müller R., Havemann K. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. (2000);65:287–300. doi: 10.1046/j.1432-0436.2000.6550287.x. [DOI] [PubMed] [Google Scholar]
- 14.Gang E.J., Jeong J.A., Han S., Yan Q., Jeon C., Kim H. In vitro endothelial potential of human UC blood-derived mesenchymal stem cells. Cytotherapy. (2006);8:215–27. doi: 10.1080/14653240600735933. [DOI] [PubMed] [Google Scholar]
- 15.Girdlestone J., Limbani V., Cutler A., Navarrete C. Efficient expansion of mesenchymal stromal cells from umbilical cord under low serum conditions. Cytotherapy. (2009) doi: 10.3109/14653240903079401. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Gnecchi M., Melo L.G. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol. Biol. (2009);482:281–294. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- 17.Grove J.E., Bruscia E., Krause D.S. Plasticity of bone marrow-derived stem cells. Stem Cells. (2004);22:487–500. doi: 10.1634/stemcells.22-4-487. [DOI] [PubMed] [Google Scholar]
- 18.Hofstetter C.P., Schwarz E.J., Hess D., Widenfalk J., El Manira A., Prockop D.J., Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA. (2002);99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houlihan D.D., Newsome P.N. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology. (2008);135:438–450. doi: 10.1053/j.gastro.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 20.Ingram D.A., Mead L.E., Tanaka H., Meade V., Fenoglio A., Mortell K., Pollok K., Ferkowicz M.J., Gilley D., Yoder M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. (2004);104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 21.Jain R.K. Molecular regulation of vessel maturation. Nat. Med. (2003);9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y., Jahagirdar B.N., Reinhardt R.L., Schwartz R.E., Keene C.D., Ortiz-Gonzalez X.R., Reyes M., Lenvik T., Lund T., Blackstad M., et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. (2002);418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 23.Kabos P., Ehtesham M., Kabosova A., Black K.L., Yu J.S. Generation of neural progenitor cells from whole adult bone marrow. Exp. Neurol. (2002);178:288–293. doi: 10.1006/exnr.2002.8039. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser S., Hackanson B., Follo M., Mehlhorn A., Geiger K., Ihorst G., Kapp U. BM cells giving rise to MSC in culture have a heterogeneous CD34 and CD45 phenotype. Cytotherapy. (2007);9:439–450. doi: 10.1080/14653240701358445. [DOI] [PubMed] [Google Scholar]
- 25.Karussis D., Kassis I., Kurkalli B.G.S., Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J. Neurol. Sci. (2008);265:131–135. doi: 10.1016/j.jns.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Kopher R.A., Penchev V.R., Islam M.S., Hill K.L., Khosla S., Kaufman D.S. Human embryonic stem cell-derived CD34+ cells function as MSC progenitor cells. Bone. (2010);47:718–728. doi: 10.1016/j.bone.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kucia M., Reca R., Campbell F.R., Zuba-Surma E., Majka M., Ratajczak J., Ratajczak M.Z. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. (2006);20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 28.Kucia M., Wysoczynski M., Ratajczak J., Ratajczak M.Z. Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tissue Res. (2008);331:125–134. doi: 10.1007/s00441-007-0485-4. [DOI] [PubMed] [Google Scholar]
- 29.Kuwana M., Okazaki Y., Kodama H., Izumi K., Yasuoka H., Ogawa Y., Kawakami Y., Ikeda Y. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J. Leukoc. Biol. (2003);74:833–845. doi: 10.1189/jlb.0403170. [DOI] [PubMed] [Google Scholar]
- 30.Li Y., Zhang C., Xiong F., Yu M., Peng F., Shang Y., Zhao C., Xu Y., Liu Z., Zhou C., et al. Comparative study of mesenchymal stem cells from C57BL/10 and mdx mice. BMC Cell Biol. (2008);9:24. doi: 10.1186/1471-2121-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim S.Y., Kim Y.S., Ahn Y., Jeong M.H., Hong M.H., Joo S.Y., Nam K.I., Cho J.G., Kang P.M., Park J.C. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc. Res. (2006);70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Martin-Rendon E., Brunskill S.J., Hyde C.J., Stanworth S.J., Mathur A., Watt S.M. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur. Heart J. (2008);29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 33.Matoba S., Tatsumi T., Murohara T., Imaizumi T., Katsuda Y., Ito M., Saito Y., Uemura S., Suzuki H., Fukumoto S., et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (therapeutic angiogenesis by cell transplantation [TACT] trial) in patients with chronic limb ischemia. Am. Heart J. (2008);156:1010–1018. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Oswald J., Boxberger S., Jørgensen B., Feldmann S., Ehninger G., Bornhäuser M., Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. (2004);22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 35.Phinney D.G., Prockop D.J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair-current views. Stem Cells. (2007);25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 36.Pitas R.E., Boyles J., Mahley R.W., Bissell D.M. Uptake of chemically modified low density lipoproteins in vivo is mediated by specific endothelial cells. J. Cell Biol. (1985);100:103–117. doi: 10.1083/jcb.100.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. (1999);284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 38.Quirici N., Soligo D., Caneva L., Servida F., Bossolasco P., Deliliers G.L. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br. J. Haematol. (2001);115:186–94. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds B.A., Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. (1992);255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 40.Romagnani P., Annunziato F., Liotta F., Lazzeri E., Mazzinghi B., Frosali F., Cosmi L., Maggi L., Lasagni L., Scheffold A., et al. CD14+ CD34 low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ. Res. (2005);97:314–322. doi: 10.1161/01.RES.0000177670.72216.9b. [DOI] [PubMed] [Google Scholar]
- 41.Shiota M., Heike T., Haruyama M., Baba S., Tsuchiya A., Fujino H., Kobayashi H., Kato T., Umeda K., Yoshimoto M., et al. Isolation and characterization of bone marrow-derived mesenchymal progenitor cells with myogenic and neuronal properties. Exp. Cell Res. (2007);313:1008–1023. doi: 10.1016/j.yexcr.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 42.Torrente Y., Polli E. Mesenchymal stem cell transplantation for neurodegenerative diseases. Cell Transplant. (2008);17:1103–1113. doi: 10.3727/096368908787236576. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q.R., Wang B.H., Huang Y.H., Dai G., Li W.M., Yan Q. Purification and growth of endothelial progenitor cells from murine bone marrow mononuclear cells. J. Cell Biochem. (2008);103:21–29. doi: 10.1002/jcb.21377. [DOI] [PubMed] [Google Scholar]
- 44.Zhang S.J., Zhang H., Hou M., Zheng Z., Zhou J., Su W., Wei Y., Hu S. Is it possible to obtain “true endothelial progenitor cells” by in vitro culture of bone marrow mononuclear cells? Stem Cells Dev. (2007);16:683–690. doi: 10.1089/scd.2006.0062. [DOI] [PubMed] [Google Scholar]


