Abstract
Endosomal sorting complex required for transport (ESCRT) complexes are involved in endosomal trafficking to the lysosome, cytokinesis, and viral budding. Extensive genetic, biochemical, and structural studies on the ESCRT system have been carried out in yeast and mammalian systems. However, the question of how the ESCRT system functions at the whole organism level has not been fully explored. In C. elegans, we performed RNAi experiments to knock-down gene expression of components of the ESCRT system and profiled their effects on protein degradation and endocytosis of YP170, a yolk protein. Targeted RNAi knock-down of ESCRT-I (tsg-101 and vps-28) and ESCRT-III (vps-24, and vps-32.2) components interfered with protein degradation while knock-down of ESCRT-II (vps-25 and vps-36) and ESCRT-III (vps-20 and vps-24) components hampered endocytosis. In contrast, the knockdown of vps-37, another ESCRT-I component, showed no defect in either YP170 uptake or degradation. Depletion of at least one component from each complex - ESCRT-0 (hgrs-1), ESCRT-I (tsg-101, vps-28, and vps-37), ESCRT-II (vps-36), ESCRT-III (vps-24), and Vps4 (vps-4) - resulted in abnormal distribution of embryos in the uterus of worms, possibly due to abnormal ovulation, fertilization, and egglaying. These results suggest differential physiological roles of ESCRT-0, -I, -II, and -III complexes in the context of the whole organism, C. elegans.
Keywords: C. elegans, endocytosis, ESCRT, protein degradation, yolk protein
INTRODUCTION
Endocytosis is a regulatory mechanism in all eukaryotes essential for the internalization of membrane proteins and intracellular trafficking. Membrane proteins are endocytosed via clathrindependent or independent pathways and delivered to early endosomes. A decision is made in the early endosome as to whether the internalized receptor proteins are recycled to the plasma membrane or further trafficked to the lysosome for degradation. Many ligand molecules are also endocytosed by binding to their cognate membrane-bound receptor proteins.
The endosomal sorting complex required for transport (ESCRT) system plays multiple roles in the cell, including endosomal-tolysosomal trafficking of ubiquitinated membrane proteins for degradation, membrane deformation, cytokinesis, and viral budding (Hurley, 2010). The list of novel roles of the ESCRT system has been further extended to also include cytosolic protein and/or organelle degradation. For example, the ESCRT system is involved in macroautophagy (Filimonenko et al., 2007; Lee et al., 2007) and endosomal microautophagy (Sahu et al., 2011). Extensive genetic, biochemical, and structural studies have revealed many aspects of the architecture and functions of components in the ESCRT system. The ESCRT system comprises at least five different complexes termed ESCRT-0, -I, -II, -III, and vacuolar sorting protein 4 (Vps4) and a few associated proteins such as Bro1/Alix and Doa4. Much of our understanding of the ESCRT system comes from studies using yeast and human molecular and cell-based systems.
In Caenorhabditis elegans, a free-living soil nematode, components of the ESCRT system are well-conserved. Previous studies reveal that the CeESCRT system is involved in receptor- mediated endocytosis in worms. CeVPS-27 mutants show defects in endocytic trafficking of low-density lipoprotein receptor- related protein 1 (Roudier et al., 2005). ALX-1, C. elegans ortholog of mammalian Alix, interacts with RME-1, a key regulator of receptor recycling from the endosome to the plasma membrane (Shi et al., 2007). In addition to the common functions of the ESCRT system, some components of the CeESCRT system are also involved in development. RNA interference (RNAi) or deletion of five ESCRT component genes in C. elegans (CeHGRS-1, CeTSG-101, CeVPS-36, CeVPS-32.1, and CeVPS- 4) are reported to cause lethality at multiple developmental stages (Michelet et al., 2010; Roudier et al., 2005).
Despite the molecular and cellular functions of ESCRT revealed by substantial genetic and biochemical approaches, much less has been reported about the physiological implications of the ESCRT system in the context of whole organisms. To investigate the roles of C. elegans ESCRT components at the organism level, we employed the vitellogenin::green fluorescent protein (YP170::GFP) reporter system to monitor oocyte endocytosis of a yolk protein fused to GFP in worms treated by RNAi against each CeESCRT component. We found that the CeESCRT system performs canonical ESCRT functions in endosomal-to-lysosomal protein degradation and cell division. Interestingly, depletion of some CeESCRT-I components showed prominent effects in endosomal-to-lysosomal protein degradation while some of the CeESCRT-II components exhibited down-regulation of membrane protein internalization. In addition, we noted the involvement of CeESCRT complexes in the internalization stage of endocytosis. All CeESCRT complexes tested in this study apparently participate in the distribution of embryos. These results suggest differential physiological roles of ESCRT complexes in the context of the whole organism, C. elegans.
MATERIALS AND METHODS
Maintenance of C. elegans strains
pwIs23[vit-2::GFP] was obtained from Caenorhabditis Genetics Center (CGC) at the University of Minnesota (USA). Worms were grown on standard Nematode Growth Medium (NGM) seeded with the E. coli strain OP50 and handled according to established methods (Brenner, 1974).
Chemicals
All chemicals were purchased from Sigma-Aldrich (USA), unless otherwise described.
RNAi constructs
To construct pVps-20, pVps-28, and pTsg-101 RNAi plasmids, cDNA for each gene was amplified from yk clones (yk1719 d07.5 for vps-20 and yk1379e02.5 for vps-28) or a cDNA library with the following primers: for vps-20, yk1719d07.5-F-XhoI (5′- TGA TCG ATG ACA CAA GCT TTC C-3′) and yk1719d07.5-R-HindIII (5′-AAT TCC TTC TCA ACG CTC GAG A-3′); for vps-28, yk1379e02.5-F-HindIII (5′-ACC CAA GCT CGA GTA GGA AAA-3′) and yk1379e02.5-R-XhoI (5′-ATC TCA TCG GAA GCT TCC ATC-3′); for tsg-101, tsg-101-c-F4 (5′-ACC GGT CGG GCA CCG ACA CAT TTA TG-3′) and tsg-101-c-R4 (5′- GCT AGC GAG ATG CTC CAG AAG CTC CC-3′). Amplified cDNA was subcloned into empty L4440 vector using XhoI and HindIII restriction enzyme sites for pVps-20 and pVps-28, or AgeI and NheI for pTsg-101. These constructs were transformed into E. coli strain HT115. The transformed E. coli were then seeded on RNAi plates containing 1 mM IPTG to induce double stranded RNA (dsRNA) to initiate the RNAi effect in worms fed on these plates.
RNAi feeding
Young adult worms (P0) were placed on RNAi feeding plates containing 2 mM IPTG and seeded with Ahringer RNAi bacteria (for vps-4, vps-22, vps-24, vps-25, hgrs-1, vps-32.1, vps-32.2, vps-36, vps-37, rab-7, and rme-2) or bacteria transformed with pVps-20, pTsg-101, or pVps-28 (for vps-20, tsg-101, or vps-28). Worms were cultured for two hours on these plates to clean residual bacteria in the gut, and then transferred to new RNAi feeding plates to lay eggs. These embryos (F1) were allowed to continue growing on RNAi plates to lay further generations of worms (F2 and F3) which were then analyzed. All of the RNAi plasmids used in this study were confirmed by sequencing, and the RNAi effect for each ESCRT component was verified by investigation of developmental delay (Supplementary Fig. S1 and Supplementary Table S1).
Microscopy and data collection
RNAi-fed worms were immobilized on 2% agarose pads with 10 mM levamisole. Normaski and fluorescent images were collected with an Axio Imager A1 microscope (Carl Zeiss, Germany). Regions inside of embryos in the uterus of worms which showed an average fluorescent intensity at least 400 times higher than average background and larger than 10 pixel2 were designated as “puncta”. Round fluorescent regions outside of embryos whose intensity was higher than 4000 and larger than 10 pixel2 were defined as “aggregates”. The number and size of puncta and aggregates were counted and measured using the AxioVision image analysis program, AxioVs40 (v.4.6.3.0) (Carl Zeiss Imaging Solution GmbH, Germany). Plotting and statistical analysis of data was carried out using Prism software (v.5.01) (GraphPad Software Inc., USA). Significance of quantified data was analyzed using unpaired t test with Welch’s correction.
RESULTS
Defects in endosomal-to-lysosomal protein degradation by RNAi against CeESCRT components
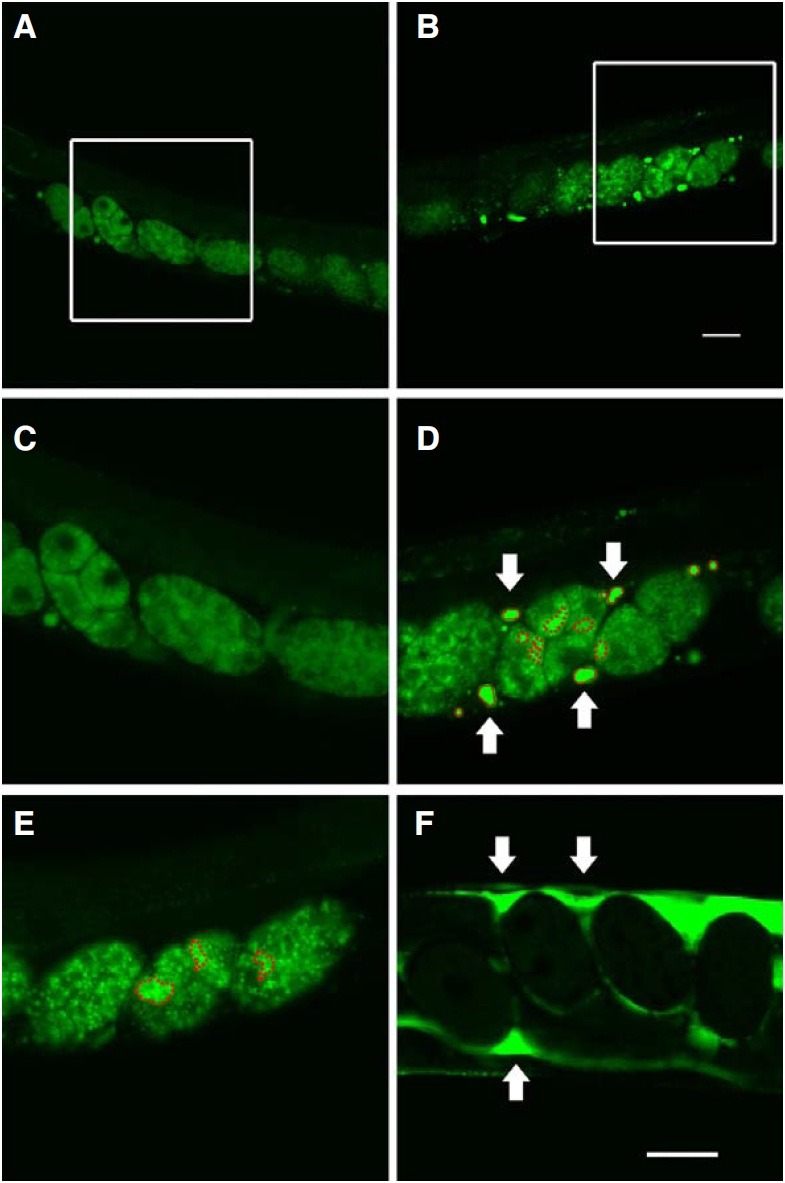
To investigate which CeESCRT components were involved in endosomal-to-lysosomal protein degradation, we performed RNAi screening in C. elegans on each of 12 CeESCRT components representing the five ESCRT complexes using YP170:: GFP as a reporter (Fig. 1). RNAi to various components of the CeESCRT system was performed on pwIs23[vit-2::gfp], a YP170::GFP-expressing transgenic line. YP170 is a yolk protein produced and secreted by the intestine and endocytosed by oocytes that are eventually fertilized to become embryos. YP170::GFP was imported into normal oocytes by endocytosis as shown by the diffuse fluorescence pattern in worms (Figs. 1A and 1C). After worms were fed with bacteria expressing dsRNA targeting ESCRT complex components for at least two generations, bright irregular puncta were often observed in embryos; with the exception of vps-32.1 embryos, which showed almost 100 % lethality (Figs. 1B and 1D). It has been previously reported that ESCRT components mediate degradation of endocytosed proteins (Audhya et al., 2007a). RNAi to rab-7 resulted in the formation of enlarged vesicles due to a block in the degradation of vesicles mediated by fusion with lysosomes (Fig. 1E) (Grant and Hirsh, 1999). We considered that these puncta represented overlapped vesicles containing yolk proteins that failed to be further degraded, presumably by a block in their fusion with lysosomes (Audhya et al., 2007b).
Fig. 1. Puncta and aggregates in worms treated with RNAi to knock-down CeESCRTs. Worms were fed with either bacteria trans- formed with L4440 empty vector (A, C) or bacteria expressing dsRNA against components of CeESCRT complexes (B, D), rab-7 (E), and rme-2 (F). Areas designated by white lines in (A) and (B) were enlarged in (C) and (D), respectively. In RNAi-treated animals, bright “puncta” (red dotted line) in embryos and “aggregates” (red solid line or white arrows) in the body cavity outside the embryos were often observed. The number and size of these fluorescent structures were quantified and presented in Figs. 2 and 3.
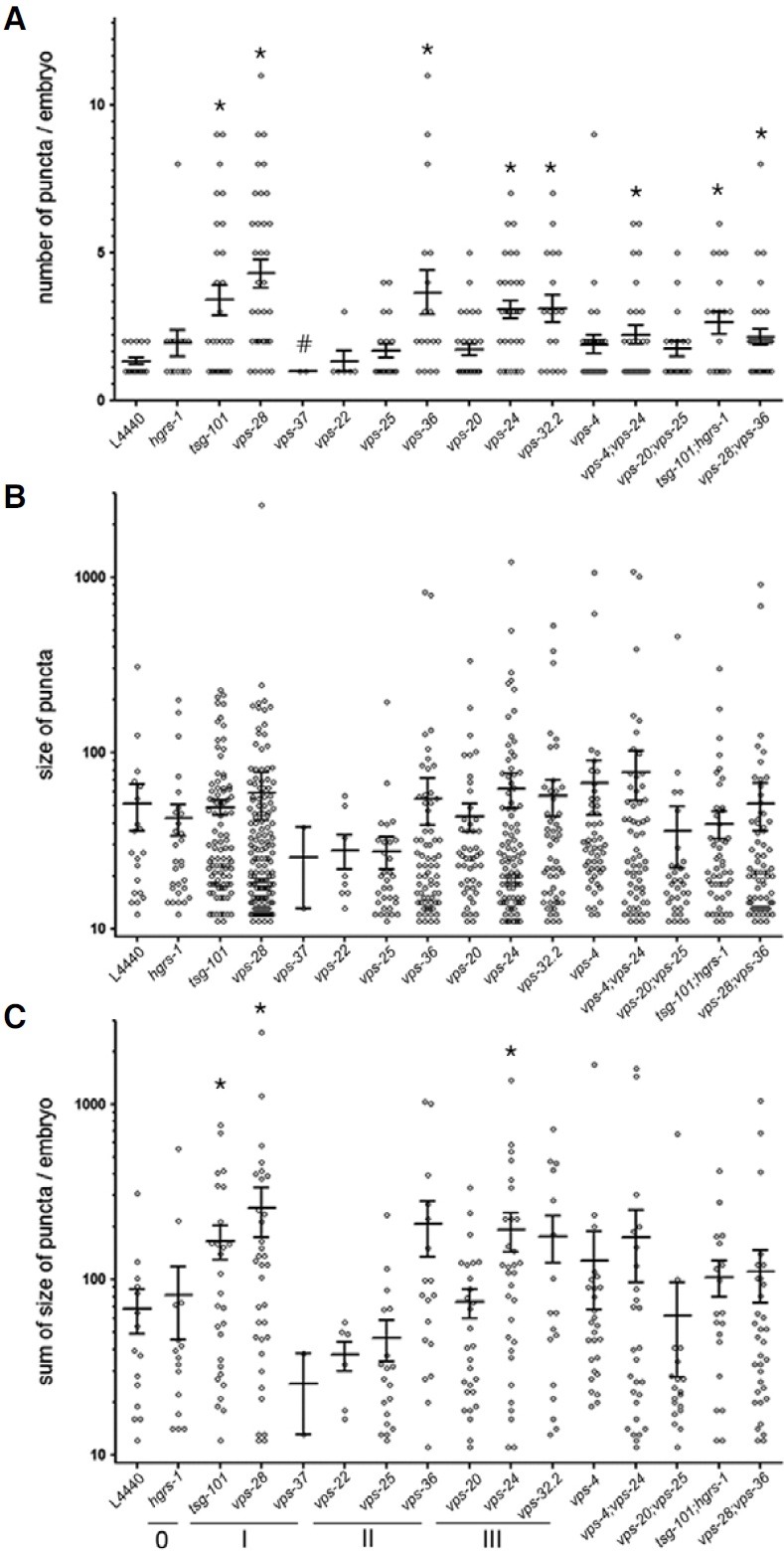
To profile the degree of defective endosomal-to-lysosomal degradation by individual CeESCRT complex components, we quantified those puncta inside embryos (Fig. 2A). The number of bright puncta was significantly increased in many of the worms treated with CeESCRT RNAi: CeESCRT-I (tsg-101 and vps-28), CeESCRT-II (vps-36), and CeESCRT-III (vps-24 and vps-32.2). Interestingly, there was a statistically significant decrease in the number of embryos treated with RNAi to vps-37. We also performed double RNAi to vps-4/vps-24 (AAA-ATPase Vps4 and ESCRT-III), vps-20/vps-25 (ESCRT- III and ESCRTII), tsg-101/hgrs-1 (ESCRT-I and ESCRT-0), and vps-28/vps-36 (ESCRT-I and ESCRT-II). With the exception of vps-20/vps-25 RNAi, all other double RNAi-fed worms showed a significant increase in the number of puncta in embryos, suggesting that vps-24 is epistatic to vps4, and tsg-101 to hgrs-1. Although the number of puncta was significantly increased by knock-down of many different CeESCRT complex components, the sizes of puncta were not significantly increased (Fig. 2B). To see whether the total level of protein degradation in an embryo was altered, we summed the sizes of the puncta in each embryo to see whether the total puncta size was increased in RNAi-fed worms (Fig. 2C). In this case, tsg-101 and vps-28 (ESCRT-I) and vps24 (ESCRT-III) RNAi-fed worms showed an increase in total puncta size. Taken together, we conclude that knock-down of ESCRT-I or -III components interfered with endoso-mal-tolysosomal degradation of endocytosed YP170::GFP.
Fig. 2. Puncta inside embryos of worms treated with RNAi to knock-down CeESCRT components. The number (A), size (B), and total puncta size (C) in each embryo were plotted for RNAi against various CeESCRT components. vps genes belonging to each of the CeESCRT-0, -I, -II, and -III complexes were underlined and labeled as 0, I, II, and III (*, p < 0.05; #, p < 0.01).
CeESCRT-II and -III are involved in endocytosis of yolk protein
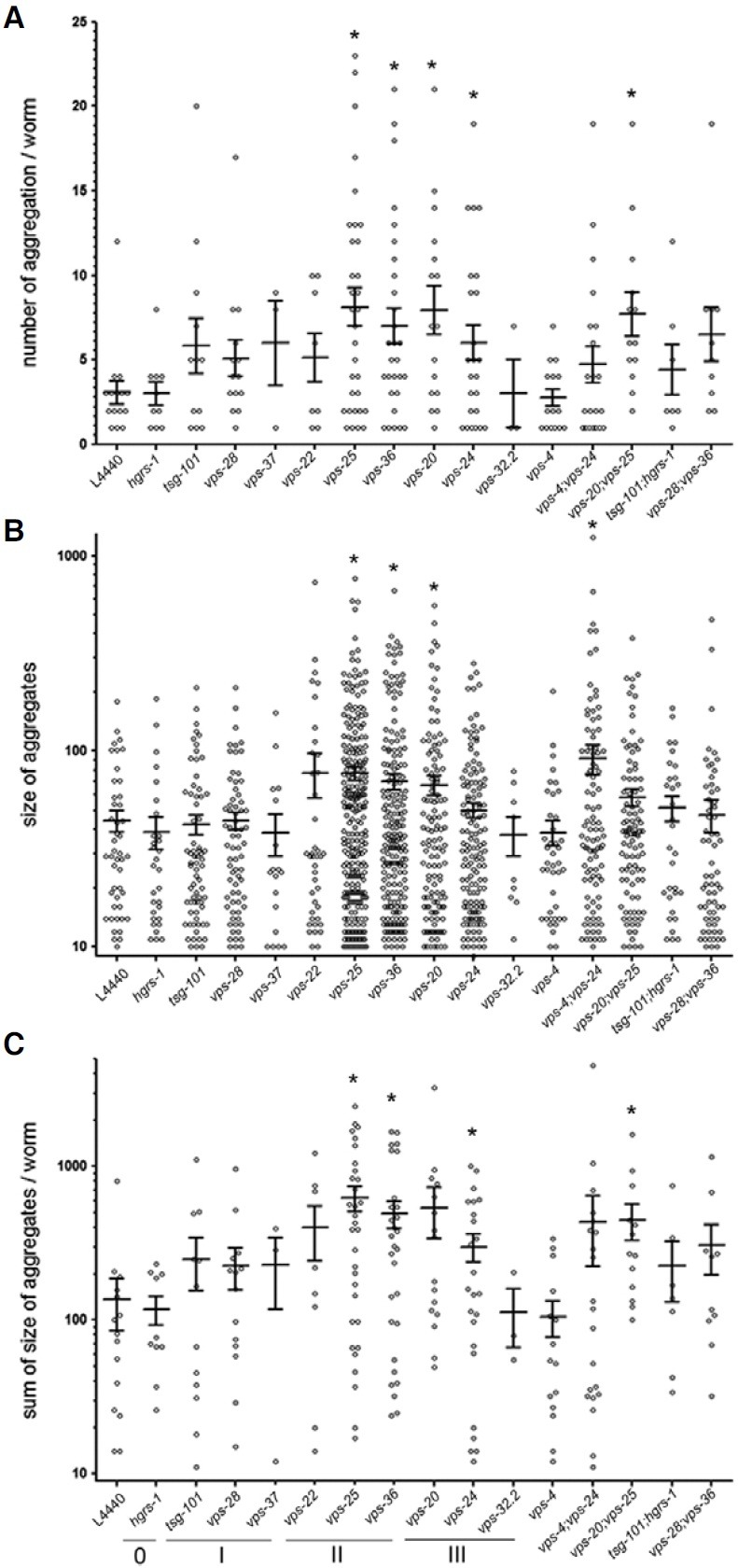
We then asked whether interfering with CeESCRT gene expression caused inefficient endocytosis of YP170::GFP. It is known that YP170::GFP that fails to be taken up by oocytes accumulates in the pseudocoelom (body cavity) as large aggregates, as shown in worms treated by RNAi against rme-2, the gene coding for the YP170 receptor (Fig. 1F) (Grant and Hirsh, 1999). Such pseudocoelomic accumulation of YP170:: GFP is interpreted as inefficient endocytosis. Similar types of aggregates often appeared in worms treated by RNAi against CeESCRT complex components (Figs. 1B and 1D), suggesting that disruption of the CeESCRT system might result in inefficient endocytosis. To see how endocytosis of YP170::GFP was affected by knock-down of each CeESCRT component, we quantified the number and size of pseudocoelomic aggregates (Fig. 3). The numbers of pseudocoelomic aggregates significantly increased in worms fed with bacteria expressing dsRNA to vps-25 and vps-36, which are CeESCRT-II complex components, and in vps-20 and vps-24, which are subunits of the CeESCRT-III complex. The size or total size (sum of the sizes) of pseudocoelomic aggregates also significantly increased in these RNAi treated worms. Double RNAi treatments, including those against vps-20, vps-25, and vps-24 also showed an increase in the accumulation of pseudocoelomic YP170::GFP aggregates, indicating that CeESCRT-II and -III complexes may play roles in the uptake of yolk proteins.
Fig. 3. Aggregates in the pseudocoelom of worms treated with RNAi against CeESCRT complexes. The number (A), size (B), and total aggregate size (C) in the pseudocoelom of each worm were plotted for RNAi against various CeESCRT components. vps genes belonging to each of the CeESCRT-0, -I, -II, and -III complexes were underlined and labeled as 0, I, II, and III (*, p < 0.05).
Defective CeESCRT components lead to arrhythmic fertilization and egg-laying
Finally, we investigated whether the CeESCRT system was involved in membrane remodeling such as cell division and found that some worms treated with RNAi against CeESCRT components showed either irregular or asymmetric distribution of embryos (Fig. 4). The ESCRT complex has been implicated in cell division by terminating cytokinesis through the facilitation of intercellular bridge abscission (Carlton and Martin-Serrano, 2007; Guizetti et al., 2011). In C. elegans, mature oocytes are ovulated and fertilized in the spermatheca, which are located at both proximal ends of a symmetrically developed gonad. Fullygrown healthy adults usually ovulate and fertilize eggs from both spermatheca approximately every embryonic cell division. Therefore, in utero, most typical worms contain one-, two-, fourcell, and so forth stage embryos in a row in symmetrical mode. In worms treated with control RNAi, regularly divided embryos were arranged symmetrically in a line toward the vulva in most animals (Figs. 4A and 4B). However, many worms treated with RNAi to knock-down gene expression of CeESCRT components retained further developed embryos, presumably due to delayed fertilization and egg-laying (Figs. 4C and 4D). In addition, they also showed asymmetric distribution of embryos in utero, indicating that both fertilization and egg-laying may have been arrhythmic at both ends of the gonad (Figs. 4E and 4F). A higher percentage of worms treated with RNAi against hgrs-1 of CeESCRT-0, CeESCRT-I components (tsg-101, vps-28, and vps-37), vps-36 of CeESCRT-II, vps-24 of CeESCRT-III, and vps-4 showed abnormal arrangement of fertilized embryos (Table 1). These irregular and asymmetric distributions of fertilized embryos in utero indicate delayed and arrhythmic ovulation, fertilization, and egg-laying, possibly caused by disturbed germline cell division in the gonad.
Fig. 4. Fertilization defect in worms treated with CeESCRT RNAi. One-, two-, and four-cell stage embryos were placed at positions ± 1, ± 2, and ± 3, respectively, of worms treated with control RNAi (A, B). Serial and symmetrical arrangement of fertilized eggs was disturbed in CeESCRT RNAi-treated animals (C, D, E, and F). Normaski (A, C, and E) and fluorescent (B, D, and F) images are shown. Worms treated with tsg-101 RNAi (C, D) or tsg-101; hgrs-1 double RNAi (E, F) are shown as representatives. Dotted line indicates the symmetric axis of the vulva and fertilized eggs are designated by numbers according to their orders of fertilization. Earlier numbers indicate newer fertilized eggs.
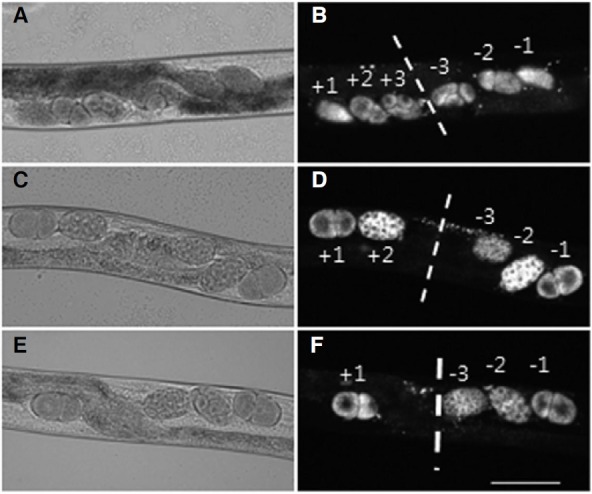
Table 1.
Worms with abnormal distribution of embryos in uterus
| ESCRT-0 | ESCRT-I | ESCRT-II | ESCRT-III | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RNAi | L4440 | hgrs1 | tsg101 | vps28 | vps37 | vps22 | vps25 | vps36 | vps20 | vps24 | vps32.2 | vps4 | vps4; vps24 | vps20; vps25 | tsg101; hgrs1 | vps28; vps36 |
| Total | 21 | 29 | 21 | 19 | 7 | 8 | 35 | 32 | 20 | 35 | 14 | 22 | 27 | 16 | 16 | 18 |
| Defective | 1 | 9 | 4 | 5 | 3 | 0 | 2 | 6 | 2 | 7 | 1 | 7 | 1 | 1 | 4 | 5 |
| % a | 4.8 | 31.0 | 19.1 | 26.3 | 42.9 | 0 | 5.7 | 18.8 | 10.0 | 20.0 | 7.1 | 31.8 | 3.7 | 6.3 | 25.0 | 27.8 |
aThe percentages of defective worms larger than three times as much as L4440 RNAi control are in bold.
DISCUSSION
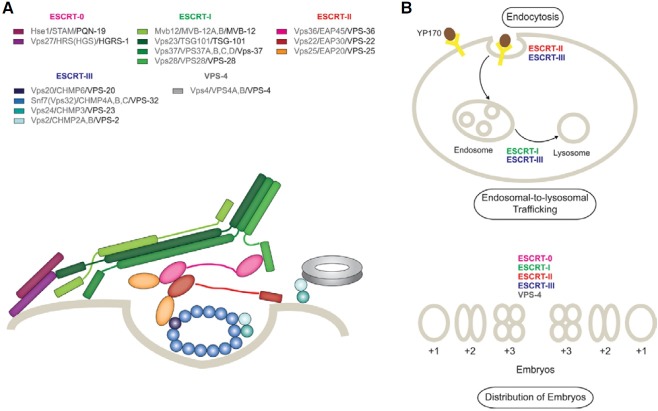
The ESCRT system comprises ESCRT-0, -I, -II, -III, and Vps4 complexes and associated proteins (Fig. 5A). It is well established that the ESCRT system is involved in endosomal-tolysosomal trafficking leading to membrane protein degradation, viral budding, and membrane remodeling such as cytokinesis (Hurley and Hanson, 2010). We utilized RNAi to deplete individual components of ESCRT complexes in C. elegans, and profiled their effects on endosomal-to-lysosomal protein degradation, endocytosis, and the distribution of embryos in utero. We found that the CeESCRT system is associated with endosomal- to-lysosomal protein degradation, consistent with the canonical function of the ESCRT system as previously reported (Audhya et al., 2007b). In particular, the effect of RNAi depletion of CeESCRT-I or CeESCRT-III was prominent in endocytosed yolk protein degradation. Interestingly, the efficient endocytosis of YP170 required ESCRT machinery, especially CeESCRT-II and -III. Finally, disruption of CeESCRT-0, -I, -II, -III components, and vps-4 caused abnormal distribution of embryos, which may have resulted from inappropriate regulation of the reproduction process. Our data provide a framework to understand ESCRT functions at the organism level, signposting the direction of subsequent mechanistic studies at molecular and cellular levels.
Fig. 5. (A) Interactions of molecular subunits of the ESCRT machinery. All the components of ESCRT complexes are depicted in different colors with legends in which their names are listed in the order of yeast/human/C. elegans. Beige line indicates membrane. (B) Summary of the functions of CeESCRT complexes from this study. ESCRT-I or -III affect the endosomal-to-lysosomal pathway, ESCRT-II and III affect the uptake of YP170, and all the complexes affect the distribution of embryos in the context of the whole organism, C. elegans. See the text for details.
Endosomal-to-lysosomal protein degradation is one of the major canonical functions of the ESCRT system. ESCRT complexes mediate the formation of multivesicular bodies (MVBs) in endosomes. MVBs are then fused to lysosomes for final degradation of cargo proteins. Depletion of CeESCRT-I, and -III (tsg-101 or vps-28, members of CeESCRT-I, and vps-24 or vps-32.2 of CeESCRT-III), caused increased fluorescence of YP170::GFP inside embryos, confirming that CeESCRT complexes are involved in endosomal-to-lysosomal protein degradation of endocytosed materials including yolk proteins. In contrast, RNAi to knock-down vps-37 caused decreased fluorescence of YP170::GFP in embryos, suggesting that vps-37 may not be vital for endosomal-to-lysosomal protein degradation in C. elegans. Yeast and mammalian orthologs of vps-37 contribute to forming both an extended stalk and a globular headpiece of the ESCRT-I complex (Kostelansky et al., 2007) (Fig. 5A). Although vps-37 is presumably a part of the triple coiled coil of the stalk of CeESCRT-I, it may be dispensable for maintaining the structural integrity of the complex. In fact, yeast Vps37 mutants disrupting the “base” of the stalk exhibited rather mild defects in ESCRT-I functions (Kostelansky et al., 2007). In C. elegans, tsg-101 and vps-28 may be sufficient to form functional ESCRT-I without vps-37, and vps-37 may have a negative regulatory role for the activity of ESCRT-I in endosomal-tolysosomal protein degradation.
Knock-down of CeESCRT-II (vps-25 or vps-36) and CeESCRTIII (vps-20 or vps-24) resulted in inefficient uptake of a yolk protein. In MVB biogenesis, ESCRT-I and -I are reported to remodel membrane into buds followed by neck scission by ESCRT-III (Wollert and Hurley, 2010). Vps25 is required for the assembly of the ESCRT-II complex and Vps36 makes contacts with ESCRT-I through a ubiquitin moiety acting as a sorting signal in the membrane (Hierro et al., 2004). In the ESCRT-III complex, Vps20 initiates the assembly of the ESCRT-III complex while Vps24 finishes scission of the budding membrane (Hurley and Hanson, 2010). It is plausible that a similar membrane remodeling mechanism may regulate the uptake of yolk protein in the plasma membrane of C. elegans. Alternatively, the inefficient uptake by knock-down of the CeESCRT complexes may have been caused by defects in recycling of yolk receptor protein. Along these lines, it has been reported that ESCRT-I (Tsg101) and -III (vps24) are required for recycling of EGFR as well as its degradation (Baldys and Raymond, 2009).
Membrane deformation is a shared feature among many different biological processes such as MVB biogenesis, viral budding, and cell division (Hurley et al., 2010). We observed that depletion of CeESCRT components caused abnormal embryo distribution in utero, manifested by the loss of rhythmic fertilization and egg-laying. In the C. elegans gonad, continuous and sequential mitosis and meiosis generate oocytes and sperm, and subsequent ovulation, fertilization, and egg-laying are tightly regulated with those extensive germline cell divisions (Hall and Altun, 2008). Eukaryotic ESCRT-I and -III complexes are implicated in cell division through cleavage furrow ingression and intercellular bridge splitting. Homologs of ESCRT-III proteins involved in cell division are also found to be involved in archaeal cell division (Lindas et al., 2008; Samson et al., 2008). Recently, it has been reported that snf-7 (vps-32), an ESCRT-III component, has a dual function in MVB formation and in activation of the transcription factor RIM101 (Weiss et al., 2009). It is possible that other ESCRT components may have nonconventional functions that are independent of their conventional roles of membrane remodeling in endocytic trafficking, virus budding, and cell division. Our data suggest that ESCRT machinery show pleiotropic effects on reproduction by supporting sufficient nutrient consumption in oocytes and appropriate regulation of reproductive organs possibly via multiple mechanisms.
We have conducted organism-level profiling of ESCRT components using C. elegans as a model organism. CeESCRT complexes are involved in endosomal-to-lysosomal protein degradation that is tightly associated with MVB formation. We also report that the CeESCRT system appears to play a role in the early stages of endocytosis, supported by inefficient uptake of YP170::GFP in RNAi-treated worms. According to our data, depletion of ESCRT-II had a more severe effect on endocytosis than endosomal-to-lysosomal trafficking, and vice versa for ESCRT-I. ESCRT-I and -II seem to act in parallel mode by templating the formation of bud necks on the surface of the membrane with their rigid multivalent membrane-binding structures (Wollert and Hurley, 2010). On the other hand, ESCRT-II is not required for viral budding or cell division which occurs at the plasma membrane of mammalian cells. In C. elegans, ESCRT-I may play a major role in the formation of bud necks which is a prerequisite step towards endosomal-to-lysosomal trafficking, while ESCRT-II may affect endocytosis by mechanisms unrelated to viral budding or cell division. Finally, most CeESCRT complex components we tested were apparently implicated in reproduction, a process in which nutrient consumption, endosomal-to-lysosomal trafficking, and cell division may play critical roles. Our results are consistent with previous ESCRT studies at the molecular and cellular level and expand them to the organism level in a systematic fashion. Further studies on the roles of the ESCRT system in the context of whole organisms using various research approaches including live imaging microscopy and epigenetics (Jung et al., 2011) will help uncover previously unknown roles of the ESCRT system in various biological processes.
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
Acknowledgments
We gratefully acknowledge the Caenorhabditis Genetic Center for pwIs23[vit-2::GFP]. We also thank Yeon-Woo Ju and Huijin Noh for their excellent technical assistance. This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (KRF-2008-314-C00224).
References
- 1.Audhya A., Desai A., Oegema K. A role for Rab5 in structuring the endoplasmic reticulum. J. Cell Biol. (2007a);178:43–56. doi: 10.1083/jcb.200701139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Audhya A., McLeod I.X., Yates J.R., Oegema K. MVB-12, a fourth subunit of metazoan ESCRT-I, functions in receptor downregulation. PLoS One. (2007b);2:e956. doi: 10.1371/journal.pone.0000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldys A., Raymond J.R. Critical role of ESCRT machinery in EGFR recycling. Biochemistry. (2009);48:9321–9323. doi: 10.1021/bi900865u. [DOI] [PubMed] [Google Scholar]
- 4.Brenner S. The genetics of Caenorhabditis elegans. Genetics. (1974);77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlton J.G., Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. (2007);316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 6.Filimonenko M., Stuffers S., Raiborg C., Yamamoto A., Malerod L., Fisher E.M., Isaacs A., Brech A., Stenmark H., Simonsen A. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J. Cell Biol. (2007);179:485–500. doi: 10.1083/jcb.200702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant B.D., Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell. (1999);10:4311–4326. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guizetti J., Schermelleh L., Mantler J., Maar S., Poser I., Leonhardt H., Muller-Reichert T., Gerlich D.W. Cortical constriction during abscission involves helices of ESCRT-IIIdependent filamens. Science. (2011);331:1616–1620. doi: 10.1126/science.1201847. [DOI] [PubMed] [Google Scholar]
- 9.Hall D.A., Altun Z.F. C. elegans Atlas. Cold Spring Harbor Laboratory Press; (2008). [Google Scholar]
- 10.Hierro A., Sun J., Rusnak A.S., Kim J., Prag G., Emr S.D., Hurley J.H. Structure of the ESCRT-II endosomal trafficking complex. Nature. (2004);431:221–225. doi: 10.1038/nature02914. [DOI] [PubMed] [Google Scholar]
- 11.Hurley J.H. The ESCRT complexes. Crit. Rev. Biochem. Mol. Biol. (2010);45:463–487. doi: 10.3109/10409238.2010.502516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurley J.H., Hanson P.I. Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat. Rev. Mol. Cell Biol. (2010);11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurley J.H., Boura E., Carlson L.A., Rozycki B. Membrane budding. Cell. (2010);143:875–887. doi: 10.1016/j.cell.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung S., Yi L., Kim J., Jeong D., Oh T., Kim C.H., Kim C.J., Shin J., An S., Lee M.S. The role of vimentin as a methylation biomarker for early diagnosis of cervical cancer. Mol. Cells. (2011);31:405–411. doi: 10.1007/s10059-011-0229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kostelansky M.S., Schluter C., Tam Y.Y., Lee S., Ghirlando R., Beach B., Conibear E., Hurley J.H. Molecular architecture and functional model of the complete yeast ESCRT-I heterotetramer. Cell. (2007);129:485–498. doi: 10.1016/j.cell.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J.A., Beigneux A., Ahmad S.T., Young S.G., Gao F.B. ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr. Biol. (2007);17:1561–1567. doi: 10.1016/j.cub.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Lindas A.C., Karlsson E.A., Lindgren M.T., Ettema T.J., Bernander R. A unique cell division machinery in the archae. Proc. Natl. Acad. Sci. USA. (2008);105:18942–18946. doi: 10.1073/pnas.0809467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michelet X., Djeddi A., Legouis R. Developmental and cellular functions of the ESCRT machinery in pluricellular organisms. Biol. Cell. (2010);102:191–202. doi: 10.1042/BC20090145. [DOI] [PubMed] [Google Scholar]
- 19.Roudier N., Lefebvre C., Legouis R. CeVPS-27 is an endosomal protein required for the molting and the endocytic trafficking of the low-density lipoprotein receptor-related protein 1 in Caenorhabditis elegans. Traffic. (2005);6:695–705. doi: 10.1111/j.1600-0854.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- 20.Sahu R., Kaushik S., Clement C.C., Cannizzo E.S., Scharf B., Follenzi A., Potolicchio I., Nieves E., Cuervo A.M., Santambrogio L. Microautophagy of cytosolic proteins by late endosomes. Dev. Cell. (2011);20:131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samson R.Y., Obita T., Freund S.M., Williams R.L., Bell S.D. A role for the ESCRT system in cell division in archaea. Science. (2008);322:1710–1713. doi: 10.1126/science.1165322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi A., Pant S., Balklava Z., Chen C.C., Figueroa V., Grant B.D. A novel requirement for C. elegans Alix/ALX-1 in RME-1-mediated membrane transport. Curr. Biol. (2007);17:1913–1924. doi: 10.1016/j.cub.2007.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss P., Huppert S., Kolling R. Analysis of the dual function of the ESCRT-III protein Snf7 in endocytic trafficking and in gene expression. Biochem. J. (2009);424:89–97. doi: 10.1042/BJ20090957. [DOI] [PubMed] [Google Scholar]
- 24.Wollert T., Hurley J.H. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. (2010);464:864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]