Abstract
The accurate distribution and segregation of replicated chromosomes through mitosis is crucial for cellular viability and development of organisms. Kinetochores are responsible for the proper congression and segregation of chromosomes. Here, we show that neural Wiskott-Aldrich syndrome protein (N-WASP) localizes to and forms a complex with kinetochores in mitotic cells. Depletion of NWASP by RNA interference causes chromosome misalignment, prolonged mitosis, and abnormal chromosomal segregation, which is associated with decreased proliferation of N-WASP-deficient cells. N-WASP-deficient cells display defects in the kinetochores recruitment of inner and outer kinetochore components, CENP-A, CENP-E, and Mad2. Live-cell imaging analysis of GFP-α-tubulin revealed that depletion of N-WASP impairs microtubule attachment to chromosomes in mitotic cells. All these results indicate that N-WASP plays a role in efficient assembly of kinetochores and attachment of microtubules to chromosomes, which is essential for accurate chromosome congression and segregation.
Keywords: chromosome, congression, mitosis, N-WASP, segregation
INTRODUCTION
Cell division is the process by which a parent cell divides into two daughter cells. The primary aim of cell division is maintenance of the original cell’s genome. Before division can occur, the genomic information stored within the chromosomes must be replicated and the duplicated genome separated precisely during mitosis. This process is crucial for genetic inheritance, cellular viability, and development of organisms. Abnormal chromosome segregation causes alterations in chromosome number, a defect commonly found in tumor cells and is potentially involved in cancer development (Jallepalli et al., 2001). Kinetochores are complex cellular structures that specify the attachments between the chromosomes and the spindle microtubules, and check that the attachment is complete. These structures are essential for accurate chromosome congression and segregation (Cleveland et al., 2003; Scholey et al., 2003; Zhu et al., 2009). Although many proteins constituting the kinetochore have been identified, the proteins responsible for accurate chromosome congression and segregation remain unclear.
The proteins of the Wiskott-Aldrich Syndrome protein (WASP) family, including WASP and N-WASP, are generally implicated in the process of the Arp2/3 complex-mediated F-actin polymerization. This process is responsible for dynamic changes in cell morphology and various intracellular events in non-dividing cells (Rohatgi et al., 1999; Takenawa et al., 2007). However, the role of the WASP proteins has not been clearly assessed during the process of mitosis when the nuclear envelope breaks down and central F-actin is disassembled. Loss of WASP activity induces defects in multiple hematopoietic lineages, including B and T lymphocytes, natural killer cells, dendritic cells, and platelets, which is characterized by the immunodeficiency, microthrombocytopenia, and eczema underlying Wiskott-Aldrich syndrome (WAS) (Burns et a., 2004; Ochs et al., 2006). In patients with WAS, platelets are markedly decreased in number (Cooper et al., 1968; Ochs et al., 1980). WASP-deficient mice also have decreased peripheral blood lymphocyte and platelet numbers, despite normal development, and showed markedly impaired T cell proliferation (Snapper et al., 1998). Therefore, it has been accepted that the activity of WASP is required for normal proliferation of certain hematopoietic cells.
N-WASP is closely related to WASP but has different tissue distributions. WASP is expressed exclusively in hematopoietic cells, whereas N-WASP is expressed ubiquitously (Derry et al., 1994; Miki et al., 1996). Accumulating evidence suggests that WASP and N-WASP have similar intracellular functions through reorganization of the actin cytoskeleton (Miki et al., 2003; Rohatgi et al., 1999). To date, most studies on N-WASP have focused on the intracellular and morphological phenomena in interphase, such as filopodium formation and vesicle transport (Roth, 2007; Takenawa et al., 2007). Interestingly, studies of an N-WASP knockout mouse indicate that N-WASP is dispensable for the general formation of actin-containing structures including filopodia (Lommel et al., 2001; Snapper et al., 2001). NWASP- deficient mouse embryos have marked developmental delay and are non-viable before embryonic day 12. Primary fibroblasts isolated from knockout embryos are unable to grow without being transformed. This suggests that N-WASP is involved in cell growth. However, little data is available regarding the function of N-WASP within cell growth. The precise in vivo functions of N-WASP have not been determined. Therefore, in this study, we examined whether N-WASP plays a role in cell division. We found that N-WASP accumulates in kinetochores of mitotic cells. Depletion of N-WASP by RNAi in HeLa cells causes diminished chromosome congression, prometaphase delay, and chromosome missegregation.
MATERIALS AND METHODS
Reagents and antibodies
The following antibodies and dyes were used: anti-α-tubulin and anti-β-tubulin (Sigma); anti-Mad2 (COVANCE); anti-CENPA (Transduction Laboratories); TOPRO3 (Molecular Probes); Hoechst 33342 (Sigma). The sequences of the stealth siRNA (Invitrogen) were as follows: N-WASP #1; 5′-UAGCUGAGCAC CCUCUCUAAUUUGA-3′; N-WASP #2; 5′-UUCUUGCCGAGG AAAGUGAAGAGGG-3′. The control siRNA sequences were designed by scrambling one of the targeted oligonucleotides of NWASP. The siRNAs were transfected into the HeLa cells using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s instructions. Antibody against N-WASP was produced as described previously (Miki et al., 1998). All other materials used were of reagent grade.
Cell culture and transfection
HeLa cells were maintained at 37℃ in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and penicillin-streptomycin. The cells were synchronized in S phase by the double-thymidine block protocol (two cycles of 12 h culture with 10 mM thymidine and 9 h of culture free of thymidine). Enrichment of mitotic cells was achieved by treatment with 1.0 μM nocodazole after release from the thymidine block. When transfection was performed during the second thymidine block, HeLa cells were washed 9 h after initiation of the second thymidine block, transfected in Opti-MEM with Lipofectamine Plus (Invitrogen) for 4 h, and cultured again in the original culture medium for 4 h in the continued presence of thymidine. Lipofectamine 2000 was used (Invitrogen) was used to transfect HeLa cells with siRNA.
Immunofluorescence
Cells collected at various time points during mitosis were fixed in -20℃ methanol for 10 min. PBS containing 1% BSA was added to the cells for 1 h to block nonspecific binding. Primary antibodies were then added and the cells incubated for 1 h at room temperature (RT), followed by washing and addition of secondary antibodies with incubation for 30 min at RT. Immunocomplexes were detected with appropriate secondary antibodies labeled with Alexa 488, Alexa 594, or Alexa 697 (Molecular Probes). DNA was stained with TOPRO3, Hoechest 33342, or DAPI. Cells were examined with an Olympus IX70 or a Zeiss LSM510 confocal imaging system.
Live-cell imaging
HeLa cells transfected with the indicated oligonucleotides or constructs were grown on glass-base dishes. After the indicated treatment of cells, images were acquired for approximately 300 min at 15-20 min intervals using the Olympus IX70 confocal imaging system.
Immunoprecipitation
Mitotic HeLa cells were harvested by shake off after nocodazole treatment and release and homogenized in RIPA buffer (10 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.5% Triton, 1% sodium deoxycholate, complete mini protease inhibitor tablets (Roche Diagnostics, USA), DNAse 20 g/ml, 100 nM Okadaic Acid). The lysates were centrifuged at 10,000 × g for 15 min and supernatant was subjected to precipitation with anti-Flag or anti-CENP-A antibodies. The immunoprecipitates were probed with antibodies to Flag, N-WASP, and CENP-A.
Cell proliferation assay
Dynamic cell proliferation was assayed using a real-time cell electronic sensing (RT-CES) system (ACEA Biosciences). HeLa cells were transfected with control and N-WASP RNAi and then synchronized to early S phase with a double thymidine block. After 60 min, the thymidine was released, during which time 6.0 × 103 cells were re-plated into RT-CES devices (16-well EPlate; ACEA Biosciences), and dynamic cell proliferation was monitored as a cell index calculated from the impedance of each well. Spreading and proliferation of the cells were continuously monitored at 30 min intervals for a period of 48-72 h.
Mitotic microtubule re-growth
GFP-α-tubulin was expressed in control and N-WASP-deficient HeLa cells and synchronized to prometaphase with nocodazole. Nocodazole was washed out and the microtubules were completely depolymerized for 30 min in an ice-water bath. Regrowth of the microtubules was then initiated in growth medium at 37℃ and the fluorescence intensity of the GFP-α-tubulin acquired for approximately 120 min at 10 min intervals using an Olympus IX70 confocal imaging system.
RESULTS AND DISCUSSION
N-WASP localizes to and forms a complex with kinetochores in mitotic cells
To determine whether N-WASP is involved in cell division, we examined the localization of endogenous N-WASP in different phases of mitotic HeLa cells. The specificity of the antibody against N-WASP was confirmed by Western blot analysis of the HeLa cell lysates. The antibody recognized a single band in the HeLa cell lysates (Fig. 1A) and the recognized band decreased upon depletion of the endogenous protein by treatment with siRNAs against N-WASP (Fig. 3A). Anti-N-WASP antibody staining showed that the subcellular distribution of N-WASP depends upon the phase of the cell cycle (Fig. 1B). The most prominent feature was the appearance of a dot-like pattern reminiscent of kinetochores on chromosomes in cells during prometaphase and metaphase. During metaphase, N-WASP signals eventually became concentrated on chromosomes condensed along the spindle equator. When the chromosomes were de-condensed, the nuclear envelope reformed, and the cell body started to segregate, it was noted that the dot-like staining disappeared and N-WASP signals appeared in the midzone in telophase and nucleus. During interphase, N-WASP staining was mostly nuclear, consistent with previous findings (Suetsugu et al., 2003; Wu et al., 2006).
Fig. 1. Immunolocalization of N-WASP at different stages of the cell cycle. (A) HeLa cell lysate was immunoblotted with purified anti-N-WASP antibody. Flag-tagged full-length N-WASP expressing plasmid (Flag-NW) and empty vector (Flag-vector) alone were also transfected into HeLa cells. Whole cell lysates for these cells were immunoblotted with anti-Flag or anti-N-WASP antibody. (B) HeLa cells were fixed with-20℃ methanol at different stages of cell cycle progression and stained for α-tubulin, TOPRO3, and N-WASP. In prometaphase and metaphase, N-WASP was seen as a pattern of dots reminiscent of kinetochores. In interphase and rophase, N-WASP is concentrated in the nucleus. In telophase and cytokinesis, intense signals of N-WASP were observed at midbody.
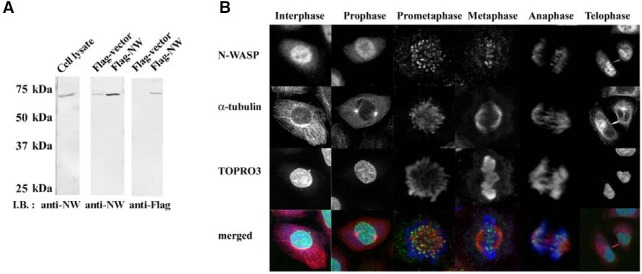
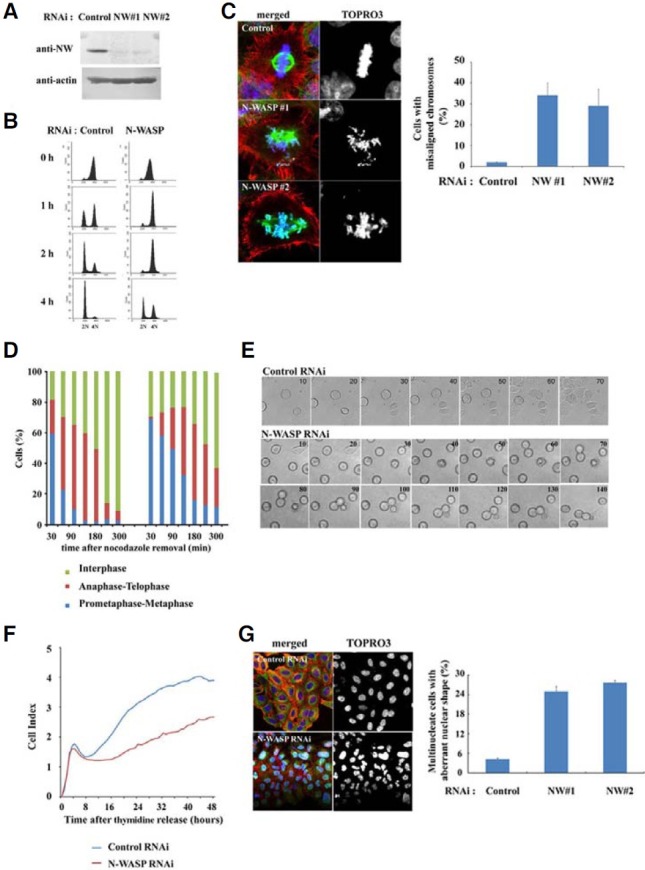
Fig. 3. Depletion of N-WASP causes misalignment and missegregation of chromosomes. (A) Treatment with siRNA against N-WASP resulted in a marked decrease in N-WASP protein levels. (B) Control and N-WASPdeficient cells were synchronized by nocodazole for 16 h. The cells were harvested at different time points from nocodazole release and analyzed for DNA contents by FACS analysis. (C) N-WASP depletion causes misalignment of chromosomes at metaphase plate and abnormal chromosome congression. Percentages of cells with chromosome misalignment were indicated. N-WASP knockdown increased the incidence of misaligned chromosomes in metaphase cells. Data are means from 100 cells in three independent experiments. (D) N-WASPknockdown induced a delay in mitosis from prometaphase to anaphase. The percentage of cells in each mitotic stage was determined by DNA and microtubule staining. The bar graphs were generated using data from three independent experiments. (E) Prolonged mitosis in N-WASPknockdown cells. Video microscopy revealed that N-WASP-deficient cells were arrested in prometaphase. Exit from prometaphase was observed over a period of 140 min. (F) Depletion of N-WASP reduced the proliferation rate of cells. HeLa cells were transfected with control or N-WASP RNAi and then synchronized at early S phase with double thymidine block. After 60 min thymidine was released, during which time the cells were replated into a RT-CES device, and real-time measurement of well impedance was monitored. Dynamic cell proliferation was monitored as a cell index calculated from the impedance of each well measured every 30 min after re-plating. (G) Multinucleate cells with macro- and micronuclei and nuclear bridges were observed during interphase of N-WASPdeficient cells. Percentages of cells with aberrant multiple nuclei produced by N-WASP-knockdown were indicated. Data are means from 100 cells in three independent experiments.
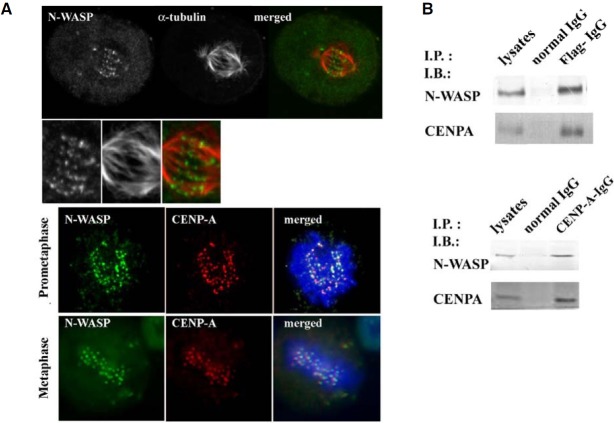
Co-staining with an antibody against CENP-A, an inner kinetochore protein, confirmed localization of N-WASP at the kinetochores. As shown in Fig. 2A, N-WASP and CENP-A were clearly colocalized on condensed mitotic chromosomes. To determine whether N-WASP is a component of the kinetochores, we overexpressed Flag-N-WASP in HeLa cells and synchronized the cells to prometaphase with nocodazole. After 30 min nocodazole was released, the tagged N-WASP was immunoprecipitated from the cell lysates with an anti-Flag antibody. The immunoprecipitated complex was blotted using specific antibodies for N-WASP and CENP-A. Endogeneous NWASP was also found to be present in immunoprecipitates with an anti-CENP-A antibody (Fig. 2B). These results indicate that the N-WASP forms a complex with CENP-A in mitosis of the cell lysates, suggesting that N-WASP may play a role in mitotic progression.
Fig. 2. N-WASP localizes to kinetochores. (A) HeLa cells at prometaphase and metaphase were fixed and immunostained for N-WASP, CENP-A, α-tubulin, and DNA. The merged image shows colocalization of N-WASP and CENPA at the kinetochores. (B) Flag-N-WASP was expressed in HeLa cells and the cells synchronized to prometaphase with nocodazole. After 30 min nocodazole was released, the lysate of the mitotic cells was immunoprecipitated with the anti-Flag antibody. Immunoprecipitated complex was blotted for N-WASP and CENPA. Additionally, immunoprecipitation with an anti-CENP-A antibody was also performed from the mitotic cell lysates. WASP forms a complex with CENPA in mitosis cell lysates.
N-WASP is required for proper chromosome congression and segregation
To investigate the role of N-WASP in mitosis, HeLa cells were transfected with RNAi for N-WASP and the cells cultured for 72 h, during which time they were subjected to a double thymidine block and a nocodazole block to synchronize the cell cycle progression. Treatment with siRNA against N-WASP resulted in a marked decrease in the protein level of N-WASP (Fig. 3A). Analyses for cell cycle stage at different time points after nocodazole release showed that depletion of N-WASP induced a significant increase in cell populations in the G2/M phase. While control cells more than 74% had completed mitosis within 2 h after nocodazole release, most of the N-WASP-deficient cells were arrested in the G2/M phase, indicating that the depletion of N-WASP leads to a delay in G2/M phase progression (Fig. 3B). Immunofluorescence analysis of mitotic cells reveled that depletion of N-WASP significantly prevented chromosome alignment during metaphase (Fig. 3C). Even 90 min after nocodazole removal, when control cells had progressed to anaphase/ telophase and interphase, the chromosomes of the NWASP- depleted cells were widely scattered; in some cells, the chromosomes remained around either pole or orientated with an apparent lack of microtubule attachment. In control cells, misaligned chromosomes were observed in only 4% of the cells. However, in N-WASP-depleted cells, cells more than 32% had misaligned chromosomes. Misaligned chromosomes were observed up to 6 h after mitotic entry. Consistent with this result, a large percentage of cells treated with siRNA against N-WASP were delayed from exiting mitosis (Fig. 3D). More than 46% of the cells remained in prometaphase for over 90 min after nocodazole release, when most of the control cells (> 90%) had progressed through mitosis into telophase and interphase.
Video microscopy allowed monitoring of the mitotic progression delay of the N-WASP-deficient cells. While most of the control cells moved from prometaphase into telophase within 60 min after nocodazole release, most N-WASP-depleted cells were arrested in metaphase during the entire observation period of 140 min (Fig. 3E). These results are likely related to the reduced cellular proliferation observed after N-WASP depletion. To further confirm the effects of the N-WASP depletion on cellular proliferation, a real-time cell electronic sensing (RT-CES) system for cell-based assays were used (Fig. 3F). This system measures the electronic impedance of sensor electrodes integrated on the bottom of the microplates. An increase in the cell index indicates that cells were spreading, undergoing enlarged morphological changes, or increasing in number (www.aceabio.com). HeLa cells were transfected with control and N-WASP RNAi and then synchronized to early S phase with a double thymidine block. After 60 min thymidine was released, during which time the cells were re-plated into RT-CES devices, and dynamic cell proliferation was monitored as a cell index calculated from the impedance of each well measured at 30 min intervals. We observed an increase in the kinetic trace corresponding to spreading of re-plated cells during the initial time period, followed by a subsequent decrease reminiscent of entry of prometaphase of cells. These results were the same for the control and N-WASP-deficient cells. However, after the consistent decrease in cell index continued until approximately 10 h, the control cells began to markedly grow over time, whereas the N-WASP-deficient cells had a significantly reduced proliferation rate. During this period, a steady state in the kinetic trace for approximately 8 h was observed. This is likely associated with prolonged mitosis in N-WASP-deficient cells. Therefore, this finding indicates that depletion of N-WASP affects cellular proliferation through mitotic delay.
Furthermore, some of the N-WASP-depleted cells with misaligned chromosomes without proper congression undergo slow progression to anaphase, and subsequently telophase. This produces multinuclear cells with micro- and macronuclei (Fig. 3G). Aberrant nuclear bridges were also frequently observed in interphase cells. These results suggest that the spindle checkpoint mechanism is also impaired in these N-WASPdeficient cells and that chromosome misalignment in N-WASPdeficient cells leads to missegregation of the chromosomes.
N-WASP contributes to kinetochores localization of mitotic kinetochore components CENP-A, CENP-E, and Mad2
A number of studies have showed that without the accurate kinetochores assembly, proper chromosome congression and the checkpoint signal cannot be established or functioned (Mao et al., 2005; Regnier et al., 2005). An inner kinetochore protein CENP-A is known to be an upstream component of a hierarchical kinetochore assembly pathway (Regnier et al., 2005). Depletion of CENP-A induces mislocalization of inner components, including CENP-I, CENP-H, and CENP-C, as well as outer kinetochores components CENP-E and Mad2, which results in defects in the kinetochore assembly, microtubule attachment, chromosome congression, and segregation (Regnier et al., 2005). CENP-E and Mad2 are key components of mitotic checkpoint signaling in many eukaryotes (Mao et al., 2005; Regnier et al., 2005). The kinesin-like motor protein CENP-E plays a role in spindle microtubule capture. A mitotic spindle-checkpoint protein Mad2 is directly involved in generation of the waitanaphase signal. They are recruited at unattached kinetochores in prometaphase cells and subsequent chromosome biorientation on the metaphase plate correlates with a significant decrease in CENP-E signals and loss of Mad2 localization.
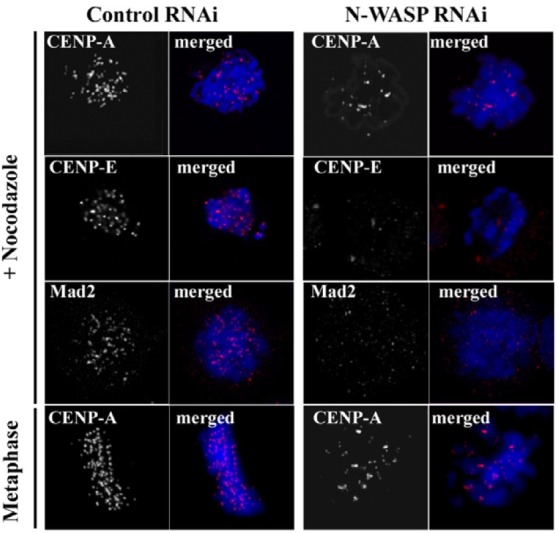
We therefore examined whether depletion of N-WASP affects the kinetochores localization of CENP-A, CENP-E and Mad2. As shown in Fig. 4, typical kinetochore staining of CENP-A observed for control cells was diffused and decreased for the N-WASP-deficient cells. Additionally, when N-WASPdeficient prometaphase cells treated with nocodazole were stained with CENP-E or Mad2, kinetochore signals of CENP-E and Mad2 were also significantly decreased compared to those in the control cells. These findings indicate that depletion of NWASP affects kinetochore recruitment of the inner kinetochore component CENP-A, as well as CENP-E and Mad2 in the outer kinetochores, resulting in kinetochore assembly defects in the N-WASP-deficient cells. The recruitment defect of Mad2 in kinetochores depleted of N-WASP also suggests that these cells are deficient in generating a Mad2-dependent checkpoint signal from unattached kinetochores, which may be responsible for chromosome missegregation in N-WASP-deficient cells. On the other hand, cell cycle analysis (Figs. 3B and 3D) have showed that N-WASP-deficient cells were still cycling despite significant delay in mitotic progression. Taken together, these results suggest that depletion of N-WASP does not impair the overall activation of the mitotic checkpoint signals. It is likely that N-WASP-deficient cells are able to activate some mitotic checkpoint but the checkpoint response is subsequently overridden by the absence Mad2 and CENP-E.
Fig. 4. Depletion of N-WASP affects kinetochore localization of CENP-A, CEPN-E, and Mad2. Control and N-WASP-deficient cells were stained for CENP-A, CENP-E, Mad2 (Red), and DNA (TOPRO3). Immunostaining of CENP-A was performed in nocodazole-treated prometaphase cells and 60 min after removal of nocodazole. Kinetochore localization of CENP-E and Mad2 were examined in nocodazoletreated prometaphase cells. CENP-A, CENP-E, and Mad2 signals were hardly detectable in N-WASP-deficient cells.
N-WASP regulates attachment between spindle microtubules and chromosomes
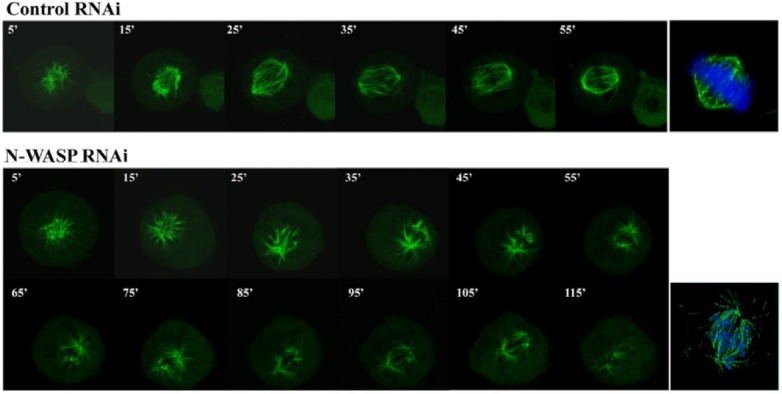
Since N-WASP-deficient cells showed a significant reduction in kinetochores-localized CENP-E, we examined whether N-WASP is required for attachment of microtubule spindles to chromosomes through microtubule spindle re-growth experiments (Fig. 5). GFP-α-tubulin was overexpressed in control and N-WASPdeficient cells, and all cells were synchronized in prometaphase with nocodazole. Nocodazole was washed out and microtubules were completely depolymerized for 30 min in an icewater bath. Re-growth of microtubules was then initiated in growth medium at 37℃. In most control cells (> 80%), GFP-α- tubulin formed well-aligned mitotic spindles within at least 60 min and chromosomes in cells are well localized within a clearly define rectangle at the cell equator. In contrast, N-WASPdepleted cells more than 43% had disorganized microtubule spindles despite bipolar spindle formation. Most notably, the microtubule ends emanating from the centrosomes were not fully aligned along the metaphase plate during the prolonged mitosis period. Chromosomes in these cells are not condensed and do not fall within the clearly defined rectangle. Therefore, this result suggests that N-WASP contributes to the formation of proper kinetochores microtubule attachments.
Fig. 5. N-WASP is required for microtubule attachment to chromosomes. GFP-α-tubulin was transfected in control and N-WASP-deficient HeLa cells and then synchronized to prometaphase with nocodazole. Nocodazole was washed out and microtubules completely depolymerized for 30 min in an ice bath. Re-growth of microtubules was then initiated in growth medium at 37℃ and the fluorescence intensity of GFP-α-tubulin acquired at 10 min intervals using an Olympus IX70 confocal imaging system. After 60 min for microtubule re-growth reaction, cells were fixed and stained for DNA (blue). Data are interpreted by mean ± SD of three independent experiments in which > 100 cells were analyzed.
In summary, our results indicate that N-WASP plays a central role in proper chromosome congression and segregation. For accurate chromosome congression and segregation, sister kinetochores must attach to the assembly properties of microtubules emanating from opposing spindle poles, which requires the integrated assembly of multiple kinetochores proteins. NWASP localizes to mitotic kinetochores and forms a complex with CENP-A essential for kinetochore assembly and function. CENP-A is a constitutive centromere protein present during the entire cell division cycle, but it is substantially recruited to the kinetochore during replication and postreplication stage in order to supply new synthesized CENP-A-containing nucleosomes to the duplicated sister centromeres (Shelby et al., 2000). Some extrinsic proteins including Mis18 and KNL2 have been implicated in the localization of CENP-A to the kinetochores (Fujita et al., 2007). When any Mis18 or KNL2 is defective, CENP-A is lost at the kinetochores (Fujita et al., 2007; Hayashi et al., 2004). Similarly, in this s tudy, we found t hat d epletion of N -WASP reduces kinetochores localization of CENP-A. The phenotypes of N-WASP RNAi cells resemble those of CENP-A RNAi cells. CENP-A-deficient cells exhibit a significant reduction in localization of inner and outer kinetochore proteins, including CENP-H, CENP-C, CENP-I, CENP-E, and Mad2, which causes defects in proper chromosomes congression, a transient prometaphase delay, and abnormal chromosome segregation (Regnier et al., 2005). Therefore, the defective phenotypes in N-WASP-deficient cells may be caused by the reduction of kinetochore CENP-A. The critical role of mitotic N-WASP may be involved in incorporation of CENP-A to kinetochores. The kinetochore is the chromosomal site that joins to microtubules during mitosis for proper segregation. N-WASP-depleted kinetochores are deficient in forming the microtubule-kinetochore interaction necessary for generating chromosome biorientation. Outer kinetochore protein CENP-E and Mad2 signals were also decreased in N-WASP-deficient cells. Therefore, it is reasonable to suppose that N-WASP contributes to the formation of kinetochore assembly and microtubule spindle attachment through association with CENP-A, which is required for accurate chromosome congression and segregation.
Our findings will help to gain an understanding of the mechanism of how N-WASP affects cell proliferation in mammalian cells, and may also provide a clue for determination of the in vivo functions of N-WASP. N-WASP may be primarily required for chromosome integrity that affects cell viability, embryonic development, birth defects, cancer, or human disease including WAS. Further studies are required to identify protein partners for N-WASP in mitosis in order to understand how N-WASP is recruited to kinetochores, and to determine the factors controlling the functions of N-WASP in mitosis.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0017199) and (2010-0005349).
References
- 1.Burns S., Cory G.O., Vainchenker W., Thrasher A.J. Mechanisms of WASp-mediated hematologic and immunologic disease. Blood. (2004);104:3454–3462. doi: 10.1182/blood-2004-04-1678. [DOI] [PubMed] [Google Scholar]
- 2.Cleveland D.W., Mao Y., Sullivan K.F. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. (2003);112:407–421. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 3.Cooper M.D., Chae H.P., Lowman J.T., Krivit W., Good R.A. Wiskott-Aldrich syndrome. An immunologic deficiency disease involving the afferent limb of immunity. Am. J. Med. (1968);44:499–513. doi: 10.1016/0002-9343(68)90051-x. [DOI] [PubMed] [Google Scholar]
- 4.Derry J.M., Ochs H.D., Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. (1994);78:635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 5.Fujita Y., Hayashi T., Kiyomitsu T., Toyoda Y., Kokubu A., Obuse C., Yanagida M. Priming of centromere for CENP-A recruitment by human hMis18alpha, hMis18beta, and M18BP1. Dev. Cell. (2007);12:17–30. doi: 10.1016/j.devcel.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T., Fujita Y., Iwasaki O., Adachi Y., Takahashi K., Yanagida M. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell. (2004);118:715–729. doi: 10.1016/j.cell.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Jallepalli P.V., Lengauer C. Chromosome segregation and cancer: cutting through the mystery. Nat. Rev. Cancer. (2001);1:109–117. doi: 10.1038/35101065. [DOI] [PubMed] [Google Scholar]
- 8.Lommel S., Benesch S., Rottner K., Franz T., Wehland J., Kuhn R. Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep. (2001);2:850–857. doi: 10.1093/embo-reports/kve197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao Y., Desai A., Cleveland D.W. Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. J. Cell Biol. (2005);170:873–880. doi: 10.1083/jcb.200505040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miki H., Takenawa T. Regulation of actin dynamics by WASP family proteins. J. Biochem. (2003);134:309–313. doi: 10.1093/jb/mvg146. [DOI] [PubMed] [Google Scholar]
- 11.Miki H., Miura K., Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. (1996);15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- 12.Ochs H.D., Thrasher A.J. The Wiskott-Aldrich syndrome. J. Allergy Clin. Immunol. (2006);117:725–738. doi: 10.1016/j.jaci.2006.02.005. quiz 739. [DOI] [PubMed] [Google Scholar]
- 13.Ochs H.D., Slichter S.J., Harker L.A., Von Behrens W.E., Clark R.A., Wedgwood R.J. The Wiskott-Aldrich syndrome: studies of lymphocytes, granulocytes, and platelets. Blood. (1980);55:243–252. [PubMed] [Google Scholar]
- 14.Regnier V., Vagnarelli P., Fukagawa T., Zerjal T., Burns E., Trouche D., Earnshaw W., Brown W. CENP-A is required for accurate chromosome segregation and sustained kinetochore association of BubR1. Mol. Cell. Biol. (2005);25:3967–3981. doi: 10.1128/MCB.25.10.3967-3981.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohatgi R., Ma L., Miki H., Lopez M., Kirchhausen T., Takenawa T., Kirschner M.W. The interaction between NWASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. (1999);97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 16.Roth M.G. Integrating actin assembly and endocytosis. Dev. Cell. (2007);13:3–4. doi: 10.1016/j.devcel.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Scholey J.M., Brust-Mascher I., Mogilner A. Cell division. Nature. (2003);422:746–752. doi: 10.1038/nature01599. [DOI] [PubMed] [Google Scholar]
- 18.Shelby R.D., Monier K., Sullivan K.F. Chromatin assembly at kinetochores is uncoupled from DNA replication. J. Cell Biol. (2000);151:1113–1118. doi: 10.1083/jcb.151.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snapper S.B., Takeshima F., Anton I., Liu C.H., Thomas S.M., Nguyen D., Dudley D., Fraser H., Purich D., Lopez-Ilasaca M., et al. N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat. Cell Biol. (2001);3:897–904. doi: 10.1038/ncb1001-897. [DOI] [PubMed] [Google Scholar]
- 20.Suetsugu S., Takenawa T. Translocation of N-WASP by nuclear localization and export signals into the nucleus modulates expression of HSP90. J. Biol. Chem. (2003);278:42515–42523. doi: 10.1074/jbc.M302177200. [DOI] [PubMed] [Google Scholar]
- 21.Takenawa T., Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell. Biol. (2007);8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 22.Wu X., Yoo Y., Okuhama N.N., Tucker P.W., Liu G., Guan J.L. Regulation of RNA-polymerase-II-dependent transcription by N-WASP and its nuclear-binding partners. Nat. Cell Biol. (2006);8:756–763. doi: 10.1038/ncb1433. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H., Fang K., Fang G. Mechanism, function and regulation of microtubule-dependent microtubule amplification in mitosis. Mol. Cells. (2009);27:1–3. doi: 10.1007/s10059-009-0014-2. [DOI] [PubMed] [Google Scholar]