Abstract
Resveratrol is a plant phenolic phytoalexin that has been reported to have antitumor properties in several types of cancers. In particular, several studies have suggested that resveratrol exerts antiproliferative effects against A549 human non-small cell lung cancer cells; however, its mechanism of action remains incompletely understood. Deregulation of microRNAs (miRNAs), a class of small, noncoding, regulatory RNA molecules involved in gene expression, is strongly correlated with lung cancer. In this study, we demonstrated that resveratrol treatment altered miRNA expression in A549 cells. Using microarray analysis, we identified 71 miRNAs exhibiting greater than 2-fold expression changes in resveratrol-treated cells relative to their expression levels in untreated cells. Furthermore, we identified target genes related to apoptosis, cell cycle regulation, cell proliferation, and differentiation using a miRNA target-prediction program. In conclusion, our data demonstrate that resveratrol induces considerable changes in the miRNA expression profiles of A549 cells, suggesting a novel approach for studying the anticancer mechanisms of resveratrol.
Keywords: A549, human non-small cell lung cancer cells, microRNA, resveratrol
INTRODUCTION
Resveratrol (3,5,4′-trihydroxystilbene) is a phenolic phytoalexin produced in various plants (e.g., grapes, legumes, berries, and peanuts) in response to stress, injury, fungal infection, and ultraviolet irradiation (Aggarwal et al., 2004; Soleas et al., 1997). Epidemiological research has revealed a link between the consumption of red wine, an important source of resveratrol, and reduced risk of cardiovascular disease in France. Consequently, resveratrol has been extensively studied, and numerous beneficial effects have been observed, including antithrombotic, cardioprotective, anti-inflammatory, antioxidative, and vasorelaxant effects (Bhat et al., 2001; Delmas et al., 2005; Kasdallah-Grissa et al., 2007; Stef et al., 2006;). In addition, resveratrol exhibits antitumor properties in several cancer types, including prostate, mammary, colon, and lung cancers (Bhat and Pezzuto, 2002; Revel et al., 2003; Schneider et al., 2000; Stewart et al., 2003). The anticancer effects of resveratrol are associated with its ability to suppress proliferation and induce apoptosis through a variety of different pathways in tumor cell lines (Aggarwal et al., 2004).
Several studies have indicated that resveratrol functions not only as a chemoprotective agent in A549 human non-small cell lung cancer (NSCLC) cells but also has therapeutic effects against existing lung carcinoma (Kim et al., 2003; Matsuoka et al., 2001; Mollerup et al., 2001). According to Kim et al. (2003), resveratrol treatment inhibits the phosphorylation of Rb protein as well as the transcriptional activities of both nuclear factorkappa B and activator protein-1 (Kundu and Surh, 2004; Sun et al., 2006). This inhibitory effect of resveratrol has been reported to stimulate apoptosis through induction of the cyclin-dependent kinase inhibitor p21CIP1/WAF1 and increased activity of caspase 3 (Kim et al., 2003). Despite such progress, more research is needed to elucidate the anticancer mechanisms of resveratrol.
Recently, microRNAs (miRNAs) were identified as important, small, and noncoding regulatory RNA molecules that posttranscriptionally modulate gene expression (Ambros and Lee, 2004; Bartel, 2004; Pillai et al., 2007; Plasterk, 2006; Zhang et al., 2007). Base pairing between miRNAs and complementary sites located in the 3′-untranslated regions of target mRNAs results in either the degradation or inhibition of mRNA translation, which subsequently reduces target gene protein levels (Ambros and Chen, 2007; Rana, 2007). miRNAs have been found to play roles in diverse biological processes, including development, differentiation, proliferation, and apoptosis (Chen et al., 2006; Cheng et al., 2005; Cho et al., 2009). Furthermore, human miRNAs exhibit aberrant expression patterns in several cancers, including lung cancer; additionally, miRNA genes are commonly found in cancer-associated genomic regions (Calin et al., 2005; He et al., 2005; Johnson et al., 2005). Although several studies have reported a correlation between miRNA deregulation and diverse lung cancers, the role of mRNAs in cancer remains incompletely understood (Eder and Scherr, 2005; Nana-Sinkam and Geraci, 2006).
Since changes in miRNA expression may play a role in the anticancer effects of resveratrol, we used miRNA microarrays to characterize the effects of resveratrol in A549 human NSCLC cells. The mRNA targets of the miRNAs altered by resveratrol in these experiments may eventually provide practical information for the management of lung cancer.
MATERIALS AND METHODS
Cell culture
The NSCLC cell line A549 was purchased from the Korean Cell Line Bank (Korea), and cultured in RPMI 1640 medium containing 10% fetal bovine serum and antibiotics at 37℃ in a humidified chamber containing 5% CO2. Cells were seeded into 60- mm culture dishes (4 × 105 cells per dish) 1 day before resveratrol treatment.
Resveratrol treatment
Resveratrol (Sigma-Alrich, USA) was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) and added to A549 cultures to obtain final concentrations of either 60 or 120 μM. DMSO was added to culture media as a vehicle control. Cells were treated for 24 h.
RNA preparation
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s protocol after the 24-h resveratrol treatment. For the microarray studies, both the quality and concentration of the RNA samples were determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, USA) and an Ultrospec 3300 Pro UV/Visible Spectrophotometer (Amersham Biosciences, USA). The recommended RNA quality parameters for microarray analysis were as follows: UV spectroscopy A260/A280 ratio of 1.8-2.0 and A260/A230 ratio greater than 1.8, 18S/28S rRNA ratio of 1.8-2.1, and RNA integrity number greater than 8.0.
Microarray analysis of miRNA profiles
A Human miRNA Microarray (V2) Kit (Agilent Technologies), containing probes for 723 human and 76 viral miRNAs, was used to analyze miRNA expression profiles. Before hybridization, 100 ng of total RNA was dephosphorylated with calf intestinal alkaline phosphatase and denatured by heating in DMSO. The dephosphorylated RNA was then labeled with cyanine 3- cytidine 3′,5′-bisphosphate using T4 RNA ligase, and the labeled RNA was purified on a Micro Bio-Spin 6 column (Bio-Rad Laboratories, USA). The purified RNA was then denatured and hybridized to the microarray probes at 55℃, and then spun at 20 rpm for 20 h in an Agilent Microarray Hybridization Chamber (Agilent Technologies). The microarray slide was subsequently washed and scanned using the Agilent scanner to obtain microarray images. The scanned signals were extracted with Feature Extraction software (Agilent Technologies), and the data were analyzed with GeneSpring GX software version 7.3 (Agilent Technologies).
Classification of miRNAs
Of the miRNAs probed by the microarray, 723 human miRNAs containing a “present” flag in at least one sample were selected for further analysis. Fold-change analysis identified miRNAs from the resveratrol-treated groups that were altered by a factor of 2 as compared with their levels in control cells.
Target prediction of miRNAs
Candidate miRNAs that were either increased or decreased in expression by more than 2-fold following resveratrol treatment were chosen for target prediction using the miRBase Targets Database (Version 5) of experimentally verified miRNA sequences. Human genes predicted as miRNA targets were analyzed with Gene Ontology in order to identify genes playing roles in apoptosis, the cell cycle, cell proliferation, or differentiation.
RESULTS AND DISCUSSION
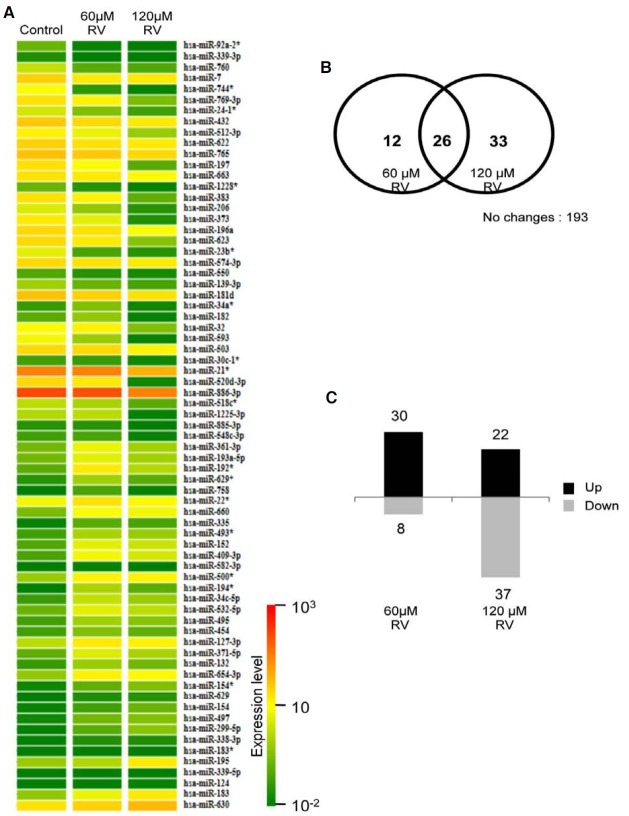
To evaluate resveratrol-induced changes in miRNA expression in A549 cells, which constitute an in vitro model of human lung adenocarcinoma, we employed miRNA microarray analysis to compare resveratrol-treated and untreated cells. The resveratrol doses used in this experiment were chosen by MTT assay to evaluate dose-dependent cell viability (data not shown). Two hundred and sixty-four out of 723 human miRNAs (36.5%) differed between the untreated and treated A549 cells (Fig. 1 and the full list is shown in Supplementary Table 1). The color bar displaying altered fluorescence intensity corresponds to miRNAs up-regulated (red colors) or down-regulated (green color) by resveratrol stimulation. Although some of the miRNAs did not show significant changes in expression, certain miRNAs were significantly influenced by resveratrol stimulation. Therefore, we carried out resveratrol-dependent miRNAs profiling by sorting according to three independent criteria (2-fold expression change, up- and down-regulated miRNAs, and putative target genes of the miRNAs).
Fig. 1. The miRNA expression profiles of A549 human non-small cell lung cancer cells after resveratrol treatment. A total of 264 human miRNAs exhibited at least a 2-fold change in expression after resveratrol treatment relative to their expression in control cells. Relative miRNA expression is represented in a scale from 1 × 10−2 (green) to 1 × 103 (red) for each treatment group [control, 60 μM resveratrol (RV), and 120 μM RV].
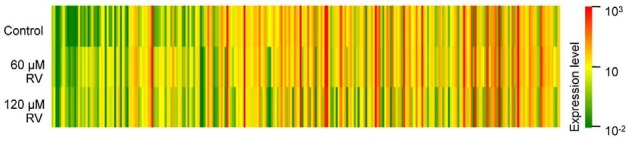
Table 1.
MicroRNAs with greater than 2-fold changes in expression after resveratrol treatment
| Resveratrol dose (μM) | No. of miRNAs | miRNA | |
|---|---|---|---|
| Upregulated | Downregulated | ||
| 60 | 12 | hsa-miR-127-3p, hsa-miR-192*, hsa-miR-193a-5p, hsa-miR-22*, hsa-miR-361-3p, hsa-miR-371-5p, hsa-miR-454, hsa-miR-495, hsa-miR-500*, hsa-miR-532-5p, hsa-miR-654-3p, hsa-miR-660 | |
| 60 and 120 | 26 | hsa-miR-132, hsa-miR-152, hsa-miR-154, hsa-miR-154*, hsa-miR-183*, hsa-miR-194*, hsa-miR-299-5p, hsa-miR-335, hsa-miR-338-3p, hsa-miR-34c-5p, hsa-miR-409-3p, hsa-miR-493*, hsa-miR-497, hsa-miR-582-3p, hsa-miR-629, hsa-miR-629*, hsa-miR-758 | hsa-miR-1228*, hsa-miR-197, hsa-miR-23b*, hsa-miR-339-3p, hsa-miR-34a*, hsa-miR-7, hsa-miR-744*, hsa-miR-760, hsa-miR-92a-2* |
| 120 | 33 | hsa-miR-124, hsa-miR-183, hsa-miR-195, hsa-miR-339-5p, hsa-miR-630 | hsa-miR-1225-3p, hsa-miR-139-3p, hsa-miR-181d, hsa-miR-182, hsa-miR-196a, hsa-miR-206, hsa-miR-21*, hsa-miR-24-1*, hsa-miR-30c-1*, hsa-miR-32, hsa-miR-373, hsa-miR-383, hsa-miR-432, hsa-miR-503, hsa-miR-512-3p, hsa-miR-518c*, hsa-miR-520d-3p, hsa-miR-548c-3p, hsa-miR-550, hsa-miR-574-3p, hsa-miR-593, hsa-miR-622, hsa-miR-623, hsa-miR-663, hsa-miR-765, hsa-miR-769-3p, hsa-miR-885-3p, hsa-miR-886-3p |
First, we determined the miRNAs that experienced greater than 2-fold changes in expression. Thirty-eight of the 723 miRNAs (5.2%) in the 60-μM resveratrol-treated group as well as 59 miRNAs (8.1%) in the 120-μM resveratrol-treated group exhibited greater than 2-fold changes in expression (Fig. 2A and 2B). Among them, 26 of the 723 miRNAs (3.6%) showed 2-fold changes in expression at both resveratrol concentrations (Fig. 2B, center and Table 2). Interestingly, some of the miRNAs (e.g., miR-299-5p, miR-194*, miR-338-3p, miR-758, miR-582-3p, and mir-92a-2*) exhibited greater than 20-fold changes in expression in resveratrol-treated cells compared with the control. These observations strongly indicate that resveratrol is able to influence the expression of specific miRNAs in a lung cancer A549 cells. The asterisk following the name indicates non-functional miRNA or passenger strand that is released from the miRNA duplex (biologically active miRNA: passenger miRNA) during miRNA biogenesis (O’Toole et al., 2006). Recent studies have suggested that miRNA* affords potential opportunities for contributing to the regulation network (Guo et al., 2010). The microarray we employed contained probes to 723 human miRNAs, including miRNA*.
Fig. 2. Dose-dependent expression profiles of miRNAs exhibiting greater than 2-fold expression changes. Seventy-one miRNAs exhibited greater than 2-fold changes in expression after resveratrol (RV) treatment. (A) Relative miRNA expression levels of each treatment group are represented in color as described in Fig. 1. (B) The Venn diagram depicts the dose-dependent responses of miRNAs to RV: 12, 33, and 26 miRNAs exhibited greater than 2-fold expression changes after treatment with 60 μM RV, 120 μM RV, and both concentrations of RV. (C) Upregulation or down-regulation of miRNAs exhibiting greater than 2-fold expression changes after RV treatment was shown to be dosedependent.
Table 2.
Average expression levels of miRNAs exhibiting greater than 2-fold expression changes in response to both concentrations of resveratrol
| miRNA | Fold changes | miRNA | Fold changes | ||
|---|---|---|---|---|---|
| 60 μM RV | 120 μM RV | 60 μM RV | 120 μM RV | ||
| hsa-miR-1228* | -2.56 | -24.48 | hsa-miR-34a* | 2.33 | 3.59 |
| hsa-miR-132 | 2.94 | 2.18 | hsa-miR-34c-5p | 3.53 | 2.76 |
| hsa-miR-152 | 2.71 | 2.43 | hsa-miR-409-3p | 3.36 | 2.95 |
| hsa-miR-154 | 8.48 | 13.74 | hsa-miR-493* | 2.46 | 2.33 |
| hsa-miR-154* | 4.86 | 6.23 | hsa-miR-497 | 7.42 | 8.74 |
| hsa-miR-183* | 2.45 | 14.40 | hsa-miR-582-3p | 21.40 | 15.70 |
| hsa-miR-194* | 42.91 | 22.62 | hsa-miR-629 | 11.58 | 17.21 |
| hsa-miR-197 | -2.58 | -7.32 | hsa-miR-629* | 3.99 | 2.15 |
| hsa-miR-23b* | -3.08 | -5.78 | hsa-miR-7 | -3.03 | -2.68 |
| hsa-miR-299-5p | 50.03 | 78.31 | hsa-miR-744* | -4.48 | -46.11 |
| hsa-miR-335 | 19.87 | 16.21 | hsa-miR-758 | 27.61 | 2.70 |
| hsa-miR-338-3p | 36.59 | 39.97 | hsa-miR-760 | -2.22 | -2.54 |
| hsa-miR-339-3p | -8.86 | -9.80 | hsa-miR-92a-2* | -24.99 | -31.75 |
RV = resveratrol
Next, we analyzed the direction of the 2-fold changes (up- or down-regulation) in miRNA expression (see above). Thirty of the 38 miRNAs (> 2-fold change in the 60-μM resveratroltreated group) and 22 of the 59 miRNAs (> 2-fold change in the 120-μM resveratrol-treated group) were up-regulated. On the other hand, eight of the 38 miRNAs and 37 of the 59 miRNAs were down-regulated (Fig. 2C). Among the 26 miRNAs (> 2- fold change at both resveratrol concentrations), eight miRNAs and seven miRNAs were up- and down-regulated, respectively, in a dose-dependent manner (Table 2). The other miRNAs (10 of 26 miRNAs) were shown to be expressed (up- or downregulated) at similar levels in the 60-μM and 120-μM resveratrol treated cells, respectively. Interestingly, miR-758 experienced the opposite expression pattern when the resveratrol concentration was increased from 60- to 120-μM (28-fold to 3-fold). This miRNA might be involved in the mechanism of action of the low dose resveratrol-mediated cellular response. However, the miRNAs that showed a dose-dependent response might be involved in the inhibition of cell proliferation. Several recent studies have reported that resveratrol inhibits cell proliferation in a dose-dependent manner in several cancer cell lines, including HL-60, HepG2, LNCap, HT1080, and A549 cells, through cell cycle arrest, cell adhesion defects, and apoptosis (Kim et al., 2003; Ma et al., 2006; Moammir et al., 2006; Park et al., 2009; Stervbo et al., 2006). Although further work on the cellular mechanisms of resveratrol-specific miRNAs should be performed, the physiological properties and changes in expression of specific miRNAs are correlated with each other in a resveratrol- concentration dependent manner. These results raise a question about the relationship between resveratrol-specific miRNAs and their putative target genes with regards to resveratrol- mediated anti-cancer properties.
Therefore, we conducted a bioinformatics analysis of resveratrol- responsive miRNAs (26 miRNAs showing a 2-fold change at both resveratrol concentrations) to identify those with potential target genes involved in resveratrol-mediated cancer preventive processes, such as apoptosis, cell cycle regulation, cell proliferation, and differentiation. Using the miRBase Target Database, we identified approximately 1,000 potential targets for each miRNA, excluding miR-1228*, which was not present in the database (data not shown). Next, we used the AmiGo web-based set tools offered by the Gene Ontology Consortium (GOC) to select the list of human genes showing experimentally confirmed function in apoptosis, cell cycle regulation, cell proliferation, and differentiation (see the “Materials and Methods”). These lists contained 178, 40, and 67 genes, respectively. Finally, the putative genes from the miRNA target prediction were compared with the set of genes from the gene ontology analysis, and the overlaps between that sets of genes are listed in Table 3. These lists contain 97, 20, and 28 genes, respectively. Some of the mRNAs were predicted to be targeted by more than one miRNA, as a single miRNA may target a number of mRNAs, and conversely, a single mRNA target may be modulated by several miRNAs (John et al., 2004). Interestingly, miR-299-5p was the most up-regulated by resveratrol (greater than 50-fold increase), and BCL6 had the highest target score among its target genes. BCL6 is a well-known protooncogene in B-cell lymphoma and breast cancer, and it has been reported to function as a transcriptional repressor (Logarajah et al., 2003; Staudt et al., 1999). Regarding the cancer chemopreventive activity of resveratrol, the expression level of BCL6 was reported to be decreased by resveratrol stimulation, resulting in increased expression of several BCL6-regulated genes, such as p27, p53, and CD69 (Faber et al., 2006). Although the cellular effect and target genes of this miRNA have not been researched, further study on miR-299-5p and BCL6 would be important for understanding the resveratrol cellular response. Moreover, we noticed the possibility that several miRNAs under this condition can potentially regulate target genes encoding known oncogenes or oncogenic proteins. Using the bioinformatics tool of Gene Set Enrichment Analysis (GSEA, www.broadinstitute.org/gsea/index.jsp), we investigated the oncogene family from the pool of listed genes in Table 3. We identified eight of those putative target genes in Table 3 as oncogenes, such as AKT1, PML, HIP1, LCK, PDGFRB, MYC, MLF1, and BCL6. Interestingly, a great number of miRNAs putatively targeting the above oncogenes were up-regulated by resveratrol (Table 2). Altogether, these results indicate that the anticancer properties resveratrol of may be derived from its effects on the expression of miRNAs targeting important regulators of cell survival.
Table 3.
Predicted targets of miRNAs exhibiting greater than 2-fold expression changes in response to both concentrations of resveratrol
| miRNA name | Functions of target genes | ||
|---|---|---|---|
| Apoptosis | Cell cycle | Cell proliferation and differentiation | |
| hsa-miR-1228* | N/A | N/A | N/A |
| hsa-miR-132 | BCL2L11, CASP8, GHRL, HIP1, IL6, LCK, PEA15, PML, RASA1, YWHAB | APC, PML | APC, ENPEP, ENPP7 |
| hsa-miR-152 | BBC3, BCL2L11, DEDD2, GZMB, IFNB1, MIF, PCSK6, SRA1, TCF7L2, TP53 | HHEX | IL29, JAG2, MIF, NF2, SRA1 |
| hsa-miR-154 | CDKN2C, DYNLL1, IGF1, IL12A, INHA, MAEA, NDUFS1, SRA1, TCF7L2, TNFSF10 | ATR, CDKN2C, IL12A | CDKN2C, EPO, MYC, SRA1 |
| hsa-miR-154* | SRGN | ATR | DERL2, IL11 |
| hsa-miR-183* | FURIN, GRIK2, IL6, PPP3CC, RASA1, TERF1 | - | IL29 |
| hsa-miR-194* | CARD8, CD40LG, CIDEB, FASLG, IGF1, NDUFA13, PCSK6, PMAIP1, SRA1 | - | DERL2, SRA1 |
| hsa-miR-197 | BCL2L10, CD70 | WDR6 | FOXP3, IL31RA, IL8RB, WDR6 |
| hsa-miR-23b* | APOH, CD27, KIAA1967, MIF, TNFSF10 | - | ENPP7, MIF |
| hsa-miR-299-5p | BCL6, CECR2, GRIK2, PMAIP1 | - | POLA1 |
| hsa-miR-335 | CDKN2C, CHEK2, FAS, NDUFS1, NUAK2, RASA1, SRGN | CDKN2C | CDKN2C, IL31RA |
| hsa-miR-338-3p | AVEN, BID, FAS, ING4, NLRP2, PCBP4 | ING4, MLF1, PCBP4 | ING4, NF2, PDGFRB, TBX2 |
| hsa-miR-339-3p | BBC3, CIDEC, ERCC3, HIP1, PCSK6, RELA, SFN | RBM38 | CD81, ENPP7, EPO |
| hsa-miR-34a* | AVEN, CD70, MAPK8, RYR2, SCG2 | - | - |
| hsa-miR-34c-5p | BAX, BID, CADM1, CDKN2A, DEDD2, PCBP4, TRAF2 | CDC23, CDKN2A, PCBP4, STRN3 | APPL2 |
| hsa-miR-409-3p | AKT1, COL4A3, DNAJB6, ERCC3, TCF7L2 | STK11 | EPO |
| hsa-miR-493* | CADM1, CHEK2, DFFB, HSPD1 | CDKN3, IL8, STRN3 | - |
| hsa-miR-497 | ALB, AVEN, BFAR, CADM1, CD70, CDKN2A, CHEK2, KIAA1967, NRG1, PROK2, SON, TNFRSF10B | CDKN2A | NF2, NRG1, PROK2 |
| hsa-miR-582-3p | AVEN, BCL2L11, BNIP1, BNIPL, CDC2, CRYAB, ERCC3, FAS, GSK3B, HSPD1, NRG1, PCSK6, RYR2 | MLF1 | BNIPL, IL31RA, NRG1 |
| hsa-miR-629 | ALB, APOE, COL4A3, DLC1, GRIK2, RTKN, TCF7L2 | - | DLC1, ENPEP, MYC, POLA1 |
| hsa-miR-629* | DLC1, GZMB, MIF | - | DLC1, MIF, MYC |
| hsa-miR-7 | ACIN1, BAD, CASP8, CRYAA, GLO1, HSPA5, INHA, PCSK6, RELA, UBE4B | NUSAP1, STK11 | ALOX15B, TBX2 |
| hsa-miR-744* | ACIN1, BAD, CD70, CRYAA, FAS, RELA, STK4 | - | TBX2 |
| hsa-miR-758 | ANGPTL4, BAD, BID, CADM1, DYNLL1, INHBA, NRG1 | INHBA | FOXP3, NRG1 |
| hsa-miR-760 | ACIN1, BRCA1, BRE, CASP10, CIDEC, HTATIP2, IL6, LGALS12 | CDT1 | ENPP7, FOXP3 |
| hsa-miR-92a-2* | BCL2L10, CASP8, CD27, CIDEC, CRYAB, INHA, PEA15, SFN, TERF1 | WDR6 | ALOX15B, FOXP3, WDR6 |
N/A: not applicable
In summary, we determined that resveratrol induces considerable changes in A549 cell miRNA expression profiles. Previous studies have provided substantial evidence regarding the role of resveratrol as a chemoprotective agent against human lung cancer, but its mechanisms of action remain incompletely understood. However, our results suggest that characterization of resveratrol-induced miRNA changes may provide a useful approach to understanding cellular responses to resveratrol. Further studies must be performed to verify the predicted miRNA targets identified in this study.
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
Acknowledgments
We are grateful to all the members of our research group for their support and advice regarding this study. This work was supported by the Ministry of Education, Science, and Technology (grants 20110018427 and 20110002841 to S. An) of the Republic of Korea
References
- 1.Aggarwal B.B., Bhardwaj A., Aggarwal R.S., Seeram N.P., Shishodia S., Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. (2004);24:2783–2840. [PubMed] [Google Scholar]
- 2.Ambros V., Chen X. The regulation of genes and genomes by small RNAs. Development. (2007);134:1635–1641. doi: 10.1242/dev.002006. [DOI] [PubMed] [Google Scholar]
- 3.Ambros V., Lee R.C. Identification of microRNAs and other tiny noncoding RNAs by cDNA cloning. Methods Mol. Biol. (2004);265:131–158. doi: 10.1385/1-59259-775-0:131. [DOI] [PubMed] [Google Scholar]
- 4.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. (2004);116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Bhat K.P., Pezzuto J.M. Cancer chemopreventive activity of resveratrol. Ann. N. Y. Acad. Sci. (2002);957:210–229. doi: 10.1111/j.1749-6632.2002.tb02918.x. [DOI] [PubMed] [Google Scholar]
- 6.Bhat K.P.L., Kosmeder J.W. 2nd., Pezzuto J.M. Biological effects of resveratrol. Antioxid. Redox. Signal. (2001);3:1041–1064. doi: 10.1089/152308601317203567. [DOI] [PubMed] [Google Scholar]
- 7.Calin G.A., Ferracin M., Cimmino A., Di Leva G., Shimizu M., Wojcik S.E., Iorio M.V., Visone R., Sever N.I., Fabbri M., et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. (2005);353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 8.Chen J.F., Mandel E.M., Thomson J.M., Wu Q., Callis T.E., Hammond S.M., Conlon F.L., Wang D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. (2006);38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng A.M., Byrom M.W., Shelton J., Ford L.P. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. (2005);33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho W.J., Shin J.M., Kim J.S., Lee M.R., Hong K.S., Lee J.H., Koo K.H., Park J.W., Kim K.S. miR-372 regulates cell cycle and apoptosis of ags human gastric cancer cell line through direct regulation of LATS2. Mol. Cells. (2009);28:521–527. doi: 10.1007/s10059-009-0158-0. [DOI] [PubMed] [Google Scholar]
- 11.Delmas D., Jannin B., Latruffe N. Resveratrol: preventing properties against vascular alterations and ageing. Mol. Nutr. Food. Res. (2005);49:377–395. doi: 10.1002/mnfr.200400098. [DOI] [PubMed] [Google Scholar]
- 12.Eder M., Scherr M. MicroRNA and lung cancer. N. Engl. J. Med. (2005);352:2446–2448. doi: 10.1056/NEJMcibr051201. [DOI] [PubMed] [Google Scholar]
- 13.Faber A.C., Chilles T.C. Resveratrol induces apoptosis in transformed follicular lymphoma OCI-LY8 cells: evidence for a novel mechanism involving inhibition of BCL6 signaling. Int. J. Oncol. (2006);29:1561–1566. [PubMed] [Google Scholar]
- 14.Guo L., Lu Z. The fate of miRNA* strand through Evolutionary anlysis: Implication for degradation as carrier strand or potential regulatory molecules? PLoS One. (2010);5:e11387. doi: 10.1371/journal.pone.0011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He L., Thomson J.M., Hemann M.T., Hernando-Monge E., Mu D., Goodson S., Powers S., Cordon-Cardo C., Lowe S.W., Hannon G.J., et al. A microRNA polycistron as a potential human oncogene. Nature. (2005);435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John B., Enright A.J., Aravin A., Tuschl T., Sander C., Marks D.S. Human MicroRNA targets. PLoS Biol. (2004);2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson S.M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K.L., Brown D., Slack F.J. RAS is regulated by the let-7 microRNA family. Cell. (2005);120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Kasdallah-Grissa A., Mornagui B., Aouani E., Hammami M., El May M., Gharbi N., Kamoun A., El-Fazaa S. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. (2007);80:1033–1039. doi: 10.1016/j.lfs.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y.A., Lee W.H., Choi T.H., Rhee S.H., Park K.Y., Choi Y.H. Involvement of p21WAF1/CIP1, pRB, Bax and NFkappaB in induction of growth arrest and apoptosis by resveratrol in human lung carcinoma A549 cells. Int. J. Oncol. (2003);23:1143–1149. [PubMed] [Google Scholar]
- 20.Kundu J.K., Surh Y.J. Molecular basis of chemoprevention by resveratrol: NF-kappaB and AP-1 as potential targets. Mutat. Res. (2004);555:65–80. doi: 10.1016/j.mrfmmm.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Logarajah S., Hunter P., Kraman M., Steele D., Lakhani S., Bobrow L., Venkitaraman A., Wagner S. BCL-6 is expressed in breast cancer and prevents mammary epithelial differentiation. Oncogene. (2003);22:5572–5578. doi: 10.1038/sj.onc.1206689. [DOI] [PubMed] [Google Scholar]
- 22.Ma X.D., Yan F., Ma A.D., Wang H.J. Resveratrol induces HepG2 cell apoptosis by depolarizing mitochondrial membrane. Nan Fang Yi Ke Da Xue Xue Bao. (2006);26:406–408. [PubMed] [Google Scholar]
- 23.Matsuoka A., Furuta A., Ozaki M., Fukuhara K., Miyata N. Resveratrol, a naturally occurring polyphenol, induces sister chromatid exchanges in a Chinese hamster lung (CHL) cell line. Mutat. Res. (2001);494:107–113. doi: 10.1016/s1383-5718(01)00184-x. [DOI] [PubMed] [Google Scholar]
- 24.Moammir H.A., Minakshi N., Vivian X.F., David F.J., Nihal A. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol. Cancer Ther. (2006);5:1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 25.Mollerup S., Ovrebo S., Haugen A. Lung carcinogenesis: resveratrol modulates the expression of genes involved in the metabolism of PAH in human bronchial epithelial cells. Int. J. Cancer. (2001);92:18–25. [PubMed] [Google Scholar]
- 26.Nana-Sinkam S.P., Geraci M.W. MicroRNA in lung cancer. J. Thorac. Oncol. (2006);1:929–931. [PubMed] [Google Scholar]
- 27.O’Toole A.S., Miller S., Haines N., Zink M.C., Serra M.J. Comprehensive thermodynamic analysis of 3’doublenucleotide overhangs neighboring Watson-Crick terminal pairs. Nucleic Acids Res. (2006);34:3338–3344. doi: 10.1093/nar/gkl428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J.S., Kim K.M., Kim M.H., Chang H.J., Baek M.K., Kim S.M., Jung Y.D. Resveratrol inhibits tumor cell adhension to endothetial cells by blocking ICAM-1 expression. Anticancer Res. (2009);29:355–362. [PubMed] [Google Scholar]
- 29.Pillai R.S., Bhattacharyya S.N., Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. (2007);17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Plasterk R.H. Micro RNAs in animal development. Cell. (2006);124:877–881. doi: 10.1016/j.cell.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 31.Rana T.M. Illuminating the silence: understanding the structure and function of small RNAs. Nat. Rev. Mol. Cell. Biol. (2007);8:23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 32.Revel A., Raanani H., Younglai E., Xu J., Rogers I., Han R., Savouret J.F., Casper R.F. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J. Appl. Toxicol. (2003);23:255–261. doi: 10.1002/jat.916. [DOI] [PubMed] [Google Scholar]
- 33.Schneider Y., Vincent F., Duranton B., Badolo L., Gosse F., Bergmann C., Seiler N., Raul F. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett. (2000);158:85–91. doi: 10.1016/s0304-3835(00)00511-5. [DOI] [PubMed] [Google Scholar]
- 34.Soleas G.J., Diamandis E.P., Goldberg D.M. Resveratrol: a molecule whose time has come? And gone? Clin. Biochem. (1997);30:91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- 35.Staudt L.M., Dent A.L., Shaffer A.L., Yu X. Regulation of lymphocyte cell fate decisions and lymphomagenesis by BCL-6. Int. Rev. Immunol. (1999);18:381–403. doi: 10.3109/08830189909088490. [DOI] [PubMed] [Google Scholar]
- 36.Stef G., Csiszar A., Lerea K., Ungvari Z., Veress G. Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance. J. Cardiovasc. Pharmacol. (2006);48:1–5. doi: 10.1097/01.fjc.0000238592.67191.ab. [DOI] [PubMed] [Google Scholar]
- 37.Stervbo U., Vang O., Bonnesen C. Time-and concentration- dependent effects of resveratrol in HL-60 and HepG2 cells. Cell Prolif. (2006);39:479–493. doi: 10.1111/j.1365-2184.2006.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart J.R., Artime M.C., O’Brian C.A. Resveratrol: a candidate nutritional substance for prostate cancer prevention. J. Nutr. (2003);133:2440S–2443S. doi: 10.1093/jn/133.7.2440S. [DOI] [PubMed] [Google Scholar]
- 39.Sun C., Hu Y., Liu X., Wu T., Wang Y., He W., Wei W. Resveratrol downregulates the constitutional activation of nuclear factor-kappaB in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet. Cytogenet. (2006);165:9–19. doi: 10.1016/j.cancergencyto.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Zhang B., Wang Q., Pan X. MicroRNAs and their regulatory roles in animals and plants. J. Cell. Physiol. (2007);210:279–289. doi: 10.1002/jcp.20869. [DOI] [PubMed] [Google Scholar]