Abstract
A proliferation-inducing ligand (APRIL), a new TNF family member, supports B-cell survival and tumor cell proliferation. APRIL is secreted as a soluble protein by macrophages, dendritic cells and activated T cells. However, factors involved in regulation of APRIL expression are as yet unknown. In this study, we investigated the effect of TGF-β1 on APRIL expression in P388D1, a mouse macrophage cell line. TGF-β1 induced APRIL mRNA expression in a time- and dose-dependent manner. One nanogram per milliliter of TGF-β1 was optimal and APRIL transcripts appeared as early as 3 h after stimulation. Based on our studies, which included overexpression of Smad3, DN-Smad3, and sh-Smad3, we found that Smad3 mediates APRIL transcription at least partially. Further, experiments using inhibitors revealed that p38MAPK and CREB are also involved in TGF-β1-induced APRIL expression. These results suggest that TGF-β1, through Smad3 and p38MAPK/ CREB signaling pathways, stimulates APRIL expression in macrophages.
Keywords: APRIL, CREB, p38 MAPK, Smad3/4, TGF-β1
INTRODUCTION
Antigen presenting cells (APCs) such as dendritic cells (DC) and macrophages play a central role in adaptive T- and B-cell responses. DCs enhance B cell proliferation and differentiation, (Dubois et al., 1997; 1999). Further, B cell-activating factor (BAFF), which belongs to the TNF family, and a proliferationinducing ligand (APRIL), are expressed by macrophages and DCs, and are critical factors for the growth and survival of both normal and malignant B cells (Fagarasan and Honjo, 2000; Litinskiy et al., 2002). Interestingly, APRIL is known to increase IgA and IgM but not IgG responses to T cell-dependent antigens (Stein et al., 2002). More specifically, APRIL is known to exclusively influence IgA isotype regulation: IgA production increases in APRIL transgenic mice (Stein et al., 2002), and conversely, IgA class switching recombination is impaired in APRIL-deficient mice (Castigli et al., 2004).
It has been shown that IFN-γ, IFN-α and CD40 ligation stimulate macrophages and DCs to express APRIL and BAFF mRNA (Litinskiy et al., 2002). In addition, we have previously shown that TGF-β1 and IFN-γ stimulate mouse macrophages to express BAFF (Kim et al., 2008). Therefore, we explored the effects of TGF-β1 on APRIL expression in mouse macrophages to see if TGF-β1 indirectly regulates B cell differentiation by influencing macrophages. Our results demonstrate that TGF-β1 can modulate mouse macrophages to express APRIL via Smad3 and p38MAPK/CREB pathways.
MATERIALS AND METHODS
Reagents
TGF-β1 was purchased from R&D Systems (USA). PD98059, SB203580, SB431542, and SP600125 were obtained from Sigma Chemical Co. (USA).
Animals
BALB/c mice were purchased from Orient. Co., Ltd. (Korea) and maintained on an 8:16 h light:dark cycle in an animal environmental control chamber (Myung Jin Inst. Co., Korea). Animal care was in accordance with the institutional guidelines of Kangwon National University.
Cell preparation and culture
The P388D1 mouse macrophage cell line was cultured in DMEM supplemented with 10% FBS (HyClone Labs, USA) and penicillin (100 U/ml)/streptomycin (100 μg/ml; Gibco BRL, USA) using a humidified CO2 incubator (Sanyo, Japan).
Normal macrophages were isolated from the peritoneal cavity of BALB/c mice following i.p. administration of 3% thioglycolate (3 ml). Peritoneal cells were harvested 72 h after injection and adherent cells were collected after 16 h incubation on petri dishes.
Plasmid constructs and transfection
Mammalian expression vectors containing Smad3 and Smad4 subcloned into N-terminal FLAG-tagged pcDNA3, were generously provided by Dr. Masahiro Kawabata (Department of Biochemistry, The Cancer Institute, Japan). The DN-Smad3 plasmid was provided by Dr. M. Kato (The Cancer Institute, Japan) (Goto et al., 1998). pRetrosuper Smad3 (Addgene plasmid 15726) which expresses Smad3 shRNA, was purchased from Addgene (USA). pCMV2-CREB (rat CREB expression plasmid) was obtained from Dr. P. R. Dobner (University of Massachusetts Medical School, USA). DN-CREB, containing an alanine substitution at the phosphorylation site serine 133 of wild type rat CREB, was constructed using QuikChange™ Site-Directed Mutagenesis (Stratagene, USA).
For transient transfection, P388D1 cells were seeded on sixwell plates and transfected using GENE SHUTTLE-20 (Qbiogene, USA) according to the manufacturer’s protocol.
RT-PCR
RNA preparation, reverse transcription, and PCR were performed as described previously (Park et al., 2001). PCR primers were synthesized by Bioneer Corp. (Korea). The primers used for mouse APRIL were: forward primer 5′-CCT CAC TTC TGA GAC CAC AGC-3′ and reverse primer 5′-GAA CAA CAG TCA AGG CAA AGC-3′. PCR was performed in parallel with β- actin primers to allow normalization of the cDNA concentration in each set of samples.
Flow cytometry
Cultured cells were washed with HBSS and resuspended in 0.01 M PBS at a density of 1 × 106 cells/ml. The cell suspension was incubated with rabbit anti-APRIL antibody (ProSci., Inc., USA) at 4℃ for 30 min. After washing, the cells were incubated with FITC-conjugated anti-rabbit IgG antibody (Becton Dickinson, USA) at 4℃ for 30 min, washed three times with 0.01 M PBS, and resuspended in 0.01 M PBS containing 1% formalin. Cytofluorometric analysis was carried out using a FACScan (Becton Dickinson).
Western blot analysis
Total cell lysates were subjected to SDS-PAGE under reducing conditions and proteins were transferred to PVDF membranes (Bio-Rad, USA). Immuno detection was performed by incubation with either rabbit anti-APRIL antibody, anti-p38MAPK, antiphospho- p38MAPK, anti-CREB, or anti-phospho-CREB (Cell Signaling Technology, USA) antibodies, followed by peroxidase- conjugated goat anti-mouse or goat anti-rabbit IgG (Pierce, USA), and visualized by chemiluminescence using a Supersignal detection kit (Pierce).
RESULTS
Effect of TGF-β1 on APRIL expression by mouse macrophages
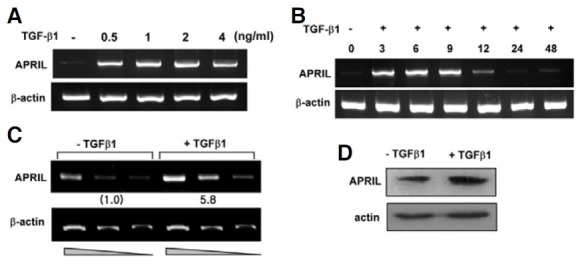
It is increasingly clear that APCs directly affect B cells: macrophages regulate B-cell responses, and TNF-like ligands APRIL and BAFF expressed by macrophages play an important role in B-cell activation and differentiation (Stein et al., 2002). Ig isotype switching is a critical event in B-cell differentiation, and TGF-β1 is a specific switching factor for the IgA and IgG2b mouse isotype (Kim and Kagnoff, 1990; McIntyre et al., 1993). Since we have previously demonstrated that TGF-β1 stimulates mouse macrophages to express BAFF (Kim et al., 2008), it was worthwhile to investigate if TGF-β1 can modulate macrophages to express another member of the same family, APRIL. Indeed, we found that TGF-β1 induced APRIL transcription in the mouse macrophage cell line, P388D1 as shown in Fig. 1A. Maximum induction was observed at the conditions of 1 ng/ml TGF-β1 and 9 h stimulation (Fig. 1B). TGF-β1 also stimulated APRIL expression in primary peritoneal macrophages at the transcriptional and intracellular protein levels (Figs. 1C and 1D). These observations indicate that macrophages, under the influence of TGF-β1, can express APRIL.
Fig. 1. TGF-β1 enhances APRIL expression in mouse macrophages. (A) Effects of TGF-β1 on APRIL mRNA expression by mouse macrophages. P388D1 cells were incubated with the indicated dose of TGF-β1 for 9 h. APRIL mRNA levels were determined by RTPCR. (B) Effect of TGF-β1 on APRIL transcripts as a function of time. TGF-β1 was added to P388D1 cell cultures for the indicated times. (C) Freshly isolated mouse peritoneal macrophages were incubated with TGF-β1 for 9 h. PCRs containing cDNA dilutions of 1:1, 1:2, and 1:4 are shown. Fold increase values represent relative amounts of APR IL cDNA n ormalized to e xpression of β-actin cDNA using Scion Image analysis (NIH software). (D) Levels of APRIL protein were determined by Western blot.
Roles of Smad3 and Smad4 in TGF-β1-induced APRIL expression
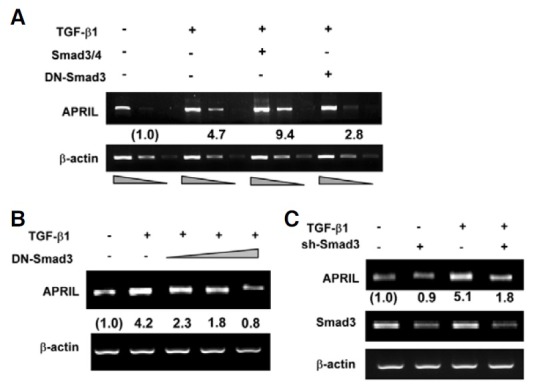
We proceeded to elucidate the mechanisms underlying TGF- β1-induced APRIL expression. As Smad3 and Smad4 are wellknown intermediates in the TGF-β signaling pathway (Massague, 1998), we asked whether they mediate APRIL expression. Overexpression of a dominant negative variant of Smad3 (DNSmad3) significantly abrogated APRIL transcription in a dosedependent manner (Figs. 2A and 2B). Furthermore, sh-Smad3, which silences Smad3 expression, also eliminated the effect of TGF-β1 on APRIL transcription (Fig. 2C).
Fig. 2. Smad3 and Smad4 mediate TGFβ1-induced APRIL expression. (A) P388D1 cells were transfected with 2 μg each of Smad3/4 or DN-Smad3 expression plasmids and stimulated with TGF-β1 for 9 h. Levels of APRIL transcripts were determined by RT-PCR. For PCR, cDNAs from each sample were prepared to 1:1, 1:3, and 1:9 dilutions. Fold increase values represent relative expression of APRIL normalized to expression of β-actin. (B, C) Cells were transfected with DN-Smad3 (2, 4, 8 μg) or Smad3 shRNA (2 μg) prior to treatment with TGF-β1 for 9 h. Levels of APRIL transcripts were measured by RT-PCR.
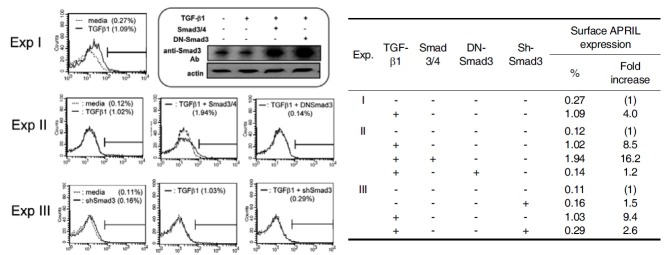
Based on the observation that Smad3 mediates TGFβ1- induced APRIL transcription, we next determine the effect of TGF-β1 and Smad3/4 at the protein level. We found that TGF- β1 actually increases the expression of membrane-bound APRIL (Fig. 3). This effect of TGF-β1 on surface APRIL expression was augmented by overexpressed Smad3/4, and abrogated by either overexpression of DN-Smad3 or Smad3 shRNA. Robust expression of both transfected Smad3 and DN-Smad3 was verified by Western blot (Fig 3, rounded box). Taken together, these results indicate that Smad-dependent pathway is involved in TGFβ1-induced APRIL expression.
Fig. 3. Effects of TGF-β1 and Smad3/4 on Surface APRIL Expression. P388D1 cells were transfected with Smad3/4 (each 2 μg), DN-Smad3 (8 μg) or Smad3 shRNA (2 μg) and incubated with TGF-β1 (1 ng/ml) for 48 h. Surface APRIL expression was analyzed by FACS by using anti-APRIL antibody. Western blot was performed to detect the expression of transfected Smad3 (rounded box).
Induction of APRIL expression by TGF-β1 involves the p38MAPK/CREB pathway
It has been demonstrated that the TGF-β signal activates Smad-independent pathways in cell type dependent, i.e MAPKs such as c-Jun N-terminal kinases (JNK) and extracellular signal- regulated kinases (ERK) (Dubois et al., 1999; Yamaguchi et al., 1995). Further, p38MAPK is a downstream molecule of TGF-β-activated kinase 1 (TAK1) in TGF-β signaling (Hanafusa et al., 1999), and activates CREB in this pathway (Johannessen et al., 2004). Therefore, we tested these possibilities using appropriate inhibitors. SB203580, a p38MAPK inhibitor, completely abolished TGFβ1-induced APRIL transcription whereas both PD98059 (ERK inhibitor) and SP600125 (JNK inhibitor) had little effect (Fig. 4A), suggesting that p38MAPK plays a critical role in induction of APRIL by TGF-β1. Moreover, overexpression of CREB, one of the downstream molecules involved in p38MAPK signaling, enhanced TGF-β1-induced APRIL mRNA expression (Fig. 4B). In contrast, overexpression of DNCREB completely abrogated the increase in APRIL transcription induced by TGF-β1. Similarly, SB203580 abolished the increase in APRIL transcription induced by TGF-β1 and overexpressed CREB (Fig. 4B). These findings were paralleled by changes in APRIL expression at the protein level (Fig. 4C-1). Robust expression of both transfected CREB and DN-CREB was verified by Western blot (Fig. 4C-2). Finally, we found that TGF-β1 induces phosphorylation of p38MAPK and CREB (Fig. 5A).
Fig. 4. Involvement of p38MAPK and CREB in TGFβ1-induced expression of APRIL. (A) TGF-β1 induces APRIL mRNA expression via p38MAPK. P388D1 cells were pre-treated with 5 μM PD98059 (an ERK inhibitor), 10 μM SP600125 (a JNK inhibitor), 10 μM SB431542 (an inhibitor of ALK4, 5, and 7), or 10 μM SB203580 (a p38 MAPK inhibitor) for 1 h, and then stimulated with TGF-β1 (1 ng/ml) for 9 h. (B, C-1) Cells were transfected with 1 μg of the expression vectors for CREB or DN-CREB prior to treatment with TGF-β1 (1 ng/ml) for 9 h. Cells were pre-incubated with 5 μM SB203580 for 1 h. Levels of APRIL transcripts and APRIL protein were measured by RT-PCR (A, B) and Western blot (C-1), respectively. Fold increase values represent relative expression of APRIL normalized to expression of β-actin. (C-2) Western blot was performed to detect the expression of transfected CREB under the same conditions as in (B) and (C-1).
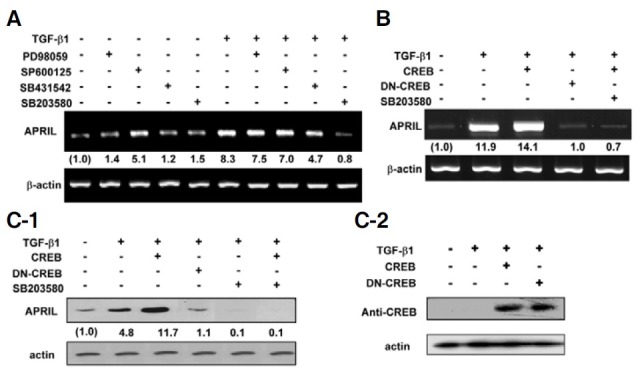
Fig. 5. TGF-β1 induces p38MAPK and CREB phosphorylation. (A) Cells were stimulated with TGF-β1 (1 ng/ml) as indicated. Cells were pre-incubated with 5 μM SB203580 for 1 h. Phosphorylated p38MAPK (ⓟ-p38), total p38MAPK, phosphorylated CREB (ⓟ-CREB), and total CREB were detected by Western blot. (B) Proposed mechanisms underlying TGFβ1-induced APRIL expression in mouse macrophages.
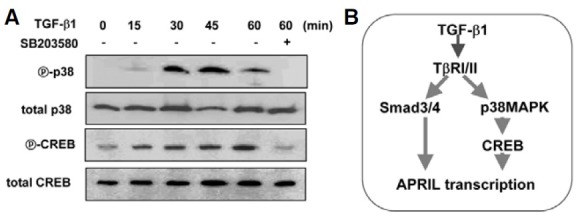
Taken together, these results indicate that the p38MAPK– CREB and the Smad pathways are involved in TGFβ1-induced APRIL expression, as proposed in Fig. 5B.
DISCUSSION
In the present study, we demonstrate that TGF-β1 stimulates mouse macrophages to produce APRIL and two different TGF- β1 signaling pathways via Smad3/4 and p38MAPK-CREB, are involved in this process. Analogous to these results, it has been reported that TGF-β1 signaling activates two independent pathways, the Smad-mediated and the TAK1-mediated pathways (Heldin et al., 1997; Moriguchi et al., 1996). In the TAK1 pathway, TGF-β1 activates TAK1-MKK6-p38MAPK (Hanafusa et al., 1999) and CREB, p38 MAPK downstream molecules (Chen and Xie, 2010; Johannessen et al., 2004). Between the two pathways, we do not distinguish at the moment which pathway is more relevant to TGFβ1-induced APRIL expression as often demonstrated by others (Hanafusa et al., 1999; Kamaraju and Roberts, 2005; Patel et al., 2010). We have previously shown that TGF-β1 induces the expression of BAFF, another TNF family member, mainly through Smad3/4 signaling (Kim et al., 2008). Thus, the mechanisms underlying APRIL and BAFF induction by TGF-β1 are slightly different, though both molecules are involved in Ig synthesis by B lymphocytes.
It is known that the DNA-binding specificity of Smad proteins is relatively low. Thus, individual Smad proteins must cooperate with other DNA binding proteins to elicit specific transcriptional responses (Feng et al., 1998; Hanai et al., 1999). In this context, we have demonstrated that Runx3 synergizes with Smad3/4 to induce Ig germ-line α transcription leading to IgA isotype switching (Park et al., 2003). In addition, HIF-1α cooperates with Smad3/4 to mediate TGFβ1-induced VEGF transcription in mouse macrophages (Jeon et al., 2007). The present data indicate that CREB could play this role in TGFβ1-inducible APRIL transcription. Consistent with this hypothesis, TGF-β1 signaling involves the phosphorylation of CREB (Potchinsky et al., 1997) and CREB cooperates with Smads to mediate TGFβ1-induced germline Ig α promoter activity (Zhang and Derynck, 2000). We note that the present report does not address this issue and that it is essential to clone the promoter region to elucidate whether this is the case. Nevertheless, we and others have been unable thus far to clone the APRIL promoter region, although we were successful in the construction of a mouse BAFF promoter reporter (Kim et al., 2008). One of the probable obstacles in determining the APRIL promoter region is the intrinsic complexity of the gene structure and its expression pattern: another TNF family gene, TWEAK, is closely linked to the APRIL gene and the two are often expressed together as a TWE-PRIL fusion protein (Bossen and Schneider, 2006). Therefore, it still remains to be determined whether and how CREB and Smad3/4 bind the promoter region of APRIL gene.
In conclusion, TGF-β1 is a well known cytokine that induce isotype switching recombination of IgA in mouse B cells (Coffman et al., 1989; Kim and Kagnoff, 1990). It is generally accepted that activated Th cells produce these cytokines which in turn directly modulate B cells. Nonetheless, there is accumulating evidence that macrophages affect B cell proliferation and differentiation (Craxton et al., 2003; Fagarasan and Honjo, 2000; Litinskiy et al., 2002). We have shown that APRIL, as well as BAFF, are produced by TGFβ1-stimulated mouse macrophages (Kim et al., 2008). BAFF and APRIL can activate B cells to express activation-induced deaminase (AID), which is a critical enzyme for Ig class switch recombination (Castigli et al., 2005; Litinskiy et al., 2002; Yamada et al., 2005). Taken together, our in vitro studies indicate that TGF-β1 induces the expression of APRIL and BAFF in macrophages and thereby exerts an important effect on Ig isotype switching in vivo.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Education, Science and Technology) (No. 2010-0012311 and The Regional Core Research Program/Medical & Bio- Material Research Center), and the 2nd stage of the Brain Korea 21 program. Studies were carried out in the Institute of Bioscience and Biotechnology at Kangwon National University.
References
- 1.Bossen C., Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin. Immunol. (2006);18:263–275. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Castigli E., Scott S., Dedeoglu F., Bryce P., Jabara H., Bhan A.K., Mizoguchi E., Geha R.S. Impaired IgA class switching in APRIL-deficient mice. Proc. Natl. Acad. Sci. USA. (2004);101:3903–3908. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castigli E., Wilson S.A., Scott S., Dedeoglu F., Xu S., Lam K.P., Bram R.J., Jabara H., Geha R.S. TACI and BAFFR mediate isotype switching in B cells. J. Exp. Med. (2005);201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y.Q., Xie X. Podophyllotoxin induces CREB phosphorylation and CRE-driven gene expression via PKA but not MAPKs. Mol. Cells. (2010);29:41–50. doi: 10.1007/s10059-010-0015-1. [DOI] [PubMed] [Google Scholar]
- 5.Coffman R.L., Lebman D.A., Shrader B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J. Exp. Med. (1989);170:1039–1044. doi: 10.1084/jem.170.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craxton A., Magaletti D., Ryan E.J., Clark E.A. Macrophage- and dendritic cell--dependent regulation of human Bcell proliferation requires the TNF family ligand BAFF. Blood. (2003);101:4464–4471. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- 7.Dubois B., Vanbervliet B., Fayette J., Massacrier C., Van Kooten C., Briere F., Banchereau J., Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J. Exp. Med. (1997);185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois B., Bridon J.M., Fayette J., Barthelemy C., Banchereau J., Caux C., Briere F. Dendritic cells directly modulate B cell growth and differentiation. J. Leukoc. Biol. (1999);66:224–230. doi: 10.1002/jlb.66.2.224. [DOI] [PubMed] [Google Scholar]
- 9.Fagarasan S., Honjo T. T-Independent immune response: new aspects of B cell biology. Science. (2000);290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 10.Feng X.H., Zhang Y., Wu R.Y., Derynck R. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/ p300 are coactivators for smad3 in TGF-beta-induced transcriptional activation. Genes Dev. (1998);12:2153–2163. doi: 10.1101/gad.12.14.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto D., Yagi K., Inoue H., Iwamoto I., Kawabata M., Miyazono K., Kato M. A single missense mutant of Smad3 inhibits activation of both Smad2 and Smad3, and has a dominant negative effect on TGF-beta signals. FEBS Lett. (1998);430:201–204. doi: 10.1016/s0014-5793(98)00658-9. [DOI] [PubMed] [Google Scholar]
- 12.Hanafusa H., Ninomiya-Tsuji J., Masuyama N., Nishita M., Fujisawa J., Shibuya H., Matsumoto K., Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J. Biol. Chem. (1999);274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- 13.Hanai J., Chen L.F., Kanno T., Ohtani-Fujita N., Kim W.Y., Guo W.H., Imamura T., Ishidou Y., Fukuchi M., Shi M.J., et al. Interaction and functional cooperation of PEBP2/CBF with Smads. Synergistic induction of the immunoglobulin germline Calpha promoter. J. Biol. Chem. (1999);274:31577–31582. doi: 10.1074/jbc.274.44.31577. [DOI] [PubMed] [Google Scholar]
- 14.Heldin C.H., Miyazono K., ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. (1997);390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 15.Jeon S.H., Chae B.C., Kim H.A., Seo G.Y., Seo D.W., Chun G.T., Kim N.S., Yie S.W., Byeon W.H., Eom S.H., et al. Mechanisms underlying TGF-beta1-induced expression of VEGF and Flk-1 in mouse macrophages and their implications for angiogenesis. J. Leukoc. Biol. (2007);81:557–566. doi: 10.1189/jlb.0806517. [DOI] [PubMed] [Google Scholar]
- 16.Johannessen M., Delghandi M.P., Moens U. What turns CREB on? Cell. Signal. (2004);16:1211–1227. doi: 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Kamaraju A.K., Roberts A.B. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-betamediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J. Biol. Chem. (2005);280:1024–1036. doi: 10.1074/jbc.M403960200. [DOI] [PubMed] [Google Scholar]
- 18.Kim P.H., Kagnoff M.F. Transforming growth factor beta 1 increases IgA isotype switching at the clonal level. J. Immunol. (1990);145:3773–3778. [PubMed] [Google Scholar]
- 19.Kim H.A., Jeon S.H., Seo G.Y., Park J.B., Kim P.H. TGF-beta1 and IFN-gamma stimulate mouse macrophages to express BAFF via different signaling pathways. J. Leukoc. Biol. (2008);83:1431–1439. doi: 10.1189/jlb.1007676. [DOI] [PubMed] [Google Scholar]
- 20.Litinskiy M.B., Nardelli B., Hilbert D.M., He B., Schaffer A., Casali P., Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. (2002);3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massague J. TGF-beta signal transduction. Annu. Rev. Biochem. (1998);67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 22.McIntyre T.M., Klinman D.R., Rothman P., Lugo M., Dasch J.R., Mond J.J., Snapper C.M. Transforming growth factor beta 1 selectivity stimulates immunoglobulin G2b secretion by lipopolysaccharide-activated murine B cells. J. Exp. Med. (1993);177:1031–1037. doi: 10.1084/jem.177.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriguchi T., Kuroyanagi N., Yamaguchi K., Gotoh Y., Irie K., Kano T., Shirakabe K., Muro Y., Shibuya H., Matsumoto K., et al. A novel kinase cascade mediated by mitogenactivated protein kinase kinase 6 and MKK3. J. Biol. Chem. (1996);271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- 24.Park S.R., Lee J.H., Kim P.H. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur. J. Immunol. (2001);31:1706–1715. doi: 10.1002/1521-4141(200106)31:6<1706::aid-immu1706>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Park S.R., Lee E.K., Kim B.C., Kim P.H. p300 cooperates with Smad3/4 and Runx3 in TGFbeta1-induced IgA isotype expression. Eur. J. Immunol. (2003);33:3386–3392. doi: 10.1002/eji.200324061. [DOI] [PubMed] [Google Scholar]
- 26.Patel P., Sekiguchi Y., Oh K.H., Patterson S.E., Kolb M.R., Margetts P.J. Smad3-dependent and -independent pathways are involved in peritoneal membrane injury. Kidney Int. (2010);77:319–328. doi: 10.1038/ki.2009.436. [DOI] [PubMed] [Google Scholar]
- 27.Potchinsky M.B., Weston W.M., Lloyd M.R., Greene R.M. TGF-beta signaling in murine embryonic palate cells involves phosphorylation of the CREB transcription factor. Exp. Cell Res. (1997);231:96–103. doi: 10.1006/excr.1996.3422. [DOI] [PubMed] [Google Scholar]
- 28.Stein J.V., Lopez-Fraga M., Elustondo F.A., Carvalho-Pinto C.E., Rodriguez D., Gomez-Caro R., De Jong J., Martinez A.C., Medema J.P., Hahne M. APRIL modulates B and T cell immunity. J. Clin. Invest. (2002);109:1587–1598. doi: 10.1172/JCI15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada T., Zhang K., Yamada A., Zhu D., Saxon A. B lymphocyte stimulator activates p38 mitogen-activated protein kinase in human Ig class switch recombination. Am. J. Respir. Cell Mol. Biol. (2005);32:388–394. doi: 10.1165/rcmb.2004-0317OC. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi K., Shirakabe K., Shibuya H., Irie K., Oishi I., Ueno N., Taniguchi T., Nishida E., Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. (1995);270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Derynck R. Transcriptional regulation of the transforming growth factor-beta -inducible mouse germ line Ig alpha constant region gene by functional cooperation of Smad, CREB, and AML family members. J. Biol. Chem. (2000);275:16979–16985. doi: 10.1074/jbc.M001526200. [DOI] [PubMed] [Google Scholar]


