Abstract
A typical 2-cysteine peroxiredoxin (2-Cys Prx)-like protein (PpPrx) that alternatively acts as a peroxidase or a molecular chaperone in Pseudomonas putida KT2440 was previously characterized. The dual functions of PpPrx are regulated by the existence of an additional Cys112 between the active Cys51 and Cys171 residues. In the present study, additional Cys residues (Cys31, Cys112, and Cys192) were added to PpPrx variants to improve their enzymatic function. The optimal position of the additional Cys residues for the dual functionality was assessed. The peroxidase activities of the S31C and Y192C mutants were increased 3- to 4-fold compared to the wild-type, while the chaperone activity was maintained at > 66% of PpPrx. To investigate whether optimization of the dual functions could enhance stress-tolerance in vivo, a complementation study was performed. The S31C and Y192C mutants showed a much greater tolerance than other variants under a complex condition of heat and oxidative stresses. The optimized dual functions of PpPrx could be adapted for use in bioengineering systems and industries, such as to develop organisms that are more resistant to extreme environments.
Keywords: dual function engineering, dual functions, extreme stress, peroxiredoxin, site-directed mutagenesis
INTRODUCTION
Peroxiredoxins (Prxs) are enzymatic antioxidants (Baier and Dietz, 1999) that are produced at high levels in cells. Prxs constitute a family of heme-free peroxidases that reduce alkyl hydroperoxides and hydrogen peroxide (H2O2), and are found in all biological kingdoms from bacteria to plant and animal (Chae et al., 1999; Link et al., 1997). The degradation reaction of 2-Cys Prx involves its two active Cys residues. Peroxidatic Cys (CysP) attacks peroxide and becomes oxidized to sulfenic acid. Oxidized CysP is attacked by the resolving Cys (CysR). Two oxidized active Cys residues are formed via intra- or intermolecular disulfide bonds after yielding water or the corresponding alcohol (An et al., 2010; 2011; Dietz, 2003; Hofmann et al., 2002).
Prxs can act either as peroxidases or as molecular chaperones, with reversible switching between the dual enzymatic functions (An et al., 2010; 2011; Chuang et al., 2006; Jang et al., 2004; 2006a). These properties enable Prxs to prevent the stress-induced misfolding or aggregation of intracellular macromolecules (Chuang et al., 2006; Jang et al., 2004; 2006a). Prxs also display diversity in structure and apparent molecular weight (MW), with 2-Cys Prxs undergoing structural conversion from low MW (LMW) to high MW (HMW) complexes. Most 2- Cys Prxs form condition-dependent HMW structures, although the physiological relevance of this oligomerization is unclear (Jang et al., 2004; 2006b; Kristensen et al., 1999; Schröder et al., 1998; Wood et al., 2003).
Two previous studies have reported physiological evidences of Prx oligomerization. Cyclin-dependent kinases (Cdks) were observed to inactivate the peroxidase activity of human Prx I (hPrxI) and hPrxII by specifically phosphorylating Thr90, thereby significantly increasing its chaperone activity (Chang et al., 2002). This process was accompanied by HMW complex formation. Moon et al. (2005) demonstrated that the 2-Cys Prx dimer-decamer transition is stimulated by hyperoxidation of the peroxidatic Cys residue and structural and functional changes. More recently, additional Cys residues were shown to promote the HMW complex formation of 2-Cys Prx, accompanied by an increase in chaperone activity (unpublished result).
The structural transitions of 2-Cys Prx by additional Cys residues can be explained by two factors. First, the additional Cys residues act as combiners by their inter- and intra-disulfide bonds and as redox sensors by the oxidation and reduction of their exposed free Cys residues (Cumming et al., 2004; unpublished result). Second, the presence of additional Cys residues between two active Cys residues promotes the change of secondary structural elements, such as the exposure of hydrophobic-, β-sheet-, or random coil-regions (unpublished result). Thus, the oligomerization of 2-Cys Prxs caused by Thr90 phosphorylation, hyperoxidation of the active Cys residues, or additional Cys insertions may be an important factor in the functional switching of Prxs.
Although Prx modifications significantly affect their structural and functional switches, Prxs predominantly exhibit chaperone activity in their HMW complex form. If 2-Cys Prxs are not modified, then they maintain the LMW form and their role as a peroxidase. The structural and functional choices of 2-Cys Prxs appear to be forced by the cell environment. This observation raises several questions. Given that the functional switches of Prx are regulated by their structural changes, why does Prx never exhibit equilibrated levels of its dual functions? Furthermore, what conditions would be required for Prx to exhibit such equilibrated levels?
The purpose of this study was to investigate whether 2-Cys Prx (PpPrx) can possess both of its dual functions in equilibrium when additional Cys residues are added. Various PpPrx variants were designed that contained multiple additional Cys derivatives that were individually inserted. The relative dual function ratio and structural changes of the PpPrx derivatives were compared. To address the physical changes of PpPrx with additional Cys residues, the optimal Cys positions needed for dual functionality were examined. We also investigated whether the additional Cys residues conferred stress-resistance to E. coli cells under extreme stress.
MATERIALS AND METHODS
Bacterial strains, media, and materials
The bacterial strains Pseudomonas putida KT2440, P. aeruginosa PAO1, and E. coli DH5α (Promega, USA), KRX (Promega), and JD22970 (NBRP, NIG, Japan) were grown aerobically at 30℃ and 37℃ in LB medium (0.5% sodium chloride, 0.5% yeast extract, and 1% tryptone) (DB, USA). These strains were used to clone the PRX genes and to perform the survival assay under extreme stress conditions. Yeast Trx and Trx reductase (TR) were prepared as described (Chae et al., 1994). Protein molecular size standards used in polyacrylamide gel electrophoresis (PAGE) were purchased from ELPIS (Korea). Ampicillin (Amp), kanamycin (Km), Imidazole, L-rhamnose, bovine serum albumin (BSA), hydrogen peroxide (H2O2; 30% v/v), and nicotinamide adenine dinucleotide phosphate (NADPH) were obtained from Sigma (USA). 1,1′-Bi(4-anilino) naphthalene- 5,5′-disulfonic acid (bis-ANS) was from Molecular Probes (Invitrogen, USA).
Cloning of PpPrx and PaPrx genes
The two PRX genes were amplified from P. putida KT2440 and P. aeruginosa PAO1 genomic DNA by polymerase chain reaction (PCR). Briefly, specific PCR reactions were carried out in 20-μl mixtures containing 10 ng of genomic DNA, 0.2 μM deoxyribonucleoside triphosphates (dNTPs), 20 pmol of each primer set for PpPrx (XhoI, 5′-CCG CTC GAG ATG AGC GTA CTC-3′; SacI, 5′-CGA GCT CTT ACA GCT TGC CAG C-3′) and PaPrx (XhoI, 5′-CCG CTC GAG ATG AGC GTA CTC GTA-3′; HindIII, 5′-CCC AAG CTT TTA CAG CTT GCT GGC- 3′), and 1 unit of Taq DNA polymerase (Promega) in a standard PCR buffer. The following conditions were used for PCR: denaturation for 1 cycle at 94℃, 60 s, 35 cycles at 94℃, 30 s; 50℃, 45 s; 72℃, 45 s, followed by 1 cycle at 72℃ for 10 min. The PCR products (615 bp) were collected, purified, and subcloned into the pGEM-T vector (Promega) to produce PpPrx and PaPrx, which were then transformed into DH5α cells. Each of the PpPrx and PaPrx fragments from pGEM-T was transferred to the pRSETa expression vector (Invitrogen) to create pRSETa:: PpPrx and pRSETa::PaPrx.
Site-directed mutagenesis of PpPrx by PCR
The additional Cys (Cys112)-excluded mutant protein, C112S, was generated by PCR. Cys112 was specifically replaced in PpPrx by Ser using PCR-mediated site-directed mutagenesis with pRSETa::PpPrx as the template. The complementary primers contained a single-base mismatch that converts the codon for Cys to Ser.
The additional Cys-added five mutant proteins (C112S, S31C/ C112S, Y192C/C112S, S31C, Y192C, and S31C/Y192C) were generated by PCR. Ser31 and Tyr192 were individually substituted in PpPrx and C112S by the additional Cys (S31C and Y192C, respectively) using PCR-mediated site-directed mutagenesis with pRSETa::PpPrx and pRSETa::C112S as the template, respectively. The complementary primers contained a single-base mismatch that converts the codons for Ser and Tyr to Cys. Briefly, specific PCR was carried out in 20-μl mixtures containing 10 ng of plasmid DNA (pRSETa::PpPrx and pRSETa:: C112S), 0.2 μM dNTPs, 20 pmol of each primer [C112S (top, 5′-GAC CCA CGA AAT CTC CAA AGC CTA CGA CG-3′; bottom, 5′-CGT CGT AGG CTT TGG AGA TTT CGT GGG TC-3′), S31C (top, 5′-TTC AAC CTG GCC TGC GCC ATC AAG GGC- 3′; bottom, 5′-GCC CTT GAT GGC GCA GGC CAG GTT GAA- 3′), and Y192C (top, 5′-GGC GTT GCT GCC TGC CTG AGC GAG AAC-3′; bottom, 5′-GTT CTC GCT CAG GCA GGC AGC AAC GCC-3′)], and 1 unit of prime star-taq DNA polymerase (Takara Bio, Japan) in a PCR buffer. The following conditions were used for PCR: denaturation for 1 cycle at 94℃, 60 s, 18 cycles at 94℃, 30 s; 75℃, 5 min, followed by 1 cycle at 72℃ for 10 min. The PCR products (3506 bp) were collected and purified, treated with DpnI (Promega) for exclusion of original DNA as the template, and then transformed into KRX cells. The point mutations were confirmed by nucleotide sequencing after plasmid isolation.
Expression and purification of His-tagged various Prx proteins in E. coli
KRX cells were transformed with pRSETa::PaPrx, pRSETa:: PpPrx, pRSETa::C112S, pRSETa::S31C/C112S, pRSETa:: Y192C/C112S, pRSETa::S31C, Y192C, or pRSETa::S31C/ Y192C. Cells were cultured at 30℃ overnight in 5 ml of LB medium supplemented with Amp (100 μg/ml), and then transferred to 500 ml of fresh LB medium in a shaking incubator. When the absorbance of the culture at 600 nm (A600nm) reached 0.4, expression was induced by L-rhamnose was added at a final concentration 0.2%. After incubation for an additional 8 h, the cells were collected by centrifugation, frozen in liquid nitrogen, and stored at -70℃ until use.
His6-fused Prxs were purified using a native Ni-NTA column (Peptron, Korea), and eluted with a linear gradient of 200 to 500 mM imidazole in phosphate-buffered saline (PBS; pH 8.0). After dialysis against 50 mM HEPES (pH 8.0), the protein concentration was measured using the Bradford method (Bradford, 1976), with BSA as the standard. The purity of the purified recombinant Prxs according to SDS-PAGE was > 99%.
Peroxidase activity assay
The thioredoxin-dependent peroxidase activity of purified Prxs was measured as described previously, with minor modifications (Cheong et al., 1999; Jang et al., 2004; Jeong et al., 2000; Lee et al., 1997). The seven PpPrx derivative proteins were incubated in 50 mM HEPES (pH 8.0) containing 200 μM NADPH, 3 μM yeast Trx, and 1.5 μM yeast TR. The reaction mixture was incubated at 30℃ for 5 min, followed by the addition of a 10-μl aliquot of H2O2 at various concentrations. NADPH oxidation was monitored for the next 6 min by the decrease in A340nm as measured by an EVOLUTION 300 UV-VIS spectrophotometer (Thermoscientific, USA).
Molecular chaperone activity assay
The molecular chaperone activity was determined as described previously by assessing the ability of recombinant Prxs to inhibit the thermal aggregation of substrate proteins (Cheong et al., 1999; Chuang et al., 2006; Lee et al., 1997; Moon et al., 2005). Briefly, 1 μM malate dehydrogenase (MDH) was mixed with various concentrations of the seven PaPrx derivative proteins in a degassed 50 mM HEPES (pH 8.0) solution. The reaction mixture was incubated at 43℃ for 15 min. The increase in light scattering as a result of the thermal aggregation of substrate proteins was monitored at 360 nm with an EVOLUTION 300 UV-VIS spectrophotometer equipped with a thermostatic cell holder (Thermoscientific).
Fluorescence measurement
Hydrophobic domain exposure of the Prx proteins was investigated using a SFM25 spectrofluorometer (Kontron, Germany) to examine the binding of 10 μl of 10 mM bis-ANS to 100 μg of each of the five Prx proteins. Spectra were accumulated five times. The excitation wavelength was set at 380 nm and emission spectra were monitored from 400 to 600 nm (Sharma et al., 1998).
Circular dichroism (CD) spectroscopy
The five Prx proteins in 10 mM sodium phosphate buffer (pH 7.4) were used for Far UV-CD spectral analysis with a Jasco J- 715 spectropolarimeter (Jasco, UK). Spectra were accumulated five times (Ito et al., 2001).
Survival assay of PpPrx derivative-transformed E. coli under extreme stress
JD22970 cell lines transformed with the seven PpPrx derivatives (ΔPRX E. coli) were individually grown aerobically at 37℃ in 5 ml of LB medium supplemented with Amp (100 μg/ml) and Km (50 μg/ml). The cells were transferred to 50 ml of fresh LB medium in a shaking incubator. When the A600nm of the culture reached 0.5, the cells were collected by centrifugation. The cells were resuspended to a density of 1.0 at 600 nm and were serially diluted from 10-1 to 10-4. The diluted cells were incubated on LB plates with 1 mM H2O2 at 37℃ (for oxidative stress), without H2O2 at 50℃ (for heat stress), or with 1 mM H2O2 at 50℃ (for extreme stress) (Gnanasekar et al., 2009).
RESULTS
Comparison of PpPrx and PaPrx based on amino acid sequences
Previously, we characterized PpPrx from P. putida KT2440, which consisted of 200 amino acids. Based on the enzymatic analysis results, this PpPrx has dual functions as a dominant chaperone and a recessive peroxidase activity (An et al., 2011). The PaPrx protein from P. aeruginosa PAO1 also contains 200 amino acids, and has dual functions as a dominant peroxidase and a recessive chaperone (An et al., 2010). In the present study, PaPrx and PpPrx were aligned, revealing a sequence homology of 93%. Each protein contained two conserved VCP tripeptides (Cys51 as catalytic Cys and Cys171 as resolving Cys) at the same position, which formed intermolecular disulfide bonds essential for the peroxidase activity. However, PpPrx contains an additional Cys112 within the two VCP motifs, whereas PaPrx has Ala at the same position (Fig. 1).
Fig. 1. Amino acid sequence alignment of PpPrx and PaPrx. (A) Alignment of the amino acid sequences of P. putida PpPrx (2-Cys Prx) with homologous PaPrx from P. aruginosa. Gray indicates identical amino acids in all sequences. Empty spaces indicate mismatch sequences. Asterisk indicates the highly conserved tripeptide (VCP) that is related to the catalytic function. The additional Cys (Cys112) is designated by a square. GenBank accession numbers of the proteins are as follows: PpPrx (AAN66709) and PaPrx (AAG06917).

Comparison of the peroxidase and chaperone activities of PpPrx and PaPrx
We next compared the molecular chaperone and peroxidase activities of PpPrx and PaPrx in vitro. The molecular chaperone activities of the two Prxs were investigated using MDH at 43℃ (Supplementary Fig. 1A). PpPrx showed low H2O2 catabolic peroxidase activity, whereas PaPrx exhibited an approximately 4- to 5-fold higher peroxidase activity than PpPrx. PpPrx exhibited an approximately 40- to 50-fold higher chaperone activity than PaPrx.
Comparison of the secondary structures of PpPrx and PaPrx
To explain the reciprocal activities of PpPrx and PaPrx, far-UV CD spectra were used to estimate their secondary structural components. Far-UV CD spectra obtained from the wavelength scans of the Prxs were used to compare the structural differences between PpPrx and PaPrx. The differences in secondary structure components due to the existence of the additional Cys (Cys112) were as follows (Supplementary Fig. 1B): the β-sheet content increased from 21.4% to 39.9%, α-helix content decreased from 32.9% to 14.4%, random coil content increased from 27.6% to 45.8%, and the turn content decreased from 17.4% to 0%. These data suggested that the additional Cys acts as a key modulator of both the function and structure of Prx proteins.
Design of PpPrx with equilibrated dual functions by including additional Cys residues
Two separate enzymatic and structural comparisons were performed between PpPrx and PaPrx, which revealed specific details of their Cys112 residues. These comparisons allowed us to construct a mechanism for the transition from low to high chaperone activity. The mechanism was based on the following three observations: (1) PpPrx has an approximately 40- to 50- fold higher chaperone activity than PaPrx; (2) PpPrx has Cys112 instead of Ala between the two VCP motifs; (3) PpPrx displays predominantly β-sheet and random coil elements with few α- helix and turn elements, whereas PaPrx displays predominantly α-helix and turn elements with few β-sheet and random coil elements; and (4) the predicted secondary structure of PpPrx indicates that Cys112 is located in an α-helical element between the two VCP motifs (Fig. 2A).
Fig. 2. Secondary structure prediction and various additional Cys derivatives of PpPrx. (A) The PpPrx secondary structure was predicted using a bioinformatics tool (http://npsa-pbil.ibcp.fr/). Spirals and arrows represent the α-helix and β-sheet motifs of PpPrx, respectively. Positions of the additional Cys residues (Cys31, Cys112 and Cys192) and active Cys residues (Cys51 as peroxidatic Cys, Cys171 as resolving Cys) are indicated by gray and black arrows, respectively, on the PpPrx secondary structure. (B) Schematic representation of 7 PpPrx derivatives with excluded or included additional Cys. Substituted Cys and Ser are indicated by embossed circles.
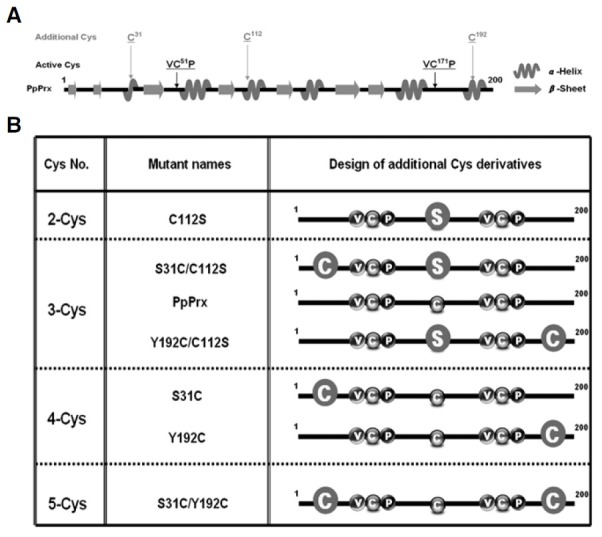
The location of Cys112 in a α-helical element could induce the exposure of β-sheet and random coil elements, thus affecting chaperone activity (unpublished result). Therefore, the number and location of the α-helical elements in PpPrx were examined. PpPrx had six α-helical elements. Two and four of these elements were located outside and inside the two active Cys residues, respectively (Fig. 2A). To improve the enzyme activities of PpPrx, various Cys derivatives were designed in which additional Cys residues were inserted in each of the two α-helical elements located outside of the active Cys residues of PpPrx (Fig. 2B).
Influence of additional Cys residues on the dual functions
To investigate the critical influence of additional Cys residues (Cys31, Cys112, and Cys192) on the dual enzymatic functions (i.e., chaperone or peroxidase), a series of experiments were performed in vitro. The molecular chaperone activities of six mutant proteins were compared to those of the respective PpPrx, whose activity was set to 100% (Fig. 3). The peroxidase activities of the six PpPrx derivatives were compared to those of the respective C112S, whose activity was set to 100% (Fig. 3).
Fig. 3. Additional Cys-mediated functional change of PpPrx. Comparison of additional Cys-mediated changes in enzymatic efficiency (peroxidase and chaperone activities). The relative activities of PpPrx derivative proteins were compared to those of the respective dominant activities. Chaperone activity of PpPrx and peroxidase activity of C112S-PpPrx were each set to 100%. Data shown are the means of at least three independent experiments.
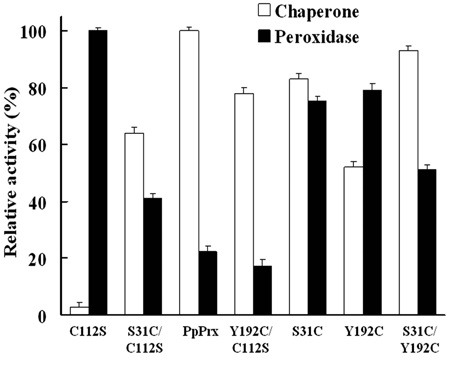
The C112S mutant displayed low chaperone activity, similar to PaPrx. PpPrx and other PpPrx proteins containing additional Cys residues exhibited an approximately 20- to 50-fold higher activity than C112S. However, the H2O2 catabolic peroxidase activity of C112S was higher than that of the other PpPrx derivatives containing single or multiple additional Cys residues. Although variants with additional Cys derivatives had significantly lower peroxidase activities than C112S, the double additional Cys mutants (S31C or Y192) maintained an about 80% peroxidase activity compared with C112S. This comparison suggests that the additional Cys residues affected the dual functions of the PpPrx derivatives.
Influence of additional Cys residues on Prx oligomerization
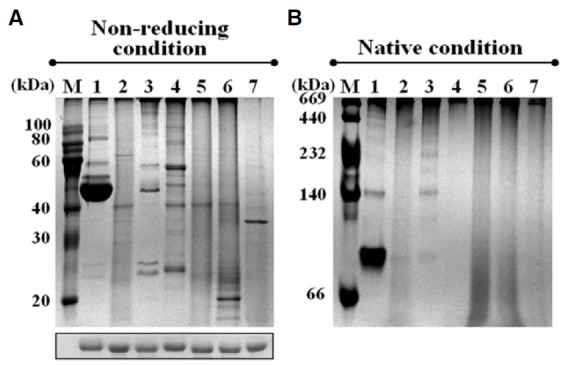
We next addressed the critical influence of the additional Cys residues (Cys31, Cys112, and Cys192) on the protein structure of PpPrx, specifically on the inter- and intradisulfide bond sites under nonreducing and native conditions. When the protein structures of PpPrx and its derivatives were analyzed on nonreducing and native PAGE gels, they showed quite different structural patterns (Figs. 4A and 4B). PpPrx displayed various oligomeric protein complexes containing both HMW complexes and a few LMW forms (lane 3), while C112S produced predominantly dimers and fewer HMW complexes than other PpPrx proteins (lane 1). In S31C/C112S and Y192C/C112S, the LMW species of C112S were shifted to HMW complexes by additional Cys residues (Cys31 or Cys192) (lanes 2 and 4). Conversely, most of the LMW structure species of PpPrx were shifted to HMW complexes if additional Cys residues were located anywhere on the three α-helical elements (Fig. 4). The double additional Cys mutants (S31C or Y192) contained abundant LMW species compared with C112S, consistent with the peroxidase activity of the double additional Cys mutants (Fig. 3).
Fig. 4. Additional Cys-mediated structure change of PpPrx derivatives. (A) PpPrx derivative proteins were separated by 12% nonreducing PAGE (upper image) or 12% reducing PAGE (lower image) and (B) 10% native PAGE. Proteins were stained with Coo-massie Brilliant Blue R-250. Lane M, marker; lane 1, C112S; lane 2, S31C/C112S; lane 3, PpPrx; lane 4, Y192C/C112S; lane 5, S31C; lane 6, Y192C; lane 7, S31C/Y192C.
Influence of additional Cys residues on the secondary structure and hydrophobicity
To determine why the structural and functional alterations of the PpPrx derivatives depended on the insertion of additional Cys residues (Cys31, Cys112 and Cys192), far-UV CD spectra were used to estimate and compare the secondary structural components between C112S and other PpPrx derivatives (Fig. 5). Changes in secondary structural elements due to the existence of additional Cys were as follows (Fig. 5); the β-sheet content increased from 21.4% to 55%, random coil content increased from 29.7% to 45.8%, α-helical content decreased from 31.5% to 6.6%, and the turn content decreased from 17.4% to 0%. In particular, the β-sheet content markedly increased when additional Cys residues were added, whereas the α-helix and turn contents markedly decreased.
Fig. 5. Additional Cys-mediated secondary structural change of PpPrx derivatives. The comparison of the secondary structure index values (%) was based on the far UV-CD spectra of the seven PpPrx derivative proteins. Data shown are the means of at least three independent experiments.
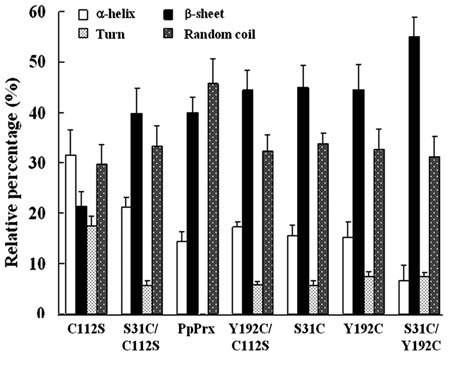
These changes in secondary structural elements enhanced chaperone activity compared with C112S. However, the double additional Cys mutants (S31C and Y192C) maintained a high efficiency of peroxidase activity, unlike the single additional Cys mutants (PpPrx, S31C/C112S and Y192/C112S). These results suggest that the double additional Cys addition gives PpPrx a high efficiency of equilibrated dual functions, and that the additional Cys residues act as a key modulator of protein function and structure of PpPrx derivatives.
To protect target substrates against stress-induced aggregation, chaperones bind to nonnative states of substrate proteins through hydrophobic interactions (Ganea, 2001; Jang et al., 2006a). The hydrophobicity of C112S and other PpPrx derivatives containing additional Cys residues were compared by measuring the binding of the bis-ANS fluorophore. Bis-ANS is widely used to detect hydrophobic regions on the surface of proteins (Sharma et al., 1998; Shi et al., 1994). The fluorescence levels of bis-ANS bound to C112S, single additional Cys mutants (S31C/C112S and Y192C/C112S), or the triple additional Cys mutant (S31C/Y192C) were significantly lower than that of bis-ANS bound to PpPrx (Fig. 6). The fluorescence spectra of bis-ANS bound to the double additional Cys mutants (S31C and Y192C) were slightly reduced compared to PpPrx (Fig. 6). This result suggests that the position of Cys112 in PpPrx greatly increased the exposure of hydrophobic domains, resulting in protein polymerization into HMW complexes and providing binding sites for the partially denatured substrate proteins. Other positions of additional Cys (Cys31 and Cys192) partially reduced the exposure of hydrophobic domains, and these mutants exhibited decreased hydrophobicity (Fig. 6) and chaperone activity (Fig. 3) compared with PpPrx.
Fig. 6. Additional Cys-mediated change in the hydrophobicity of the PpPrx derivatives. Changes in the surface hydrophobicities of the PpPrx derivative proteins due to the additional Cys. Fluorescence spectra of bis-ANS bound to 100 μg/ml of each PpPrx derivative protein. Controls were measured in the absence of PpPrx protein. Data shown are the means of at least three independent experiments.
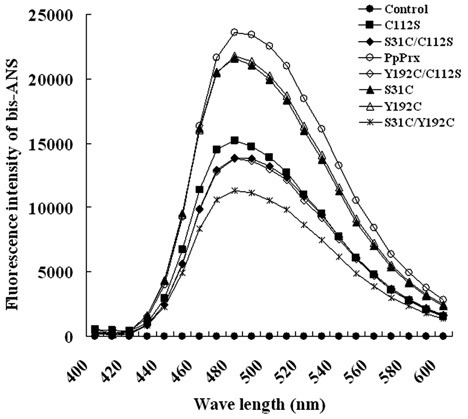
Additional Cys residues provide enhanced tolerance to ΔPRX E. coli under heat and oxidative stresses
We investigated the degree to which each additional Cys mutant protein contributed to the stress tolerance of ΔPRX E. coli cells. Survival rates were compared under heat-, oxidative- or complex-stress conditions. Heat shock was induced by incubation at 50℃ and oxidative stress was induced by incubation at 37℃ with 1 mM H2O2. The control used was ΔPRX E. coli cells harboring empty vector. Six additional Cys derivatives (C112S, S31C/C112S, PpPrx, Y192C/C112S, S31C and Y192C) showed similar survival rates as ΔPRX E. coli cells harboring empty vector under the normal and 1 mM H2O2 conditions (Fig. 7). The C112S protein displayed low chaperone activity and abundant peroxidase activity (Fig. 3). This result may suggest that the peroxidase capacity changes of PpPrx did not contribute to the survival rate of ΔPRX E. coli against H2O2 stress, and that PpPrx was not a major H2O2 scavenger in the E. coli antioxidant system. However, C112S showed a lower survival rate than other additional Cys derivatives under heat stress. This result might be related to the low chaperone activity of C112S (Fig. 3).
Fig. 7. In vivo observation of PpPrx mutant phenotypes under extreme stress. Growth temperature (as a heat stress), H2O2 concentration (as an oxidative stress), and the fold dilution are indicated. The growth phenotypes of E. coli transformed with PpPrx derivatives were observed after 1 day under the indicated conditions. After cultivation in liquid LB medium at 37℃, the cells were resuspended at a density of 1.0 at 600 nm. The cells were serially diluted from 10-1 to 10-4. Diluted cells were incubated on an LB plate with 1.6 mM H2O2 at 37℃ (for oxidative stress), on an LB plate without H2O2 at 50℃ (for heat stress), or on LB plate with 1.6 mM H2O2 at 50℃ (for extreme stress).
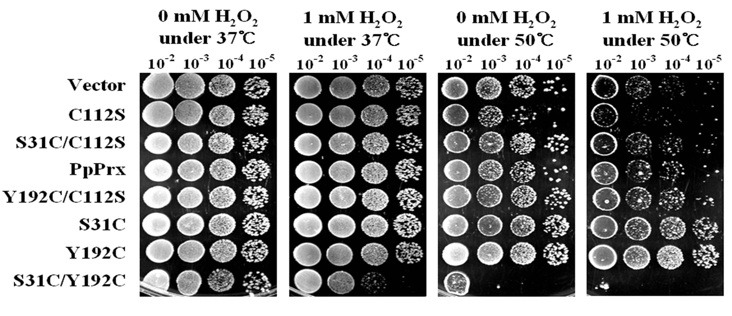
Among the PpPrx derivatives, the single Cys mutants (S31C/ C112S and Y192C/C112S) and the double Cys mutants (S31C and Y192C) displayed higher survival rates than C112S under heat stress. The enhanced stress tolerance of ΔPRX E. coli by additional Cys derivatives was clearly shown under the complex- stress condition. The S31C and Y192C mutants exhi-bited dramatically increased survival rates compared with other PpPrx derivatives (Fig. 7). S31C/Y192C exhibited hypersensitivity to all stress conditions, although the level of chaperone activity was maintained at 93% compared with PpPrx (Fig. 3). The survival rates of S31C and Y192C indicate that the equilibrium of the dual functions was modulated by the additional Cys residues. The equalized dual functions could enhance the tolerance of ΔPRX E. coli cells against the complex-stress condition.
DISCUSSION
The 2-Cys Prx proteins are members of a ubiquitous family of peroxidases that exhibit antioxidant activity as peroxidases and molecular chaperones and participate in redox-sensitive signaling (Jang et al., 2004; 2006a; Wood et al., 2003). Most 2-Cys Prxs form condition-dependent oligomeric structures, although the physiological relevance of the association or dissociation of these proteins is unclear (Jang et al., 2004; 2006a; Moon et al., 2005). Oligomerization may be dependent on the redox state, with reduction of the enzyme resulting in oligomerization (Schröder et al., 1998). Yeast Prxs undergo conversion to a multimeric structure by heat stress (Jang et al., 2004), and the chaperone activity of hPrxI is enhanced by the phosphorylation of Thr90 (Jang et al., 2006b). Although the chaperone activity is maximally increased by 6- to 10-fold following these modifications, the mechanism responsible for the increased activity is unknown (Jang et al., 2004; 2006b; Moon et al., 2005).
In this study, we found that PpPrx and PaPrx have a highly conserved sequence-based alignment, and that they both have dual functions as peroxidases and molecular chaperones. In spite of these similarities, PpPrx had an approximately 40- to 50-fold stronger chaperone activity than PaPrx, and contained Cys112 between two active Cys residues. By contrast, PaPrx exhibited low chaperone activity and absent additional Cys residues at the same position.
We investigated how Cys112 could affect the structure and dual functions of PpPrx. C112S exhibited a similar functional tendency as PaPrx (Supplementary Fig. 1A). According to the secondary structure prediction, Cys112 is located on an α-helical element of PpPrx (Fig. 2A). Thus, the influence of additional Cys residues (Cys31, Cys112 and Cys192) on the functional and structural changes of PpPrx was investigated using various additional Cys derivatives on other α-helical elements in PpPrx. The C112S mutant showed lower chaperone activity than single or multiple additional Cys residue mutants. Additional Cys derivatives had significantly lower peroxidase activities than C112S. In particular, S31C and Y192 maintained an approximately 80% peroxidase activity compared with C112S (Fig. 3).
Compared to C112S, mutants with additional Cys derivatives tended to form variously sized oligomeric HMW complexes and tended to display higher chaperone activities. By contrast, C112S displayed mostly LMW structures and low chaperone activity (Figs. 3 and 4). The substitution of additional Cys residues (Cys31 and Cys192) in the α-helical elements of C112S caused the mutants to behave like PpPrx, which has a high chaperone activity. Thus, the secondary structure elements and hydrophobicity were significantly transformed by the additional Cys residues (Cys31, Cys112 and Cys192), and provided a structural explanation for the increased chaperone activity.
The additional Cys residues also significantly increased the exposure of β-sheet and random coil elements on the protein surface, whereas the exposure of α-helical and turn elements was decreased (Fig. 5). We also investigated the influence of hydrophobicity on the chaperone function. The Cys112 of PpPrx greatly increased the exposure of hydrophobic domains; however, other positions of additional Cys residues (Cys31 and Cys192) partially reduced the exposure of hydrophobic domains. Consequently, switches in the functional and structural characters were determined partly by the position of additional Cys residues (Cys31, Cys112 and Cys192).
Finally, we investigated whether the stress tolerance of ΔPRX E. coli cells was significantly enhanced by PpPrx engineered with additional Cys residues. S31C and Y192C exhibited dramatically higher survival rates under the complex-stress condition than the other derivatives (Fig. 7). The enhanced stress tolerance of S31C and Y192C suggested that the equilibrium of the dual functions could be significantly modulated by the use of additional Cys residues (Cys31, Cys112, and Cys192). Consequently, the equalized dual functions could enhance the resistance of ΔPRX E. coli cells against the complex-stress condition (Fig. 7).
In conclusion, we have provided a guideline for conserved 2- Cys Prx protein engineering by additional Cys residues, and have demonstrated that additional Cys is a critical factor in the structural and functional switching. These results suggest that equilibrated dual functions could be adapted for use in bioengineering systems and industries. Furthermore, it may be possible to develop organisms that are more resistant to extreme environments.
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
Acknowledgments
This project was performed under the Nuclear R&D Program of the Ministry of Science and Technology (http://www.mest.go.kr).
References
- 1.An B.C., Lee S.S., Lee E.M., Lee J.T., Wi S.G., Jung H.S., Park W., Chung B.Y. A new antioxidant with dual functions as a peroxidase and chaperone in Pseudomonas aeruginosa. Mol. Cells. (2010);29:145–151. doi: 10.1007/s10059-010-0023-1. [DOI] [PubMed] [Google Scholar]
- 2.An B.C., Lee S.S., Lee E.M., Lee J.T., Wi S.G., Jung H.S., Park W., Chung B.Y. Functional switching of a novel prokaryotic 2-Cys peroxiredoxin (PpPrx) under oxidative stress. Cell Stress Chaperones. (2011);16:317–328. doi: 10.1007/s12192-010-0243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baier M., Dietz K.J. Alkyl hydroperoxide reductase: the way out of the oxidative breakdown of lipids in chloroplast. Trends Plant Sci. (1999);4:166–168. doi: 10.1016/s1360-1385(99)01398-9. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. (1976);72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Chae H.Z., Robison K., Poole L.B., Church G., Storz G., Rhee S.G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. USA. (1994);91:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chae H.Z., Kim H.J., Kang S.W., Rhee S.G. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res. Clin. Pract. (1999);45:101–112. doi: 10.1016/s0168-8227(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 7.Chang T.-S., Jeong W., Choi S.Y., Yu S., Kang S.W. Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation. J. Biol. Chem. (2002);277:25370–25376. doi: 10.1074/jbc.M110432200. [DOI] [PubMed] [Google Scholar]
- 8.Cheong N.E., Choi Y.O., Lee K.O., Kim W.Y., Jung B.G., Chi Y.H., Jeong J.S., Kim K., Cho M.J., Lee S.Y. Molecular cloning, expression, and functional characterization of a 2 Cys-peroxiredoxin in Chinese cabbage. Plant Mol. Biol. (1999);40:825–834. doi: 10.1023/a:1006271823973. [DOI] [PubMed] [Google Scholar]
- 9.Chuang M.H., Wu M.S., Lo W.L., Lin J.T., Wong C.H., Chiou S.H. The antioxidant protein alkyl hydroperoxide reductase of helicobacter pylori switches from a peroxide reductase to a molecular chaperone function. Proc. Natl. Acad. Sci. USA. (2006);103:2552–2557. doi: 10.1073/pnas.0510770103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cumming R.C., Andon N.L., Haynes P.A., Park M.K., Fischer W.H., Schubert D. Protein disulfide bond formation in the cytoplasm during oxidative stress. J. Biol. Chem. (2004);279:21749–21758. doi: 10.1074/jbc.M312267200. [DOI] [PubMed] [Google Scholar]
- 11.Dietz K.J. Plant peroxiredoxins. Annu. Rev. Plant Biol. (2003);54:93–107. doi: 10.1146/annurev.arplant.54.031902.134934. [DOI] [PubMed] [Google Scholar]
- 12.Ganea E. Chaperone-like activity of alpha-crystallin and other small heat shock proteins. Curr. Prot. Pept. Sci. (2001);2:205–225. doi: 10.2174/1389203013381107. [DOI] [PubMed] [Google Scholar]
- 13.Gnanasekar M., Dakshinamoorthy G., Ramaswamy K. Translationally controlled tumor protein is a novel heat shock protein with chaperone-like activity. Biochem. Biophys. Res. Commun. (2009);386:333–337. doi: 10.1016/j.bbrc.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann B., Hecht H.-J., Flohé L. Peroxiredoxins. Biol. Chem. (2002);383:347–364. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- 15.Ito H., Kamei K., Iwamoto I., Inaguma Y., Nohara D., Kato K. Phosphorylation-induced change of the oligomerization state of alpha B-crystallin. J. Biol. Chem. (2001);276:5346–5352. doi: 10.1074/jbc.M009004200. [DOI] [PubMed] [Google Scholar]
- 16.Jang H.H., Lee K.O., Chi Y.H., Jung B.G., Park S.K., Park J.H., Lee J.R., Lee S.S., Moon J.C., Yun J.W., et al. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell. (2004);117:625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Jang H.H., Chi Y.H., Park S.K., Lee S.S., Lee J.R., Park J.H., Moon J.C., Lee Y.M., Kim S.Y., Lee K.H., et al. Structural and functional regulation of eukaryotic 2-Cys peroxiredoxins including the plant ones in cellular defense signaling mechanisms against oxidative stress. Physiol. Plant. (2006a);126:549–559. [Google Scholar]
- 18.Jang H.H., Kim S.Y., Park S.K., Jeon H.S., Lee Y.M., Jung J.H., Lee S.Y., Chae H.B., Jung Y.J., Lee K.O., et al. Phosphorylation and concomitant structural changes in human 2-Cys peroxiredoxin isotype I differently regulate its peroxidase and molecular chaperone functions. FEBS Lett. (2006b);580:351–355. doi: 10.1016/j.febslet.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Jeong W.J., Cha M.K., Kim I.H. A new member of human Tsa/AhpC as thioredoxin-dependent thiol peroxidase. J. Biochem. Mol. Biol. (2000);33:234–241. [Google Scholar]
- 20.Kristensen P., Rasmussen D.E., Kristensen B.I. Properties of thiol-specific anti-oxidant protein or calpromotin in solution. Biochem. Biophys. Res. Commun. (1999);262:127–131. doi: 10.1006/bbrc.1999.1107. [DOI] [PubMed] [Google Scholar]
- 21.Lee G.J., Roseman A.M., Saibil H.R., Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. (1997);16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Link A.J., Robison K., Church G.M. Comparing the predicted and observed properties of proteins encoded in the genome of Escherichia coli K-12. Electrophoresis. (1997);18:1259–1313. doi: 10.1002/elps.1150180807. [DOI] [PubMed] [Google Scholar]
- 23.Moon J.C., Hah Y.S., Kim W.Y., Jung B.G., Jang H.H., Lee J.R., Kim S.Y., Lee Y.M., Jeon M.K., Kim C.W., et al. Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J. Biol. Chem. (2005);280:28775–28784. doi: 10.1074/jbc.M505362200. [DOI] [PubMed] [Google Scholar]
- 24.Schröder E., Willis A.C., Ponting C.P. Porcine naturalkiller- enhancing factor-B: oligomerisation and identification as a calpain substrate in vitro. Biochim. Biophys. Acta. (1998);1383:279–291. doi: 10.1016/s0167-4838(97)00217-3. [DOI] [PubMed] [Google Scholar]
- 25.Sharma K.K., Kaur H., Kumar G.S., Kester K. Interaction of 1,1′-bi(4-anilino)naphthalene-5,5′-disulfonic acid with alpha-crystallin. J. Biol. Chem. (1998);273:8965–8970. doi: 10.1074/jbc.273.15.8965. [DOI] [PubMed] [Google Scholar]
- 26.Shi L., Palleros D.R., Fink A.L. Protein conformational changes induced by 1,1′-bis(4-anilino-5-naphthalenesulfonic acid): preferential binding to the molten globule of DnaK. Biochemistry. (1994);33:7536–7546. doi: 10.1021/bi00190a006. [DOI] [PubMed] [Google Scholar]
- 27.Wood Z.A., Schroder E., Robin H.J., Poole L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. (2003);28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 28.Yang K.-S., Kang S.W., Woo H.A., Hwang S.C., Chae H.Z., Kim K., Rhee S.G. Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site Cys to Cys-sulfinic acid. J. Biol. Chem. (2002);277:38029–38036. doi: 10.1074/jbc.M206626200. [DOI] [PubMed] [Google Scholar]


