Abstract
Platelet aggregation plays crucial roles in the formation of hemostatic plugs and thrombosis. Although it was recently shown that canonical Wnt signaling negatively regulates platelet aggregation, the role of non-canonical Wnt signaling remains unknown. Here, we observed that Wnt5a, one of the non-canonical Wnts, positively regulated platelet aggregation. Platelet aggregation was potentiated by the addition of Wnt5a to collagen- or U46619-induced rat platelet rich plasma (PRP). Treatment with Wnt5a to U46619- stimulated PRP resulted in an increase in the level of phosphorylated Akt, whereas phosphorylation of PKCδ and JNK1 was unaffected. In addition, inhibition of PI3K blocked the potentiating effect of Wnt5a. Taken together, these results suggest that Wnt5a potentiates U46619- induced platelet aggregation via the PI3K/Akt pathway.
Keywords: Akt1, platelet aggregation, platelet-rich plasma, U46619, Wnt
INTRODUCTION
Platelet aggregation, in which a platelet attaches to another platelet, is the major process of hemostatic plug formation or thrombosis at sites of vascular damage. Previously, it was demonstrated that several receptor-mediated signaling mechanisms are involved in platelet aggregation (Jackson, 2007). Platelet aggregation is stimulated by soluble agonists such as collagen, adenosine diphosphate (ADP), U46619 (9, 11-dieoxy- 11α, 9α-methanoepoxyprostaglandin F2α; thromboxane A2 mimetic), or thrombin (Jackson, 2007). Previous reports demonstrated that activation of PLC-γ coupled with collagen receptors leads to increase in the levels of intracellular Ca2+, diacylglycerol (DAG), and inositol triphosphate (IP3) (Surin et al., 2008). DAG then activates protein kinase C (PKC) isoforms, which leads to secretion of granules on collagen-induced platelet aggregation (Harper and Poole, 2010; Surin et al., 2008). Components in the secreted granules, including ATP, ADP, serotonin, and Ca2+, are essential for induction of secondary platelet aggregation (Holmsen, 1991). In addition, multiple agonists are known to stimulate phosphoinositol 3-kinase (PI3K) in platelets, subsequently activating Akt via phosphorylation (Li et al., 2003).
Wnt signaling is classified into canonical and non-canonical pathways based on the involvement of β-catenin (Veeman et al., 2003). Canonical Wnt signaling is referred as the Wnt/β- catenin pathway since the regulation of cytoplasmic β-catenin is a key step in signal transduction. Recently, it was demonstrated that several components of Wnt/β-catenin signaling are present in platelets. It was shown specifically that pre-treatment with recombinant Wnt3a, one of the canonical Wnts, inhibits platelet aggregation (Steele et al., 2009). On the other hand, the role of non-canonical Wnt signaling in platelet aggregation has not yet been determined. Wnt5a, a typical non-canonical Wnt, stimulates intracellular Ca2+ release, thereby activating PKC and Ca2+/calmodulin-dependent protein kinase II (CamKII) (Kuhl et al., 2000). In addition, Wnt5a activates PI3K/Akt signaling through Frizzled3 receptor in human dermal fibroblasts (Kawasaki et al., 2007) and enhances mammary cells binding to collagen (Jonsson and Andersson, 2001). Given that these functions of Wnt5a seem to overlap with signaling for platelet aggregation, it is plausible that Wnt5a plays a role in platelet aggregation. In this study, we demonstrate that Wnt5a potentiates U46619-induced rat platelet aggregation via activation of PI3K/Akt signaling.
MATERIALS AND METHODS
Reagents and instruments
Collagen was purchased from Chrono-log Corporation and U46619 (9, 11-Dieoxy-11α, 9α-methanoepoxyprostaglandin F2α; thromboxane A2 mimetic) was purchased from Sigma. Anti-phospho(Ser473)-Akt1 antibody and anti-phospho(Thr505)-PKCδ antibody were purchased from Cell Signaling Technology. Anti-phospho- JNK1 antibody was purchased from Santa Cruz Bio-tech. Recombinant Wnt5a was purchased from R&D systems. The number of platelets was determined using a hematology analyzer (Exell TM 18, Drew Scientific Inc.), whereas the degree of platelet aggregation was measured using a four-channel platelet aggregometer (Model 490-X, Chrono-Log Corp.) connected to a personal computer.
Animals
The rats (Sprague-Dawley, 250 ± 20 g) used in this study were purchased form Orient Bio (Korea). They were fed a diet of animal chow and tap water and were housed at 23 ± 0.5℃ and 60% humidity under a 12-h light-dark cycle in accordance with the Guide for the Care and Use of Laboratory Animals of Seoul National University. Animal experiments were conducted under the license given by the animal ordinance issued by the Institute of Laboratory Animal Resources of Seoul National University and according to the guidelines of the Seoul National University Institutional Animal Care and Use Committee (approval no. SNU050502-13).
Rat blood collection
Rat blood was collected from hearts after surgery using a syringe containing 0.1 volumes of 2.2% sodium citrate. Collected blood was centrifuged at 200 × g for 10 min at room temperature to obtain supernatant platelet-rich plasma (PRP). Platelet-poor plasma (PPP) was obtained from the residue after centrifugation at 1500 × g for 20 min. Supernatant PRP was diluted with PPP and physiological saline to adjust the final platelet number to 4.0-4.5 × 108 platelets/ml with the aid of a platelet counter.
Human blood collection
A few Korean men who had not taken any medicine for 2 weeks prior volunteered for the study. They were given information con-cerning the purpose of the experiment. The research protocol was approved by the Institutional Review Board of Seoul National University Hospital, Korea.
Human blood was collected from the antecubital vein using 3.8% sodium citrate (9:1, v/v). PRP was centrifuged at 200 × g for 10 min, whereas PPP was collected from the residue by centrifugation at 1500 × g for 20 min. The number of platelets was adjusted to 300-350 × 106/ml by adding PPP to PRP.
Platelet aggregation
Platelet aggregation was monitored by a turbidimetric method using an optical aggregometer. PRP (500 μl) was incubated at 37℃ and stirred continuously at 1,200 rpm. Platelet aggregation was recorded after the final addition of an aggregating agent relative to PPP, which represented 100% light transmission, whereas PRP represented 0% light transmission. Reduction in the turbidity of PRP was observed as platelet aggregation proceeded. PRP was equilibrated at 37℃ for 3 min prior to the initiation of each experiment. Briefly, 5 μl of sample or vehicle (buffer) was added, followed by addition of an aggregating agent [collagen (0.7 μg/ml)] at 30 s. U46619 (3 μM)-induced platelet aggregation was measured in the presence of a threshold concentration of collagen (0.5 μg/ml), which was added 30 sec before the addition of the agent.
Reverse transcriptase - PCR
Rat and human PRP were washed twice with PBS. RNA was isolated with TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. cDNA was generated using ImProm-II™ Reverse Transcriptase (Promega). The cDNA were amplified with the primers indicated in Supplementary Table 1.
Western blotting
After the reaction samples were centrifuged, the precipitated platelets were washed twice with PBS. The platelets were then lysed in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% TritonX-100, 50 mM NaF, 2 mM EDTA, 100 μM Naorthovanadate, 1 mM PMSF, 5 μg/ml of leupeptin, 1 μM pepstatin A). The lysates were centrifuged at 13,000 rpm for 15 min at 4℃, after which the supernatants were removed. The protein samples were then resolved by SDS-PAGE and analyzed by Western blotting. PVDF membrane (Pall Life Sciences) was used for transfer, and ECL reagent (Roche or ELPIS Biotech.) was used to detect the chemiluminescent signal.
RESULTS AND DISCUSSION
Presence of Wnt receptors in platelets
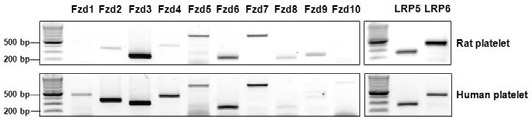
Since the function of Wnt5a depends on its receptors, we investigated whether or not receptors for Wnt, Frizzleds (Fzd), and LRP5/6 (low-density lipoprotein receptor-related protein) are present in rat and human platelets using RT-PCR analysis (Fig. 1). In rat platelets, all known Frizzled receptors except Fzd1 and 10 were detected. Fzd3, 5, 6, and 7 displayed higher expression compared to Fzd2, 4, 8, and 9 (Fig. 1; upper panel). All Fzd receptors were present in human platelets, whereas expression of Fzd8, 9, and 10 was relatively low (Fig. 1; lower panel). Expression of both LRP5 and 6, Wnt co-receptors, was observed in rat and human platelets. Fzd3 was the most abundant receptor in rat and human platelets, suggesting that Fzd3 might play a crucial role in platelet function.
Fig. 1. Wnt receptors are present in rat and human platelets. RNA from rat and human platelets was used for RT-PCR. The indicated receptors were amplified using the primers listed in Supplementary Table 1.
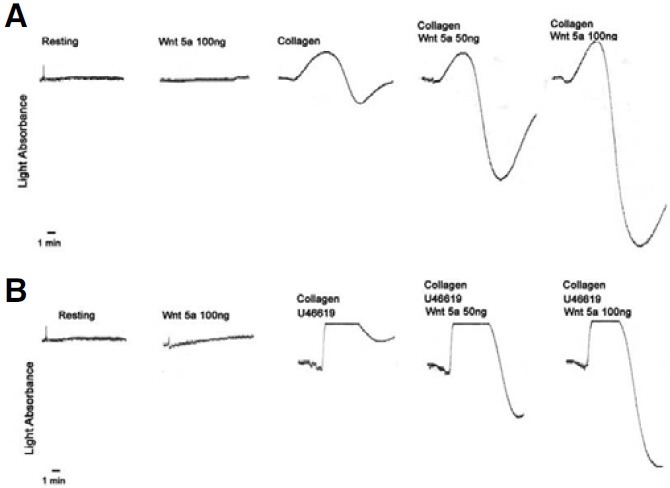
Potentiation of collagen or U46619-induced platelet aggregation by Wnt5a
Steele et al. (2009) previously showed that Wnt5a has no inhibitory effect on platelet aggregation. However, they did not examine the potentiative effect of Wnt5a on platelet aggregation. Given that Wnt5a leads to PKC activation (Koyanagi et al., 2009), Akt phosphorylation (Kawasaki et al., 2007), and inhibition of canonical Wnt signaling (Topol et al., 2003), Wnt5a may augment platelet aggregation. To test this possibility, we investigated whether or not Wnt5a can induce aggregation of platelets on rat PRP. The degree of platelet aggregation was analyzed based on the level of light absorption. As shown in Fig. 2, recombinant Wnt5a alone did not lead to aggregation of platelets in rat PRP. To test the potentiative effect of Wnt5a, we used collagen or U46619 at the threshold concentration in which platelet aggregation does not occur. Further, U46619 was used in the presence of a low concentration of collagen since the threshold concentration of collagen must be surpassed for U46619-induced platelet aggregation in rat PRP (Hanasaki et al., 1987; Nakano et al., 1987). Interestingly, Wnt5a potentiated platelet aggregation in a dose-dependent manner at the threshold concentration of collagen (0.7 μg/ml) (Fig. 2A) or collagen (0.5 μg/ml) plus U46619 (3 μM) (Fig. 2B).
Fig. 2. Effects of Wnt5a on collagen- or U46619-induced rat platelet aggregation. The degree of platelet aggregation was measured based on the level of light absorption. Rat PRP was stimulated by (A) collagen (0.7 μg/ml) with or without Wnt5a (50 or 100 ng) or (B) U46619 (a thromboxane A2 mimetic, 3 μM) with or without Wnt5a (50 or 100 ng). Collagen (0.5 μg/ml) was added 30 s prior to stimulation. The aggregation traces are representative of three separate experiments.
Wnt5a enhances phosphorylation of Akt1 in U46619- induced platelet aggregation
It has been shown that the PI3K/Akt pathway is activated by Wnt5a through Fzd3 in human dermal fibroblasts (Kawasaki et al., 2007). Akt isoform 1 is known to be responsible for platelet aggregation (Chen et al., 2004). In our results, Fzd3 was highly expressed compared to other Fzds in rat and human platelets (Fig. 1). These results suggest that Wnt5a might potentiate platelet aggregation via activation of PI3K/Akt signaling. We next tested the effect of recombinant Wnt5a on the level of phosphorylated Akt1 during platelet aggregation. Both Wnt5a and U46619 alone slightly induced phosphorylation of Akt1 in rat platelets. However, phosphorylation of Akt1 was remarkably increased in the presence of both Wnt5a and U46619 (Fig. 3), and the amount of increase paralleled the degree of platelet aggregation, as shown in Fig. 2B.
Fig. 3. Wnt5a enhances phosphorylation of Akt1 in rat platelets. Rat PRP was stimulated by collagen and U46619 with or without Wnt5a and analyzed by Western blotting using the indicated antibodies. The β-actin was used as a loading control. Collagen (0.5 μg/ml) was added 30 s prior to U46619-induced stimulation.
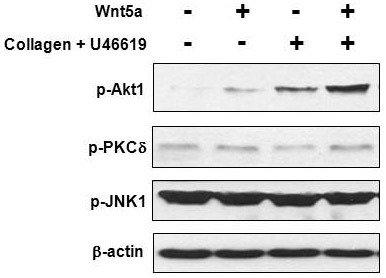
In addition to Akt1 activation, Wnt5a can relay signaling via activation of JNK and PKC in the planar cell polarity (PCP) pathway (Brade et al., 2006). Among PKC family proteins, PKCδ is implicated in the regulation of the convergent extension movement of Xenopus embryos by the Wnt/JNK pathway as well as the regulation of cardiac gene expression by Wnt5a in human circulating progenitor cells (Kinoshita et al., 2003; Koyanagi et al., 2009). In our Western blot experiment, however, neither Wnt5a, U46619, nor Wnt5a with U46619 stimulated PKCδ, based on the level of phosphorylated PKCδ, in rat platelets (Fig. 3). In addition, no detectable difference was observed in JNK phosphorylation when Wnt5a was added during U46619-induced rat platelet aggregation. These results suggest that Wnt5a specifically induces the activation of Akt1, but not PKCδ or JNK, in U46619-induced rat platelet aggregation.
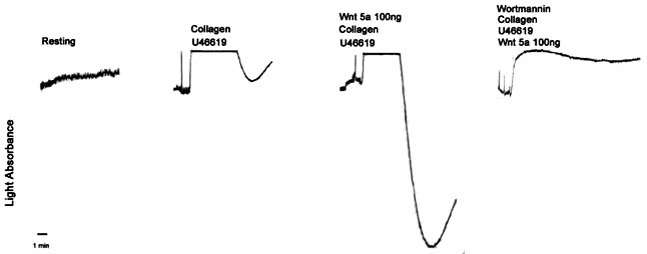
Potentiative effect of Wnt5a on rat platelet aggregation is blocked by Wortmannin
To confirm that the potentiative effect of Wnt5a is mediated by activation of PI3K/Akt signaling, we performed the same experiment shown in Fig. 2B using Wortmannin, a PI3K inhibitor. In the presence of Wortmannin, platelet aggregation potentiated by Wnt5a was completely blocked (Fig. 4). Similarly, the potentiative effect of Wnt5a was blocked by LY294001, another PI3K inhibitor (data not shown). These data suggest that Wnt5a could potentiate U46619-induced platelet aggregation via activation of PI3K/Akt signaling.
Fig. 4. Wortmannin blocks potentiation of platelet aggregation by U46619 and Wnt5a. PRP was stimulated with U46619 (3 μM) with or without Wnt5a (100 ng). Wortmannin (10 μM) or vehicle was added 30 s prior to stimulation. Collagen (0.5 μg/ml) was added 30 s prior to stimulation. The aggregation traces are representative of three separate experiments.
Wnt5a is known to be expressed in melanoma. Especially, Wnt5a is strongly expressed in metastatic melanoma cells (Weeraratna et al., 2002). This suggests that Wnt5a most likely participates in the metastasis of cancer cells. To metastasize, cancer cells must not only invade blood vessels but also overcome stresses such as blood shear forces and immune responses (Fidler et al., 2010; Gupta and Massague, 2006). Although it is known that Wnt5a promotes the invasion of cancer cells into blood vessels (Pukrop et al., 2006), the role of Wnt5a during circulation of cancer cells remains unknown. Platelet aggregation is a critical step for metastatic cells to survive in the circulation. It was previously shown that metastatic cells protect themselves from shear forces and immune cells by using aggregated platelets as a shield (Gupta and Massague, 2006). In this study, we showed that most of the Wnt receptors are present in platelets and that Wnt5a functions positively in collagenor U46619-induced platelet aggregation. Although our study could not provide evidence of Wnt5a secretion by circulating metastatic cells, it is still possible that Wnt5a might be involved in metastatic progression via induction of platelet aggregation after invasion into blood vessels.
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
Acknowledgments
This work was supported by a grant from the University of Seoul 2010 Research Fund to E. Jho.
References
- 1.Brade T., Manner J., Kuhl M. The role of Wnt signalling in cardiac development and tissue remodelling in the mature heart. Cardiovasc. Res. (2006);72:198–209. doi: 10.1016/j.cardiores.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Chen J., De S., Damron D.S., Chen W.S., Hay N., Byzova T.V. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood. (2004);104:1703–1710. doi: 10.1182/blood-2003-10-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fidler I.J., Balasubramanian K., Lin Q., Kim S.W., Kim S.J. The brain microenvironment and cancer metastasis. Mol. Cells. (2010);30:93–98. doi: 10.1007/s10059-010-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta G.P., Massague J. Cancer metastasis: building a framework. Cell. (2006);127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Hanasaki K., Nakano T., Arita H. Two phasic generation of thromboxane A2 by the action of collagen on rat platelets. Thromb. Res. (1987);46:425–436. doi: 10.1016/0049-3848(87)90130-7. [DOI] [PubMed] [Google Scholar]
- 6.Harper M.T., Poole A.W. Diverse functions of protein kinase C isoforms in platelet activation and thrombus formation. J. Thromb. Haemost. (2010);8:454–462. doi: 10.1111/j.1538-7836.2009.03722.x. [DOI] [PubMed] [Google Scholar]
- 7.Holmsen H. Signal transducing mechanisms in platelets. Proc. Natl. Sci. Counc. Repub. China B. (1991);15:147–152. [PubMed] [Google Scholar]
- 8.Jackson S.P. The growing complexity of platelet aggregation. Blood. (2007);109:5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson M., Andersson T. Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J. Cell Sci. (2001);114:2043–2053. doi: 10.1242/jcs.114.11.2043. [DOI] [PubMed] [Google Scholar]
- 10.Kawasaki A., Torii K., Yamashita Y., Nishizawa K., Kanekura K., Katada M., Ito M., Nishimoto I., Terashita K., Aiso S., et al. Wnt5a promotes adhesion of human dermal fibroblasts by triggering a phosphatidylinositol-3 kinase/Akt signal. Cell. Signal. (2007);19:2498–2506. doi: 10.1016/j.cellsig.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita N., Iioka H., Miyakoshi A., Ueno N. PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. (2003);17:1663–1676. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koyanagi M., Iwasaki M., Haendeler J., Leitges M., Zeiher A.M., Dimmeler S. Wnt5a increases cardiac gene expressions of cultured human circulating progenitor cells via a PKC delta activation. PloS one. (2009);4:e5765. doi: 10.1371/journal.pone.0005765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhl M., Sheldahl L.C., Park M., Miller J.R., Moon R.T. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. (2000);16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 14.Li Z., Zhang G., Le Breton G.C., Gao X., Malik A.B., Du X. Two waves of platelet secretion induced by thromboxane A2 receptor and a critical role for phosphoinositide 3- kinases. J. Biol. Chem. (2003);278:30725–30731. doi: 10.1074/jbc.M301838200. [DOI] [PubMed] [Google Scholar]
- 15.Nakano T., Terawaki A., Arita H. Signal transduction in collagen-stimulated rat platelets is composed of three stages. J. Biochem. (1987);101:1169–1180. doi: 10.1093/oxfordjournals.jbchem.a121981. [DOI] [PubMed] [Google Scholar]
- 16.Pukrop T., Klemm F., Hagemann T., Gradl D., Schulz M., Siemes S., Trumper L., Binder C. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc. Natl. Acad. Sci. USA. (2006);103:5454–5459. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steele B.M., Harper M.T., Macaulay I.C., Morrell C.N., Perez- Tamayo A., Foy M., Habas R., Poole A.W., Fitzgerald D.J., Maguire P.B. Canonical Wnt signaling negatively regulates platelet function. Proc. Natl. Acad. Sci. USA. (2009);106:19836–19841. doi: 10.1073/pnas.0906268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surin W.R., Barthwal M.K., Dikshit M. Platelet collagen receptors, signaling and antagonism: emerging approaches for the prevention of intravascular thrombosis. Thromb. Res. (2008);122:786–803. doi: 10.1016/j.thromres.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Topol L., Jiang X., Choi H., Garrett-Beal L., Carolan P.J., Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol. (2003);162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veeman M.T., Axelrod J.D., Moon R.T. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. (2003);5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 21.Weeraratna A.T., Jiang Y., Hostetter G., Rosenblatt K., Duray P., Bittner M., Trent J.M. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. (2002);1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]


