Abstract
Quantitative trait loci (QTLs) that control the tissue culture response in soybean were detected by using 184 recombinant inbred lines (RILs) derived from two varieties: Kefeng No.1 and Nannong 1138-2. The molecular map consisting of 834 molecular markers using this population covered space 2307.83 cM of the genome throughout 24 linkage groups. The performance of tissue culture in soybean was evaluated by two indices: callus induction frequency (CIF) and somatic embryos initiation frequency (SEIF). They were expressed as the number of explants producing callus/ the number of total explants and the number of explants producing somatic embryos/ the number of total explants, respectively. The RIL lines showed continuous segregation for both indices. With the composite interval mapping (CIM) described in Windows QTL Cartographer Version 2.5, three quantitative trait loci (QTLs) were identified for the frequency of callus induction, on chromosomes B2 and D2, accounting for phenotypic variation from 5.84% to 16.60%; four QTLs on chromosome G were detected for the frequency of somatic embryos initiation and explained the phenotypic variation from 7.79% to 14.16%. The information of new QTLs identified in the present study will contribute to genetic improvement of regeneration traits with marker-assisted selection (MAS) in soybean.
Keywords: quantitative trait loci, recombinant inbred line population, somatic embryogenesis, soybean (Glycine max (L.) Merr.), tissue culture response
INTRODUCTION
In order to improve the quality of the crops and to develop new varieties for crop breeding, it is important to use biotechnological methods, including selection of somaclonal variants and genetic transformation. However, a highly efficient regeneration system is a necessary prerequisite for successful utilization of such methods (Bolibok and Rakoczy-Trojanowska, 2006; Bolibok et al., 2007). So far, a number of regeneration systems and transformation systems have been established for many plant species. However, these systems are characterized by instability and low efficiency. Moreover, the regeneration ability and transformation efficiency of plants is considerably restricted by genotype-dependence (Mano et al., 2002; Krakowsky et al., 2006). To date, research has focused on factors such as the media components, culture conditions and explants types to develop and optimize methods for plant regeneration and transformation. However, the genetic basis of tissue culture responses has not been investigated in detail (Krakowsky et al., 2006). Genetic studies of tissue-culture traits, such as callus growth, embryogenesis and differentiation, will make it possible to transfer genes controlling desirable tissue-culture traits into recalcitrant cultivars or species (Mano et al., 2002). With advances in molecular biology, traditional quantitative genetics has progressed to the stage of molecular quantitative genetics, thus providing a new route to study complex quantitative traits. The use of molecular markers has enabled the identification of quantitative trait loci (QTLs) involved in the expression of important agronomic traits. Furthermore, the construction of genetic maps has provided a tool for identification of the number, significance and location of QTLs (Bolibok et al., 2007; Flores Berrios et al., 2000). Recent reports have identified the QTLs for tissue culture response in many plant species, such as in rice (He et al., 1998; Kwon et al., 2002; Taguchi-Shiobara et al., 1997; 2006), maize (Armstrong et al., 1992; Krakowsky et al., 2006; Murigneux et al., 1994), barley (Komatsuda et al., 1995; Mano et al., 2002), wheat (Jia et al., 2007; Torp et al., 2001), rye (Bolibok et al., 2007), sunflower (Flores Berrios et al., 2000), tomato (Koornef et al., 1993) and alfalfa (Yu and Pauls, 1993).
Soybean is a major crop worldwide, which contributes significantly to the global economy as an important source of dietary oil and protein (Wilcox, 2004). Therefore, improvement of soybean characteristics via regeneration and transformation techniques is of critical importance. Although soybean is one of the most recalcitrant crops (Hofmann et al., 2004; Khalafalla et al., 2006; Paz et al., 2006), many soybean regeneration and transformation methods have been established over the years. However, the efficiency of these techniques is considerably restricted by genotype-dependence (Paz et al., 2004; Reichert et al., 2003; Sairam et al., 2003; Yang et al., 2009). Detailed genetic studies are required to identify the genes or QTL associated with the tissue culture response. This study aimed to identify QTLs of the in vitro response of soybean using immature zygotic embryo explants.
MATERIALS AND METHODS
Plant materials
A population (NJRIKY) of recombinant inbred lines provided by the National Center for Soybean Improvement, Nanjing Agricultural University was used in this study. The population containing 184 F8:11 recombinant inbred lines was developed from the crosses between Kefeng No.1 and Nannong 1138-2 by the single-seed descent method. The NJRIKY population was sown on two different dates in 2008 (early: June 10th; late: June 25th) under normal soybean cultivation conditions at the Jiangpu Experiment Station in Nanjing, China.
Tissue culture procedures
Approximately 15 days after flowering, immature pods containing cotyledons of 4-5 mm in length and other sizes required for various experiments were collected, then surface-sterilized in 3% NaClO solution, containing 2-3 drops of Tween-20 for 20 min, and rinsed five times with sterilized water. According to Lazzeri et al. (1985), immature zygotic embryo explants were obtained by removing the embryonic axis. Then, the explants were placed with the adaxial surface in contact with the inducing medium containing Murashige and Skoog (MS) (Murashige and Skoog, 1962) macro-salts, MS micro-salts, B5 vitamins (Gamborg et al., 1968), 40 mg L-1 2,4-D and 1 g L-1 L-asparagine. Phytagel at 0.2% w/v was used as the solidifying agent. The pH was adjusted to 5.8 with 1 N HCl before autoclaving. Cultures were incubated under a 18-h photoperiod per day rhythm and a light intensity of 10 umol photons m-2s-1 provided by continuous cool-white fluorescent light at 26 ± 1℃.
In this study, two characteristics (callus induction and somatic embryogenesis ability) were selected to evaluate the tissue culture response of soybean. These two characteristics were calculated using the following parameters: callus induction frequency = number of explants producing calluses/number of total explants; somatic embryos initiation frequency = number of explants producing somatic embryos/number of total explants. After incubation for 4 weeks, the callus induction frequency (CIF) and the somatic embryos initiation frequency (SEIF) were recorded for the population. The experiment was carried out twice using materials sown on two different dates. In each experiment, an initial minimum of 100 explants of each line were cultured. Due to contamination during the incubation period, six lines that contained less than 100 explants were disregarded in the following mapping analysis.
Genetic linkage map
The previously published genetic map for the population covered 4151.2 cM and consisted of 25 linkage groups (Qi et al., 2008). Each linkage group contained 19.5 markers with an average 8.6 cM between adjacent markers. In order to improve the density of this map, SSR and EST-SSR markers were integrated. The genetic map was developed using JoinMap 3.0 (Van-Ooijen and Voorrips, 2002) and the mapping distance (cM) was derived based on Kosambi function (Kosambi, 1943). This linkage map contained 834 molecular markers on 24 molecular linkage groups and spanned 2307.83 cM of the soybean genome with an average interval distance of 2.77 cM, the average markers per group of 34.75. The grouping and order of the majority of markers on the map was consistent with the public soybean genetic linkage map.
Statistical analysis and QTL analysis
In order to improve the normality of distribution, the phenotypic data were normalized using three kinds of transformation methods: square-root transformations, logarithmic transformations and arc-sin transformations. The analysis of variance (ANOVA) and genetic correlation between the two traits were performed on the phenotypic data using the PC-SAS, Version 9.0. Broad-sense heritability (h2) for each trait was defined as: h2 = σ2g/{(σ2g/r) + σ2e}, where σ2g, σ2e and r represent genetic variance, error variance and the number of replications, respectively. The original data normalized by arc-sin transformation were used for QTL mapping. QTL analysis was resolved according to the method of composite interval mapping (CIM) by using the computer package WinQTL Cartographer Version 2.5 (Wang et al., 2006). In this study, empirical thresholds were calculated using the permutation test (1,000 permutations, overall error level 5%) for CIM (Churchill and Doerge, 1994) and the LOD threshold value of 2.5 was used to suggest the presence of a putative QTL. Confidence intervals were set as the map interval corresponding to a 1-LOD decline on either side of the LOD peak.
RESULTS
Phenotypic data analysis
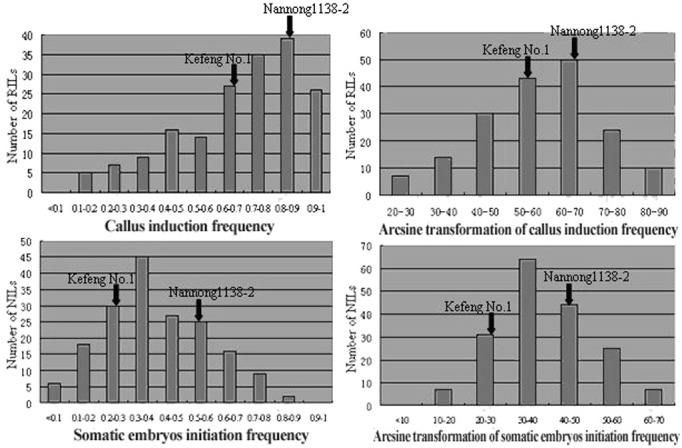
Significant differences were observed between the two parents of NJRIKY for both callus induction and somatic embryogenesis ability. The callus induction frequency and the somatic embryos initiation frequency were respectively 0.69 and 0.25 for Kefeng No.1, and 0.86 and 0.51 for Nannong1138-2 (Table 1). Nannong1138- 2 exhibited a better tissue culture response compared with Kefeng No.1. The results of ANOVA revealed the existence of highly significant variation among the lines for the two traits investigated (Table 2). The frequency of tissue culture response varied between lines, ranging from 0.14 to 1.00 with a 0.71 average for callus induction, and 0.04 to 0.85 with a 0.41 average for somatic embryos initiation (Table 1). Transgressive segregations were observed in the RIL population for the two traits studied. The broad-sense heritability calculated for the two traits was 0.93 and 0.82, respectively. High correlation (r = 0.61) was observed between callus induc-tion and somatic embryogenesis ability. Original data were compared with the transformed data due to the non-normal frequency distribution for RILs. Arc-sin transformation resulted in normal (or almost normal) frequency distribution in all cases (Table 3, Fig. 1). Consequently, the original data normalized by arc-sin transformation was used for QTL analysis.
Table 1.
Parental values and population parameters of the two characteristics
| Traits | Parent | RILs | ||
|---|---|---|---|---|
| Kefeng No.1 | Nannong1138-2 | Mean | Min-Max | |
| Callus induction frequency (CIF) | 0.69 | 0.86 | 0.71 | 0.14-1.00 |
| Somatic embryos initiation frequency (SIF) | 0.25 | 0.51 | 0.41 | 0.04-0.85 |
Table 2.
Analysis of variance for callus induction frequency and somatic embryos initiation frequency in RILs
| ANOVA of callus induction frequency | ANOVA of somatic embryos initiation frequency | ||||||
|---|---|---|---|---|---|---|---|
| Source of variation | DF | Mean square | F | Source of variation | DF | Mean square | F |
| Line | 177 | 431.96 | 66.03** | Line | 177 | 252.65 | 136.32** |
| Replication | 1 | 26.53 | 4.06 | Replication | 1 | 270.31 | 145.84** |
| Error | 177 | 6.54 | Error | 177 | 1.85 | ||
| Total | 355 | Total | 355 | ||||
** indicates significantly different at 0.01 probability level.
Table 3.
The original phenotypic data and transformed values of callus induction frequency (CIF) and somatic embryos initiation frequency (SEIF)
| Trait | Transformation | Mean value | Standard error | Skew | Kurtosis |
|---|---|---|---|---|---|
| Callus induction frequency (CIF) | Nt | 0.69 | 0.02 | -0.69 | -0.37 |
| A | 57.81 | 1.10 | -0.21 | -0.28 | |
| L | 1.81 | 0.01 | -1.52 | 2.04 | |
| S | 0.82 | 0.01 | -1.06 | 0.49 | |
| Somatic embryos initiation frequency (SIF) | Nt | 0.41 | 0.01 | 0.29 | -0.51 |
| A | 38.87 | 0.84 | 0.11 | -0.32 | |
| L | 1.55 | 0.02 | -1.02 | 1.21 | |
| S | 0.62 | 0.01 | -0.28 | -0.31 | |
Nt, non-transformed data; A, arcsine transformation; L, logarithmic transformation; S, square root transformation
Fig. 1. Frequency distributions for RILs of two characteristics with non-transformed data and arcsine transformation data.
QTL analysis
QTL for CIF
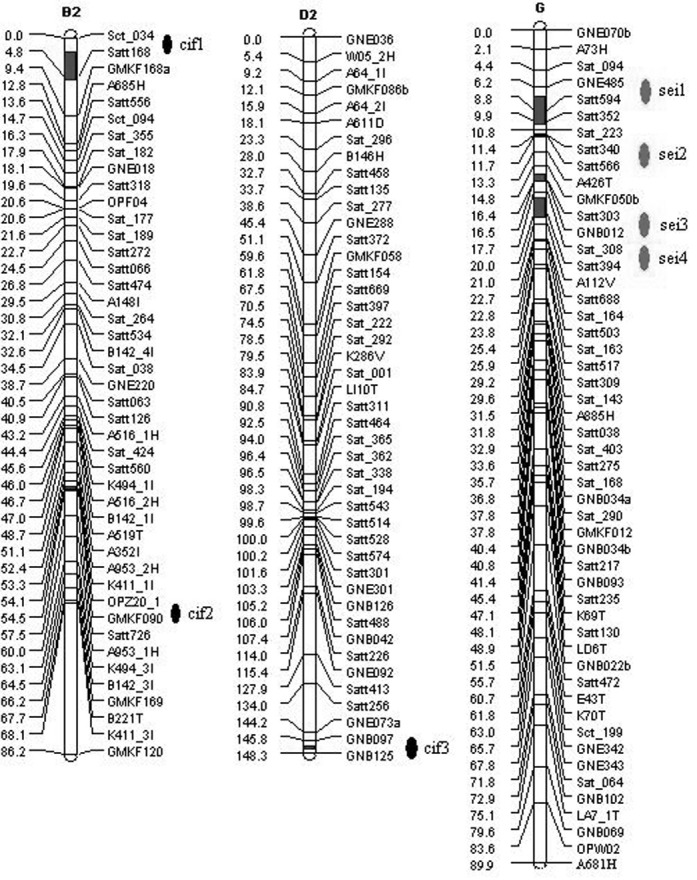
Three putative QTLs related to callus induction frequency (CIF) were detected and named cif1, cif2, cif3 (Table 4). Two QTLs, cif1 and cif2, were located on linkage group B2, and cif3 was on linkage group D2 (Fig. 2). Cif1 was associated with the largest effect, accounting for 16.6% of the total phenotypic variance, and the additive effect of the Kefeng No.1 allele increased callus induction. The other two QTLs, cif2 and cif3, explained 5.84% and 6.28% of the total phenotypic variance, respectively.
Table 4.
QTLs of callus induction frequency (CIF) and somatic embryos initiation frequency (SEIF) detected in NJRIKY using QTL cartographer Version 2.5
| Trait | QTL | Chromosome | Nearest flanking marker | Distance | Position | LOD | Additive effect | Variance explained | Interval |
|---|---|---|---|---|---|---|---|---|---|
| CIF | cif1 | B2 | Sct_034-Satt168 | 4.01-0.82 | 4.01 | 7.69 | 7.7477 | 0.1660 | 1.8-5.3 |
| CIF | cif2 | B2 | OPZ20_1-GMKF090 | 0.01-0.35 | 54.14 | 2.86 | -4.5561 | 0.0584 | 54.0-54.5 |
| CIF | cif3 | D2 | GNB097-GNB125 | 2.01-0.40 | 147.85 | 3.14 | 3.7355 | 0.0628 | 146.9- |
| SEIF | sei1 | G | GNE485-Satt594 | 2.01-0.59 | 8.19 | 2.65 | 3.5926 | 0.0816 | 7.2-10.4 |
| SEIF | sei2 | G | Satt340-Satt566 | 0.01-0.22 | 11.45 | 3.67 | 3.9544 | 0.0823 | 11.3-11.6 |
| SEIF | sei3 | G | Satt303-GNB012 | 0.01-0.02 | 16.46 | 3.31 | 4.4767 | 0.1416 | 15.6-16.5 |
| SEIF | sei4 | G | Sat_308-Satt394 | 2.01-0.31 | 19.67 | 2.95 | 3.6439 | 0.0779 | 18.2-20.4 |
Fig. 2. The detected QTLs on corresponding linkage groups in this experiment.
QTL for SEIF
Interestingly, four putative QTLs identified for somatic embryos initiation frequency (SEIF) (sei1, sei2, sei3 and sei4) were all located on linkage group G. However, these QTLs were characterized by low effects and accounted for similar proportions of the phenotypic variation (7.79-16.6%). The major QTL (sei3) explained 16.6% of the total phenotypic variance and the additive effect of the Kefeng No.1 allele increased somatic embryos initiation by 4.48%. The alleles contributed by Kefeng No.1 also resulted in an increment of SEIF for the other three loci (Table 4, Fig. 2).
DISCUSSION
Both the proper mapping population and high density linkage map are required for the QTL mapping. A RIL population (NJRIKY) derived from parental populations exhibiting signifycant differences in many agricultural traits was investigated in this study. Tests for regeneration ability revealed that both the NJRIKY population and the parental lines differed in callus initiation frequency and somatic embryogenesis induction. The linkage map of NJRIKY contained 834 molecular markers on 24 molecular linkage groups and spanned 2307.83 cM of the soybean genome with an average interval distance of 2.77 cM, the average markers per group of 34.75. In our opinion, the average density of the constructed map makes it a useful tool for preliminary studies aimed at determining approximate QTL locations.
Characteristics of callus induction and somatic embryogenesis ability were used to evaluate the tissue culture response of soybean were used to map QTL related to TCR (tissue culture response) in the NJRIKY population. Both characteristics have commonly been used to map genes for TCR and have been shown to be reliable predictors of the final yield of regenerated plants (Flores Berrios et al., 2000b; He et al., 1998; Manninen et al., 2000). Skewed distributions were observed for all the characteristics analyzed in this study, which was in accordance with previous reports describing mapping of the tissue culture response (Manninen et al., 2000; Mano et al., 2002; Torp et al., 2001). This was predicted on the basis of the known recalcitrance of soybean plant with respect to TCR. Following comparison with other data transformation methods, Bliss transformation was selected to improve the normality of distribution (Table 3).
QTL for tissue culture responses have been mapped for numerous plant species, the majority of which were conducted in economically important monocot plant species such as rice, barley and maize. Although mechanisms of regeneration in dicots have been studied extensively by means of Mendelian genetic studies (Henry et al., 1994), few reports describe QTLs studies of tissue culture capability performed in dicotylodenous plants, such as in tomato, Arabidopsis, sunflower, poplar and broccoli (Bolibok and Rakoczy-Trojanowska, 2006). In this study, callus induction frequency (CIF) and somatic embryos initiation frequency (SEIF) were investigated in terms of the percentages of explants producing calluses and the percentage of explants producing somatic embryos, respectively. With the method of composite interval mapping (CIM) described in Windows QTL Cartographer Version 2.5, 3 quantitative trait loci (QTL) were identified for the frequency of callus induction on chromosomes B2 and D2, accounting for phenotypic variation from 5.84% to 16.60%; 4 QTLs which are all located on chromosome G were revealed for the frequency of somatic embryos initiation and explained the phenotypic variation from 7.79-14.16%. These data indicate that there may be a gene cluster controlling the initiation of soybean somatic embryos on chromosone G. The construction of the public soybean linkage map makes it possible to compare the QTLs of different traits. Panthee et al. (2004) reported that Marker Satt168 (LG B2) was linked to a QTL governing nitrogen accumulation in soybean seeds at the R7 growth stage. In this study, Marker Satt168 (LG B2) was also found to be associated with a QTL controlling the soybean callus initiation (Table 4, Fig. 2), thus indicating that callus induction is related to nitrogen accumulation in soybean seeds.
Taguchi-Shiobara et al. (1997) detected five putative QTLs for the average number of regenerated shoots per callus (NRS) and four putative QTLs for the regeneration rate (RR), using 245 RFLP markers and 98 BC1F5 lines derived from two varieties (Nipponbare and Kasalath). He et al. (1998) studied the genetic basis of anther culturability and identified five QTLs for callus induction frequency on chromosomes 6, 7, 8, 10 and 12. In maize Wan et al. (1992) identified six regions on chromosomes 1, 2, 3, 6 and 8 involved in the embryo-like structures (ELS) or regenerable callus formation. In barley, QTLs controlling callus growth (CG), subsequent shoot differentiation ratio (SD) and green shoot ratio (GS) in immature embryo culture were identified using 99 recombinant inbred lines (RILs) (Mano et al., 2002). The identification of QTLs for the in vitro culture response of winter rye immature embryos and immature inflorescences was also carried out by Bolibok et al. (2007). Numerous studies conducted on genetic control of plant tissue culture responses have shown that tissue culture performance is quantitatively controlled (Taguchi-Shiobara et al., 2006). The results of this study indicated that the inheritance of soybean regeneration ability is also controlled by several major effect QTLs with relatively low phenotypic variance (5.84-16.60%). The objective of QTL mapping of targeted traits is to utilize the detected traits to improve the soybean tissue culture response. An important method to achieve this is the use of molecular markers linked the QTL to enable marker assisted selection and pyramiding of targeted quantitative traits. Meanwhile, the distances between the molecular markers linked to the identified QTLs in the present study are generally short (< 5 cM), and it opens the way to physical mapping, map-based cloning of these QTL regions and the isolation of genes for soybean tissue culture response. Further verification of the QTL detected in this study, in addition to identification of other major QTL or genes governing soybean regeneration ability through cloning and functional analysis will provide relevant information for enhancement of the soybean tissue culture response. Moreover, this information would lead soybean breeders not only to improve soybean tissue culture response, but also to combine these qualities with other valuable traits to achieve the integrated improvement of soybean agronomic traits.
Acknowledgments
The authors thank Drs. D. L. Fu and Kati. Wu for their valuable help in improving the manuscript. This work was supported by the National Key Basic Research Program (2006CB1017, 2009CB1184, and 2010CB1259), the National Hightech R & D Program (2006AA1001 and 2009AA1011) and the MOE 111 Project (B08025).
References
- 1.Armstrong C.L., Romero-Severson J., Hodges T.K. Improved tissue culture response of an elite maize inbred through backcross breeding and identification of chromosomal regions important for regeneration by RFLP analysis. Theor. Appl. Genet. (1992);84:755–762. doi: 10.1007/BF00224181. [DOI] [PubMed] [Google Scholar]
- 2.Bolibok H., Rakoczy-Trojanowska M. Genetic mapping of QTLs for tissue-culture response in plants. Euphytica. (2006);149:73–83. [Google Scholar]
- 3.Bolibok H., Gruszczynska A., Hromada-Judycka A., Rakoczy-Trojanowska M. The identification of QTLs associated with the in vitro response of rye (Secale cereale L.). Cell. Mol. Biol. Lett. (2007);12:523–535. doi: 10.2478/s11658-007-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churchill G., Doerge R.W. Empirical threshold values for quantitative triat mapping. Genetics. (1994);138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores Berrios E.L., Sarrafi A., Fabre F., Alibert G., Gentzbittel L. Genotypic variation and chromosomal location of QTLs for somatic embryogenesis revealed by epidermal layers culture of recombinant inbred lines in the sunflower (Helianthus annuus L.). Theor. Appl. Genet. (2000);101:1307–1312. [Google Scholar]
- 6.Flores Berrios E.L., Gentzbittel H., Kayyal G., Alibert A., Sarrafi A. AFLP mapping of QTLs for in vitro organogenesis traits using recombinant inbred lines in sunflower (Helianthus annuusL.). Theor. Appl. Genet. (2000);101:1299–1306. [Google Scholar]
- 7.Gamborg O.L., Miller R.A., Ojima K. Nutrient requirement of suspension cultures of soybean root cells. Exp. Cell. Res. (1968);50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- 8.He P., Shen L., Lu C., Chen Y., Zhu L. Analysis of quantitative trait loci which contribute to anther culturability in rice (Oryza sativa L.). Mol. Breed. (1998);4:165–172. [Google Scholar]
- 9.Henry Y., Vain P., Buyser J.D. Genetic analysis of in vitro plant tissue culture responses and regeneration capacities. Euphytica. (1994);79:45–58. [Google Scholar]
- 10.Hofmann N., Nelson R.L., Korban S.S. Influence of medium components and pH on somatic embryo induction in three genotypes of soybean. Plant Cell, Tissue and Organ Culture. (2004);77:157–163. [Google Scholar]
- 11.Jia H., Yi D., Yu J., Xue S., Xiang Y., Zhang C., Zhang Z., Zhang L., Ma Z. Mapping QTLs for tissue culture response of mature wheat embryos. Mol. Cells. (2007);23:323–330. [PubMed] [Google Scholar]
- 12.Khalafalla M.M., El-Shemy H.A., Rahman S.M., Teraishi M., Hasegawa H., Terakawa T., Ishimoto M. Efficient production of transgenic soybean (Glycine max [L] Merrill) plants mediated via whisker-supersonic (WSS) method. African J. Biotech. (2006);5:1594–1599. [Google Scholar]
- 13.Komatsuda T., Taguchi-Shiobara F., Oka S., Takaiwa F., Annaka T., Jacobson H.J. Transfer and mapping of the shoot differentiation locus Shd1 in barley chromosome 2. Genome. (1995);38:1009–1014. doi: 10.1139/g95-133. [DOI] [PubMed] [Google Scholar]
- 14.Koornneef M., Bade J., Hanhart C., Horsman K., Schel J., Soppe W., Verkerk R., Zabel P. Characterization and mapping of a gene controlling shoot regeneration in tomato. Plant J. (1993);3:131–141. [Google Scholar]
- 15.Kosambi D.D. The estimation of map distances from recombination values. Ann. Hum. Genet. (1943);12:172–175. [Google Scholar]
- 16.Krakowsky M.D., Lee M., Garay L., Woodman-Clikeman W., Long M.J., Sharopova N., Frame B., Wang K. Quantitative trait loci for callus initiation and totipotency in maize (Zea mays L.). Theor. Appl. Genet. (2006);113:821–830. doi: 10.1007/s00122-006-0334-y. [DOI] [PubMed] [Google Scholar]
- 17.Kwon Y.S., Kim K.M., Eun M.Y., Sohn J.K. QTL mapping and associated marker selection for the efficacy of green plant regeneration in anther culture of rice. Plant Breeding. (2002);12:10–16. [Google Scholar]
- 18.Lazzeri P.A., Hildebrand D.F., Collins G.B. A procedure for plant regeneration from immature cotyledon tissue of soybean. Plant Mol. Biol. Rep. (1985);3:160–167. [Google Scholar]
- 19.Manninen O.M. Associations between anther-culture response and molecular markers on chromosomes 2H, 3H and 4H of barley (Hordeum vulgare L.). Theor. Appl. Genet. (2000);100:57–62. [Google Scholar]
- 20.Mano Y., Komatsuda T. Identification of QTLs controlling tissue-culture traits in barley (Hordeum vulgare L.). Theor. Appl. Genet. (2002);105:708–715. doi: 10.1007/s00122-002-0992-3. [DOI] [PubMed] [Google Scholar]
- 21.Murashige T., Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. (1962);15:473–497. [Google Scholar]
- 22.Murigneux A., Bentollila S., Hardy T., Baud S., Guitton C., Jullien H., Ben Tahar S., Freyssinet G., Beckert M. Genotypic variation of quantitative trait loci controlling in vitro androgenesis in maize. Genome. (1994);37:970–976. doi: 10.1139/g94-137. [DOI] [PubMed] [Google Scholar]
- 23.Panthee D.R., Pantalone V.R., Sams C.E., Saxton A.M., West D.R., Rayford W.E. Genomic regions governing soybean seed nitrogen accumulation. J. Am. Oil Chem. Soc. (2004);81:77–82. [Google Scholar]
- 24.Paz M.M., Shou H., Guo Z., Zhang Z., Banerjee A.K., Wang K. Assessment of conditions affecting Agrobacteriummediated soybean transformation using the cotyledonary node explant. Euphytica. (2004);136:167–179. [Google Scholar]
- 25.Paz M.M., Martinez J.C., Kalvig A.B., Fonger T.M., Wang K. Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium- mediated soybean transformation. Plant Cell Rep. (2006);25:206–213. doi: 10.1007/s00299-005-0048-7. [DOI] [PubMed] [Google Scholar]
- 26.Qi B., Korir P., Zhao T., Yu D., Chen S., Gai J. Mapping quantitative trait loci associated with Aluminum toxin tolerance in NJRIKY recombinant inbred line population of soybean (Glycine max). J. Integr. Plant Biol. (2008);50:1089–1095. doi: 10.1111/j.1744-7909.2008.00682.x. [DOI] [PubMed] [Google Scholar]
- 27.Reichert N.A., Young M.M., Woods A.L. Adventitious organogenic regeneration from soybean genotypes representing nine maturity groups. Plant Cell, Tissue and Organ Culture. (2003);75:273–277. [Google Scholar]
- 28.Sairam R.V., Franklin G., Hassel R., Smith B., Meeker K., Kashikar N., Parani M., Abed D.A., Ismail S., Berry K., et al. A study on the effect of genotypes, plant growth regulators and sugars in promoting plant regeneration via organogenesis from soybean cotyledonary nodal callus. Plant Cell Tissue and Organ Culture. (2003);75:79–85. [Google Scholar]
- 29.Taguchi-Shiobara F., Komatsuda T., Oka S. Comparison of two indices for evaluating regeneration ability in rice (Oryza sativa L.) through a diallel analysis. Theor. Appl. Genet. (1997);94:378–382. [Google Scholar]
- 30.Taguchi-Shiobara F., Yamamoto T., Yano M., Oka S. Mapping QTLs that control the performance of rice tissue culture and evaluation of derived near-isogenic lines. Theor. Appl. Genet. (2006);112:968–976. doi: 10.1007/s00122-005-0200-3. [DOI] [PubMed] [Google Scholar]
- 31.Torp A.M., Hansen A.L., Andersen S.B. Chromosomal regions associated with green plant regeneration in wheat (Triticum aestivum L.) anther culture. Euphytica. (2001);119:377–387. [Google Scholar]
- 32.Van-Ooijen J.W., Voorrips R.E. JOINMAP 3.0, Software for the calculation of genetic linkage maps. Plant Research Int. Wageningen; the Netherlands: (2002). [Google Scholar]
- 33.Wan Y., Rocheford T.R., Widholm J.M. RFLP analysis to identify putative chromosomal regions involved in the anther culture response and callus formation of maize. Theor. Appl. Genet. (1992);85:360–365. doi: 10.1007/BF00222882. [DOI] [PubMed] [Google Scholar]
- 34.Wang S., Basten C.J., Zeng Z.B. Windows QTL Cartographer 2.5. Department of Statistics. North Carolina State; Raleigh: (2006). [Google Scholar]
- 35.Wilcox J.R., Boerma H.R.,, Specht J.E., World distribution and trade of soybean. In Soybeans: Improvement, Production and Uses, Agronomy Monograph 16. 3rd eds. American Society of Agronomy/Crop Science Society of America/Soil Science Society of America; Madison: (2004). pp. 1–13. [Google Scholar]
- 36.Yang C., Zhao T., Yu D., Gai J. Somatic embryogenesis and plant regeneration in Chinese soybean (Glycine max (L.) Merr.) – impacts of mannitol, abscisic acid, and explant age. In vitro cellular & developmental biology - Plant. (2009);45:180–188. [Google Scholar]
- 37.Yu K., Pauls K. Identification of a RAPD marker associated with somatic embryogenesis in alfalfa. Plant Mol. Biol. (1993);22:269–277. doi: 10.1007/BF00014934. [DOI] [PubMed] [Google Scholar]


