Abstract
The generation of various subtypes of neurons and glial cells at the right time and place is crucial for the proper development of the vertebrate CNS. Although the mechanisms and factors for the regulation of neuronal diversity in the CNS have been well studied, the mechanisms regulating the sequential production of neuronal and glial cells from neural precursors remain poorly understood. This study shows that Tcf3, a member of the Lef/Tcf family of proteins, is required to inhibit the premature oligodendroglial fate specification of spinal cord precursors using the transgenic zebrafish, which expresses a dominant repressor form of Tcf3 under the control of a heat-shock inducible promoter. In addition, the data revealed that Tcf3 function in oligodendroglial fate specification is mediated independently of canonical Wnt signaling. Altogether, these results show a novel function for Tcf3 in regulating the timing of oligodendroglial fate specification in the spinal cord.
Keywords: neurogenesis, oligodendrocytes, spinal cord, Tcf3, zebrafish
INTRODUCTION
A fundamental feature of neural development in the vertebrate CNS is that different types of neurons and glia are generated in a precisely regulated temporal sequence: neurons appear first, followed by glial cells such as oligodendrocytes and astrocytes. The specification of motor neurons and oligodendrocytes from the motoneuron precursor (pMN) domain in the ventral spinal cord is one of the most extensively studied examples of the sequential production of neurons and glia. In the developing spinal cord, motor neurons and oligodendrocytes are produced from precursor cells in the ventral spinal cord. These precursor cells express the basic-helix-loop-helix transcription factor, olig2, which is induced by Hedgehog signaling in the pMN domain. Olig2-expressing precursors initially generate motor neurons during neurogenesis, followed by oligodendrocyte progenitor cells (OPCs) once neurogenesis is complete (Lu et al., 2002; Ono et al., 2009; Park et al., 2002b; 2004; Zhou and Anderson, 2002; Zhou et al., 2001). The precise mechanisms underlying the sequential production of motor neurons and oligodendrocytes from common precursors are not well understood, but are thought to include changes in both the intrinsic properties of neural progenitors and their signaling environment.
Canonical Wnt signaling is known to regulate proliferation and patterning in the developing spinal cord (Chesnutt et al., 2004; Ille et al., 2007; Megason and McMahon, 2002; Muroyama et al., 2002; Zechner et al., 2003). In zebrafish, canonical Wnt signaling is required initially for cellular proliferation throughout the entire spinal cord, and later for patterning in the dorsal progenitor domain of the developing spinal cord (Bonner et al., 2008). Interestingly, the regulation of proliferation and patterning by Wnt signaling in the developing spinal cord occurs as separate events mediated by Tcf3 and Tcf7, respectively, members of the Lef/Tcf family of transcription factors (Bonner et al., 2008).
Lef/Tcf transcription factors function as the downstream components of the canonical Wnt signaling pathway and mediate the transcriptional output of Wnt signaling upon binding with β-catenin (Hartmann, 2007; van Noort and Clevers, 2002). Among the Lef/Tcf proteins, Tcf3 functions as a transcriptional repressor, thus the Tcf3 knockout mouse shows phenotypes with increased Wnt function (Merrill et al., 2004). However, several lines of in vivo evidence have shown that Tcf3 acts as a repressor of target genes that are activated not by Wnt signaling, but by other mechanisms (Korinek et al., 1998; Pereira et al., 2006), indicating that Tcf3 function can be regulated by other mechanisms independent of Wnt signaling. A recent study has shown that Wnt-independent Tcf3 function is required to inhibit premature neurogenesis in spinal progenitors by inhibiting Sox4a expression, suggesting that Tcf3 plays a crucial function in regulating the timing of neurogenesis (Gribble et al., 2009). In this study, we show that Tcf3 function is also required for the inhibition of the premature oligodendroglial fate of the spinal precursors, using transgenic zebrafish expressing a dominant repressor form of Tcf3 (ΔTcf3) under the control of a heat-shock inducible promoter. In addition, we found that Tcf3 function in the regulation of gliogenesis is mediated independently of canonical Wnt signaling. Collectively, these results, together with previous reports (Gribble et al., 2009), suggest a novel function for Tcf3 in regulating the timing of neurogenesis and gliogenesis in the spinal cord.
MATERIALS AND METHODS
Fish lines
Wild-type AB, Tg(olig2:egfp)vu12 (Shin et al., 2003), Tg(hsp70: tcf3-gfp) (Lewis et al., 2004), Tg(hsp70:axin2-egfp), and headless (hdl) mutant zebrafish (Kim et al., 2000) were used for this study.
Generation of Tg(hsp70:axin2-egfp) transgenic fish
To construct hsp70:axin2-egfp recombinant DNA, we first amplified a 2.4 kb of zebrafish axin2 cDNA using RT-PCR with mRNA purified from the 24 h old zebrafish embryos, and cloned it into pBluescript II KS plasmid vector. Next, we digested cloned DNA with BamHI and ClaI restriction enzymes, and sub-cloned axin2 cDNA into hsp70-EGFP-pCS2+ vector. To amplify axin2, we used forward (5′-GGATCCGAGCTGCA AGAGCCATGAAT-3′) and reverse (5′-ATCGATGTCCATTCT GTCCACTTTGCC-3′) PCR primers.
Bromodeoxyuridine (BrdU) labeling and immunohistochemistry
Manually-dechorionated embryos were labeled with BrdU (Roche) by incubating them for 20 min on ice in a solution of 10 mM BrdU and 15% DMSO in embryo medium (EM) (15 mM NaCl, 0.5 mM KCl, 1 mM CaCl2, 1 mM MgSO4, 0.15 mM KH2PO4, 0.05 mM NH2PO4, and 0.7 mM NaHCO3) at 24 hpf. The embryos were then placed in EM, incubated for 20 min at 28.5℃, and fixed using 4% paraformaldehyde in PBS. Embryos were processed for immunohistochemistry to detect Sox10 expression, treated for 1 h with 2 M HCl, and were then processed for anti-BrdU immonohistochemistry. For immunohistochemstry, we used the following primary antibodies: a mouse anti-BrdU [G3G4, 1:1,000, Developmental Studies Hybridoma Bank (DSHB), USA], a rabbit anti-Sox10 (1:1000) (Park et al., 2005), a mouse anti-Zrf1 (1:400, University of Oregon Monoclonal Antibody Facility), and a mouse anti-HuC/D antibodies (16A11, 1:20, Molecular Probes).
Heat-induced gene expression
To induce expression of dominant repressor form of Tcf3 (ΔTcf3), we intercrossed Tg(hsp70:tcf3-GFP) adults, raised embryos at 28.5℃, transferred tem to EM at 40℃ for 30 min at the desired stage, and continued incubation at 28.5℃ until appropriate stages for fixing.
TUNEL staining
To detect apoptotic cell death, TUNEL assay was performed using In Situ Cell Death Detection Kit (Roche) according to the manufacturer’s instructions. TUNEL labeling was done with 10mm-thick cryosections.
RESULTS
Forced expression of ΔTcf3 induces Sox10 expression in the neural precursors of the spinal cord
Previously, it has been shown that Tcf3 function is required to inhibit neurogenesis by repressing sox4a expression in the spinal cord of zebrafish embryos. In this study, Tcf3 loss-of-function mediated by a morpholino oligonucleotide targeted against tcf3 (tcf3 MO) caused premature neurogenesis in the progenitors of the spinal cord. Since Tcf3 functions as a transcriptional repressor in the spinal cord, knock-down of tcf3 by an MO induces the expression of several tcf3 target genes including sox4a and sox11, which are normally repressed by Tcf3 (Gribble et al., 2009). These data suggest that constitutive repression of tcf3 target gene expression by a dominant-repressor form of Tcf3 may inhibit normal neurogenesis in the spinal cord. Since gliogenesis normally occurs after neurogenesis, we hypothesized that the inhibition of neurogenesis by the constitutive repression of Tcf3 function may cause premature gliogenesis in spinal progenitors. To test this idea, a transgenic line, Tg(hsp70:tcf3-GFP), was used in which the N-terminal domain of Tcf3 was replaced with green fluorescent protein (GFP), thus eliminating the β-catenin-binding domain and producing a dominant repressor form of Tcf3, called ΔTcf3. This fusion gene was then placed under the control of the zebrafish hsp70 promoter for the conditional expression of ΔTcf3 by heat-shock induction (Lewis et al., 2004).
The expression of Sox10, a marker for the oligodendrocyte lineage, which includes oligodendrocyte progenitor cells (OPCs) (Park et al., 2002a; 2005), was first tested after the induction of ΔTcf3 in Tg(hsp70:tcf3-GFP) embryos. Normally, OPC specification begins at 36 h post fertilization (hpf), and thus there are no Sox10+ cells in the spinal cord of the wild-type embryo at 32 hpf (Fig. 1A) while Sox10 immunoreactivity can be observed at 48 hpf in the wild-type embryo (Fig. 1E). However, in the spinal cord of Tg(hsp70:tcf3-GFP) embryos, heat-shocked at 24 hpf, labeling with an anti-Sox10 antibody and an antibody against Hu, a neuronal marker, at 32 hpf, revealed that ΔTcf3 expression can induce premature expression of Sox10 only in Hu- neural precursor cells near the midline ventricle (Figs. 1B and 1B′). Interestingly, heat-shock induction of ΔTcf3 at the postnatal stage did not induce Sox10 expression in Hu- neural precursors in the spinal cord (Figs. 1C-1D′). At 3.5 days post fertilization (dpf), most of the Sox10+ oligodendrocyte lineage cells were located in the white matter of the spinal cord in wild-type embryos (Fig. 1C, indicated by arrowheads) and in Tg(hsp70: tcf3-GFP) embryos (Fig. 1D, arrowheads), and even though tcf3-GFP was expressed in Hu- precursor cells, Sox10 expression was not observed in the precursor zone (Fig. 1D′, bracketed area). Together, these data indicated that constitutive repression of tcf3 target genes by ΔTcf3 induces premature expression of Sox10, a marker for oligodendrocyte lineage cells, in spinal precursor cells only during embryonic stages of development.
Fig. 1. Expression of ΔTcf3 induces Sox10 expression in spinal cord neural precursors. All images are transverse sections of the spinal cord, dorsal side up. (A-D′) Labeling of wild-type embryos (A, C) and Tg(hsp70:tcf3-GFP) embryos (B, B′, D, and D′) with anti-Hu (blue) and anti-Sox10 antibodies (red) to mark neurons and OPCs, respectively, and with EGFP (green) representing ΔTcf3 expression in Tg(hsp70:tcf3-GFP) embryos (B′, D′). White brackets indicate the Hu- precursor zone near the ventricle of the spinal cord (D, D′). Arrowheads indicate endogenous Sox10+ OPCs (C-D′). (E-F′) Labeling of Tg(olig2:egfp) (E) and Tg(olig2:egfp);Tg(hsp70:tcf3-gfp) embryos (F, F′) with anti-Sox10 antibody (red) and EGFP (green). Arrows indicate endogenous Sox10+olig2+ OPCs, arrowheads indicate ectopically-induced Sox10+ olig2- cells (E-F′). Data were obtained from 20 sections from each of five control and five heat-shocked transgenic embryos for each time point. Scale bar: 20 μm.
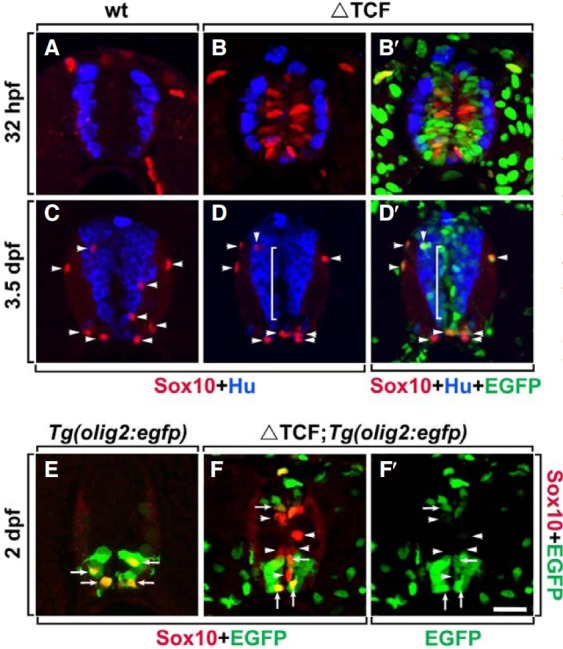
Next, we investigated whether ectopically-induced Sox10+ cells can differentiate into mature oligodendrocytes. To test this, the expression of olig2, a basic-helix-loop-helix transcription factor required for oligodendrocyte development (Park et al., 2002a; 2004), was examined in Sox10+ cells induced by ΔTcf3. Tg(hsp70:tcf3-GFP);Tg(olig2:egfp) zebrafish were generated by mating Tg(hsp70:tcf3-GFP) fish with Tg(olig2:egfp) fish, which express EGFP under the control of the olig2 promoter (Park et al., 2004; Shin et al., 2003). In the spinal cord of Tg(olig2:egfp) embryos, all Sox10+ OPCs expressed olig2-EGFP (Fig. 1E, arrows). However, in Tg(hsp70:tcf3-GFP);Tg(olig2:egfp) embryos, ventrally-located Sox10+ cells were olig2-EGFP+ (indicated by arrows in Figs. 1F and 1F′), but most of the dorsallylocated Sox10+ cells were EGFP- (indicated by arrowheads in Figs. 1F and 1G). These data indicated that Sox10+ cells, ectopically induced by ΔTcf3, did not induce olig2, which is required for oligodendrocyte development. Consistent with these results, cells that expressed ΔTcf3-induced Sox10 failed to express MBP, a marker for mature oligodendrocytes (Brosamle and Halpern, 2002) (data not shown). Together, these results indicated that Sox10+ cells induced by ΔTcf3 failed to generate into mature oligodendrocytes.
Neural precursors stop proliferating and undergo apoptotic cell death in response to ΔTcf3 expression
Since spinal precursor cells, expressing Sox10 induced ectopically by ΔTcf3, failed to differentiate into mature oligodendrocytes, the next step was to investigate the fate of neural precursors following ΔTcf3 expression. To test the proliferation of neural precursors after the induction of ΔTcf3 expression, ΔTcf3 was induced in Tg(hsp70:tcf3-GFP) embryos at 24 hpf, then the embryos were treated with the thymidine analog BrdU, and labeled with an anti-BrdU antibody at 27 hpf, 3 h after ΔTcf3 induction. In wild-type embryos, most of the precursor cells near the ventricle of the spinal cord were BrdU+ proliferating cells (Fig. 2A); however, the spinal cord of ΔTcf3-expressing embryos showed a dramatic reduction in the number of BrdU+ cells (Fig. 2B). Interestingly, although Sox10 immunoreactivity started to appear in a few cells, there were no Sox10+, BrdU+ cells, suggesting that those cells capable of expressing Sox10 stop proliferating before Sox10 is expressed (Fig. 2B). BrdU labeling at 32 hpf, 8 hours after ΔTcf3 induction, revealed only a few BrdU+ cells in ΔTcf3-expressing embryos, while most of the cells in the precursor zone were Sox10+, BrdU-, indicating that ΔTcf3-expressing cells stop proliferating before expressing Sox10 (Figs. 2C and 2D).
Fig. 2. Spinal precursors stop proliferation and undergo apoptotic cell death in response to ΔTcf3 expression. All images are transverse sections of the spinal cord, dorsal side up. (A-D) Double labeling of wild-type (A, C) and Tg(hsp70:tcf3-GFP) embryos (B, D) with anti-BrdU (green) and anti-Sox10 (red) antibodies. (E, F) Double labeling of wild-type (E) and Tg (hsp70:tcf3-GFP) embryos (F) with anti-Zrf-1 (green) and anti-Sox10 (red) antibodies. (G, H) TUNEL staining (red) of wild-type (G) and Tg(hsp70:tcf3-GFP) embryos (H) to detect apoptotic cell death. EGFP (green) indicates ΔTcf3 expression in response to heat-shock (H). Arrowheads indicate TUNEL+ cells (G, H). Data were obtained from 20 sections from each of five control and five heat-shocked transgenic embryos for each time point. Scale bar: 20 μm.
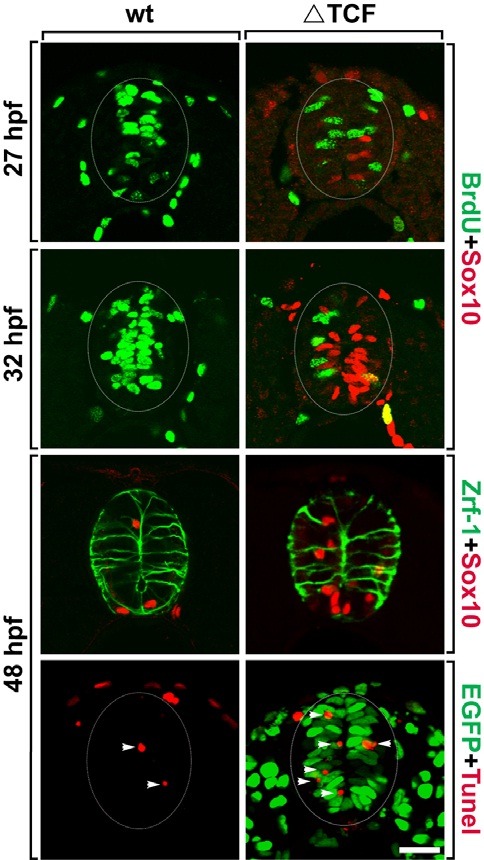
Next, we tested whether ΔTcf3 expression affected radial glial precursors using a Zrf-1 antibody, a marker for radial glial processes. In wild-type embryos, Zrf-1+ radial glial processes extended in an apical to basal orientation and approximately four Sox10+ OPCs per section were visible at 48 hpf (Fig. 2E). The ΔTcf3-expressing embryos had increased numbers of Sox10+ cells but showed the expected number of radial glial processes with normal morphology, indicating that ΔTcf3 expression does not affect radial glial precursor cells (Fig. 2F). Since ΔTcf3-expressing cells stopped proliferating and expressed Sox10, but failed to differentiate into mature oligodendrocytes, a TUNEL staining assay was performed to test whether ΔTcf3-expressing precursor cells undergo apoptotic cell death. Only one or two TUNEL+ cells were visible per section of the spinal cord in wild-type embryos (Fig. 2G), while ΔTcf3-expressing embryos had about three times the number of TUNEL+ cells in the precursor zone of the spinal cord, indicating that precursors, which express Sox10 in response to ΔTcf3, fail to differentiate into oligodendrocytes and finally undergo apoptotic cell death (Fig. 2H).
ΔTcf3-induced Sox10 expression is independent of Wnt signaling
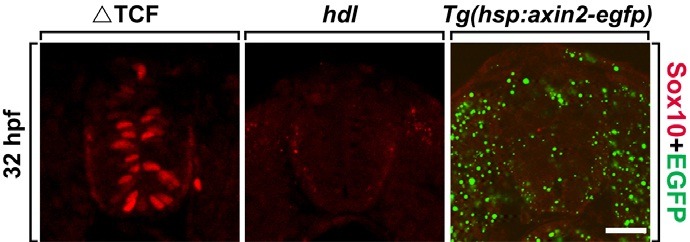
Tcf3 is thought to be responsible for mediating the transcriptional output of canonical Wnt signaling by acting as a transcriptional activator upon binding with stabilized β-catenin. Since ΔTcf3 functions as a dominant repressor form of Tcf3 and is known to inhibit canonical Wnt signaling (Lewis et al., 2004), next we investigated whether the induction of Sox10 expression by ΔTcf3 is due to an alteration in Wnt signaling. To test this idea, Sox10 expression was first investigated in the headless (hdl) mutant, which has a mutation in the tcf3 gene and shows a Tcf3 loss-of-function phenotype (Kim et al., 2000). Consistent with this phenotype, no Sox10+ cells were seen in the spinal cord of the hdl mutant embryos (Fig. 3B). Finally, we tested whether the inhibition of Wnt signaling could induce Sox10 expression in the spinal cord by generating Tg(hsp: axin2-egfp) zebrafish that expressed Axin2, an inhibitor of Wnt signaling, fused with EGFP under the control of the zebrafish heat-shock 70 promoter. Thus, the inducible expression of axin2 meant that Wnt signaling could be inhibited conditionally. However, heatshock induction of axin2 in the Tg(hsp:axin2-egfp) embryo failed to induce premature Sox10 expression in the spinal cord, suggesting that the induction of premature Sox10 expression by ΔTcf3 occurs independently of Wnt signaling (Fig. 3C).
Fig. 3. Modulation of Wnt signaling does not induce Sox10 expression. All images are transverse sections of the spinal cord, dorsal-side up. (A, B) Tg(hsp70:tcf3-GFP) embryo (A) and headless mutant embryo (B) labeled with anti-Sox10 antibody (red). (C) Labeling of Tg(hsp:axin2-egfp) embryo with anti- Sox10 antibody (red). EGFP (green) expression represents Axin2-EGFP expression. Scale bar: 20 μm.
DISCUSSION
Previous study has shown that loss of Tcf3 function in response to a Tcf3 MO induced premature neurogenesis in zebrafish spinal precursors (Gribble et al., 2009). Since Tcf3 functions as a transcriptional repressor, Tcf3 loss-of-function causes unregulated constitutive activation of Tcf3 target genes, including Sox4a. Therefore, these data indicate that correct regulation of target gene expression by Tcf3 is required to regulate the timing of neurogenesis such that neurons are generated at the appropriate time during embryogenesis. The present study has demonstrated that expression of a dominant repressor form of Tcf3 (ΔTcf3) induced the expression of Sox10, a marker for oligodendrocyte lineage cells, in the neural precursors of zebrafish spinal cord (Figs. 1A-1B′). The ΔTcf3-expressing spinal precursors then stopped proliferating (Figs. 2A and 2B) and, instead of differentiating into neurons, exhibited an oligodendroglial character by expressing Sox10. Consistent with the function of ΔTcf3 as a dominant repressor form of Tcf3, ΔTcf3 expression constitutively inhibited the expression of Tcf3-target genes required for neurogenesis, including Sox4a (Gribble et al., 2009). These data suggested that, in addition to the regulation of the timing of neurogenesis, the correct regulation of Tcf3 function is also required for the inhibition of oligodendroglial fate specification and for the generation of glial cells at the appropriate time during CNS development.
Although spinal precursor cells expressed Sox10 in response to ΔTcf3, Sox10+ cells failed to differentiate into mature oligodendrocytes (Fig. 2). Previously, it has been shown that olig2-expressing neural precursor cells in the pMN domain of the ventral spinal cord sequentially generate motor neurons and oligodendrocytes. In particular, pMN precursor cells first generate motor neurons during neurogenesis, and later produce OPCs after motor neuron formation is complete (Lu et al., 2002; Park et al., 2002b; Zhou and Anderson, 2002). Interestingly, it has been shown that a change from neurogenesis to oligodendrogenesis is mediated by an increase in the concentration of Shh, indicating that the modulation of Shh concentration is crucial for the switch of spinal precursor cells from the motor neuron to OPC fate (Danesin et al., 2006). Therefore, we hypothesized that the failure of ΔTcf3-induced Sox10+ cells to differentiate into mature oligodendrocytes is due to a deficiency in the amount of Shh. Consequently, the lack of a differentiation factor might cause premature Sox10+ cells to undergo apoptotic cell death (Figs. 2G and 2H).
In canonical Wnt signaling, Tcf3 functions as a repressor of Wnt target gene expression and as an activator upon binding with β-catenin. The expression of ΔTcf3 inhibited Wnt signaling since the β-catenin-binding site of ΔTcf3, which is required to change the transcriptional repressor form of Tcf3 into an activator, was replaced with GFP (Lewis et al., 2004). A previous study demonstrated that Wnt signaling, mediated by Tcf3, regulated the proliferation but not patterning of spinal precursor cells (Bonner et al., 2008). Consistent with this function of Tcf3 in Wnt signaling, in the current study, the number of BrdU+ cells decreased dramatically in response to ΔTcf3 (Figs. 2A and 2B), implying that ΔTcf3 expression inhibited spinal precursor proliferation. However, the inhibition of Wnt signaling by Axin2 did not induce Sox10 expression in spinal cord precursors (Fig. 3C), suggesting that the induction of Sox10 by ΔTcf3 occurs independently of canonical Wnt signaling. These results are consistent with a previous report demonstrating that Tcf3 function in the inhibition of spinal cord neurogenesis was mediated independently of canonical Wnt signaling (Gribble et al., 2009). Collectively, these results show a novel function for Tcf3 in regulating the timing of oligodendroglial fate specification in the spinal cord.
Acknowledgments
We thank Richard Dorsky for generously providing reagents and fish. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0075120).
References
- 1.Bonner J., Gribble S.L., Veien E.S., Nikolaus O.B., Weidinger G., Dorsky R.I. Proliferation and patterning are mediated independently in the dorsal spinal cord downstream of canonical Wnt signaling. Dev. Biol. (2008);313:398–407. doi: 10.1016/j.ydbio.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brosamle C., Halpern M.E. Characterization of myelination in the developing zebrafish. Glia. (2002);39:47–57. doi: 10.1002/glia.10088. [DOI] [PubMed] [Google Scholar]
- 3.Chesnutt C., Burrus L.W., Brown A.M., Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev. Biol. (2004);274:334–347. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Danesin C., Agius E., Escalas N., Ai X., Emerson C., Cochard P., Soula C. Ventral neural progenitors switch toward an oligodendroglial fate in response to increased Sonic hedgehog (Shh) activity: involvement of Sulfatase 1 in modulating Shh signaling in the ventral spinal cord. J. Neurosci. (2006);26:5037–5048. doi: 10.1523/JNEUROSCI.0715-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gribble S.L., Kim H.S., Bonner J., Wang X., Dorsky R.I. Tcf3 inhibits spinal cord neurogenesis by regulating sox4a expression. Development. (2009);136:781–789. doi: 10.1242/dev.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartmann C. Skeletal development--Wnts are in control. Mol. Cells. (2007);24:177–184. [PubMed] [Google Scholar]
- 7.Ille F., Atanasoski S., Falk S., Ittner L.M., Marki D., Buchmann- Moller S., Wurdak H., Suter U., Taketo M.M., Sommer L. Wnt/BMP signal integration regulates the balance between proliferation and differentiation of neuroepithelial cells in the dorsal spinal cord. Dev. Biol. (2007);304:394–408. doi: 10.1016/j.ydbio.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 8.Kim C.H., Oda T., Itoh M., Jiang D., Artinger K.B., Chandrasekharappa S.C., Driever W., Chitnis A.B. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. (2000);407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korinek V., Barker N., Willert K., Molenaar M., Roose J., Wagenaar G., Markman M., Lamers W., Destree O., Clevers H. Two members of the Tcf family implicated in Wnt/ beta-catenin signaling during embryogenesis in the mouse. Mol. Cell. Biol. (1998);18:1248–1256. doi: 10.1128/mcb.18.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis J.L., Bonner J., Modrell M., Ragland J.W., Moon R.T., Dorsky R.I., Raible D.W. Reiterated Wnt signaling during zebrafish neural crest development. Development. (2004);131:1299–1308. doi: 10.1242/dev.01007. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q.R., Sun T., Zhu Z., Ma N., Garcia M., Stiles C.D., Rowitch D.H. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. (2002);109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 12.Megason S.G., McMahon A.P. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. (2002);129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- 13.Merrill B.J., Pasolli H.A., Polak L., Rendl M., Garcia-Garcia M.J., Anderson K.V., Fuchs E. Tcf3: a transcriptional regulator of axis induction in the early embryo. Development. (2004);131:263–274. doi: 10.1242/dev.00935. [DOI] [PubMed] [Google Scholar]
- 14.Muroyama Y., Fujihara M., Ikeya M., Kondoh H., Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. (2002);16:548–553. doi: 10.1101/gad.937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono K., Takebayashi H., Ikenaka K. Olig2 transcription factor in the developing and injured forebrain; cell lineage and glial development. Mol. Cells. (2009);27:397–401. doi: 10.1007/s10059-009-0067-2. [DOI] [PubMed] [Google Scholar]
- 16.Park H., Mehta A., Richardson J.S., Appel B. olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev. Biol. (2002a);248:356–368. doi: 10.1006/dbio.2002.0738. [DOI] [PubMed] [Google Scholar]
- 17.Park H.C., Mehta A., Richardson J.S., Appel B. olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev. Biol. (2002b);248:356–368. doi: 10.1006/dbio.2002.0738. [DOI] [PubMed] [Google Scholar]
- 18.Park H.C., Shin J., Appel B. Spatial and temporal regulation of ventral spinal cord precursor specification by Hedgehog signaling. Development. (2004);131:5959–5969. doi: 10.1242/dev.01456. [DOI] [PubMed] [Google Scholar]
- 19.Park H.C., Boyce J., Shin J., Appel B. Oligodendrocyte specification in zebrafish requires notch-regulated cyclindependent kinase inhibitor function. J. Neurosci. (2005);25:6836–6844. doi: 10.1523/JNEUROSCI.0981-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira L., Yi F., Merrill B.J. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell selfrenewal. Mol. Cell. Biol. (2006);26:7479–7491. doi: 10.1128/MCB.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin J., Park H.C., Topczewska J.M., Mawdsley D.J., Appel B. Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci. (2003);25:7–14. doi: 10.1023/B:MICS.0000006847.09037.3a. [DOI] [PubMed] [Google Scholar]
- 22.van Noort M., Clevers H. TCF transcription factors, mediators of Wnt-signaling in development and cancer. Dev. Biol. (2002);244:1–8. doi: 10.1006/dbio.2001.0566. [DOI] [PubMed] [Google Scholar]
- 23.Zechner D., Fujita Y., Hulsken J., Muller T., Walther I., Taketo M.M., Crenshaw E.B., 3rd, Birchmeier W., Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev. Biol. (2003);258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Q., Anderson D.J. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. (2002);109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Q., Choi G., Anderson D.J. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. (2001);31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]


