Abstract
The Octamer 4 gene (Oct4) is a master pluripotency controller that has been detected in several types of tumors. Here, we examine the expression of Oct4 in human esophageal squamous cell carcinoma (ESCC). We found that punctate Oct4 protein was expressed in most (93.7%) ESCC samples but it was not observed in esophageal mucosa. Some ESCC cells had the capacity to form tumorospheres; those with an Oct4+-rich cell phenotype had increased proliferation and Oct4 mRNA levels compared to those of differentiated cells in culture or xenograft tumors. The over-expression of Oct4 in ESCCs suggests that it is a potential target for ESCC therapy. Oct4 could be a useful tumor marker in an immunohistochemical panel designed to differentiate between ESCC and esophageal mucosa. Expression of Oct4 in tumorospheres might indicate the presence of a population of ECSCs and its expression in xenograft tumors suggests that Oct4 is also associated with tumor metastasis.
Keywords: esophageal cancer stem cell, esophageal squamous cell carcinoma, Nanog, Oct4, Sox2
INTRODUCTION
Esophageal cancer (EC) is a lethal cancer with a poor prognosis (Jemal et al., 2008); the survival rate is only 15% at 5 years and remains less than 20-30% at 2 years even after surgical resection (Jemal et al., 2008). Squamous cell adenocarcinoma is the most common type of malignant tumor in the esophagus. The prognosis for patients with esophageal squamous cell cancer (ESCC), which remains the most frequent cause of cancer-related deaths (Enzinger and Mayer, 2003), is poor because of the high rate of local and distant metastases at the time of diagnosis. It has been suggested that only a fraction of tumor cells are responsible for tumor regrowth. Cancer cells are clonal in origin and might undergo processes similar to the self-renewal and differentiation of normal stem cells (Reya et al., 2001). Multipotent cancer stem cells (CSC) might explain the histologic heterogeneity found in tumors (Gao, 2008). Zhang et al. found esophageal cancer stem-like cells (CSLC) after establishing radioresistant esophageal carcinoma cell lines (Zhang et al., 2008) that expressed Octamer 4 (Oct4). This finding prompted us to examine Oct4 expression in ESCC tissues.
Oct4 is a member of the family of POU-domain transcription factors and its expression is normally confined to pluripotent cells of the developing embryo (Nichols et al., 1998). However, Oct4 has been shown to be a master pluripotency controller that is expressed in neoplastic germ cells with pluripotent potential (Cheng et al., 2007; Looijenga et al., 2003), including seminomas (Ezeh et al., 2005; Jones et al., 2004), embryonal carcinomas (Jones et al., 2004) and dysgerminomas (Cheng et al., 2004). In adults, Oct4 protein expression in tumors has been observed in bladder cancer (Atlasi et al., 2007), breast carcinoma (Ezeh et al., 2005), oral squamous cell carcinoma (Chiou et al., 2008) and gastric cancer (Chen et al., 2009). Knockdown of Oct4 has been shown to arrest proliferation, resulting in the apoptosis of CSLCs (Hu et al., 2008). With respect to the new CSC theory, the expression of such genes is potentially correlated with tumorigenesis and might affect some aspects of tumor behavior, such as tumor recurrence or resistance to therapies; therefore, we investigated the expression of Oct4 in ESCC and its potential role in ESCC therapy.
MATERIALS AND METHODS
Patients
This study was approved by the Medical Ethics Committee of Hubei Medical University. A total of 174 Chinese patients (95 males aged 42 ± 12 years; 69 females aged 45 ± 11 years; the age range of all patients 35-76 years) were enrolled in the study. All patients provided informed written consent.
Tissue array samples
ESCC and esophageal mucosa (EM) array slides containing formalin-fixed, paraffin-embedded tissues were purchased from Shaanxi Chaoyin Biological Company (China). Each slide contained 153 tissue specimens obtained before any treatment of patients who underwent esophagectomy. The main indications were ESCC (stage I, n = 65; stage IIA, n = 29; stage IIB, n = 17; stage III, n = 9; stage IV, n = 7; total, n = 127) and EM (n = 26). The clinical staging was done according to the tumor-node metastasis classification system.
Tumorosphere culture
ESCC fresh tissues were obtained within 30 min after surgical resection (stage I, n = 14; stageIIA, n = 4; stageIIB, n = 3; total n = 21), immediately washed in PBS containing 500 U/L penicillin G (Gibco, USA) and 500 mg/L streptomycin (Gibco, USA) to remove blood cells, cut with scissors into small pieces and digested overnight in DMEM/F12 supplemented with 0.5 mg/ml collagenase IV (Gibco, USA). The unsorted cells were diluted with serum-free medium (SFM; a mixture of DMEM-F12 containing 10 ng/ml fibroblasts and 20 ng/ml epidermal growth factors (Gibco, USA), 5 ng/ml insulin (Gibco, USA), 2.75 mg/ml transferrin (Gibco, USA), 2.75 ng/ml selenium (Insulin-Transferrin- Selenium solution, Gibco, USA), 105 U/L penicillin and 100 mg/L streptomycin). Cells were plated at a density of 5 × 105 live cells/100 mm plate at 37℃ in a humidified 5% CO2 atmosphere. Tumorospheres were dissociated every 7-10 days by incubation in nonenzymatic cell dissociation solution (Sigma, USA) for 2 min at 37℃ and passaged at 1 × 103 cell/plate. Tumorosphere cells were induced to differentiate in stem cell medium by adding fetal calf serum (FCS, 10% v/v).
Immunohistochemical staining
Tissue samples were fixed in phosphate-buffered 10% formalin (pH 7.2), embedded in paraffin and 4 μm thick sections were cut. Tissue microarray sections were dewaxed in xylene and dehydrated in alcohol. Antigen retrieval was done by heating samples to 100℃ for 10 min in 0.01 M sodium citrate buffer (pH 6.0). After three rinses in PBS (5 min each), the sections were immersed in 3% H2O2 for 30 min to suppress endogenous peroxidase activity. After rinsing in PBS, the sections were incubated with normal mouse serum at 37℃ for 15 min to block nonspecific antibody binding, followed by incubation with mouse monoclonal anti-human Nanog antibody (1:100 in PBS, Santa Cruz Biotechnology, USA) for 2 h at room temperature. After three washes in PBS (5 min each), the sections were incubated with biotinylated secondary antibody (Santa Cruz Biotechnology, USA) and then rinsed with PBS. After incubation (1 h at room temperature) in a solution containing streptavidin- HRP (Kangwei Century Biotechnology, China) and rinsing in PBS, bound antibodies were visualized by reaction with 3,3′- diaminobenzidine (DAB). The tissues were counterstained with hematoxylin. Staining of Sox2 (Santa Cruz Biotechnology, USA) and Oct4 (Santa Cruz Biotechnology, USA) was done as described above.
Tumorosphere cells and tumorosphere-derived differentiated cells in cocultures were seeded on poly-D-lysine/laminin-coated coverslips (BD Biosciences, USA). The tumor cells were fixed in 4% paraformaldehyde in PBS for 6 min at room temperature, followed by two brief rinses with PBS. Fixed cells were permeabilized by incubation (room temperature) in 0.02% (v/v) Triton X-100 in PBS for 6 min. The cells were then briefly washed three times with PBS and stained using the antibodies and technique described above for tissue sections, the number of Nanog-positive cells in 300 cells were counted under light microscopy.
Semiquantitative reverse transcription PCR of Nanog, Oct4 and Sox2
Total RNA of tumorospheres and its differentiated cells or xenograft tumors was purified with TRIzol reagent (Invitrogen, USA) and reverse transcribed with a reverse transcription kit (Invitrogen, USA). We used 2 μl of each reaction for PCR in 1 μl of sense and antisense primers (Table 1), and 1 μl of β-actin primer, 2.5 μl of 25 mmol/L MgCl2, 1 μl of deoxynucleotide triphosphates (100 mM), 2.5 μl of 10× PCR buffer, 5 units of Taq DNA polymerase and 14 μl of double-distilled water in a 25 μl reaction volume. The cycling protocol was: 4 min at 94℃; 20 cycles of 94℃ for 50 s, 59℃ for 1 min, 72℃ for 50 s; and a final extension step at 72℃ for 10 min. PCR products (1 μl) were electrophoresed in 1.5% (w/v) agarose gels and the gray scale ratio of PCR products was calculated.
Table 1.
PCR primers and products
| Symbol | Primers | Products (bp) |
|---|---|---|
| Nanog | Sence: TCAGGCCCACAAATCACAGGCATAG | 193 |
| Antisence: CTATGCCTGTGATTTGTGGGCCTGA | ||
| Oct4 | Sence: AGCAACTCCGATGGGGCCTCC | 194 |
| Antisence: GCCCCACATCGGCCTGTG | ||
| Sox2 | Sence: TGGAAACTTTTGTCGGAGACG | 193 |
| Antisence: CCCCGCTCGCCATGCTAT | ||
| β-actin | Sence: CCAGAGCAAGAGAGGCATCC | 437 |
| Antisence:CCGTGGTGGTGAAGCTGTAG | ||
Tumorosphere and xenograft tumor proliferation assays
Tumorosphere cells or cells from digested xenograft tumors were plated at a density of 1,000 cells/well in 96-well microwell plates in 0.1 ml of SFM supplemented with growth factors. Cell proliferation assays were done at 24 h post-plating using the 3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetr-azolium bromide-based Colorimetric Assay Cell Proliferation Kit 1 (Roche, USA). Quantification of viable cells was done by measurement of UV absorption at 575 nm with a Versamax microplate reader.
Implantation of tumorosphere and tumorosphere-derived differentiated cells into nude mice
Tumorospheres and tumorosphere-derived differentiated cells from 21 ESCC patients were dissociated in nonenzymatic cell dissociation solution (Sigma) then washed with serum-free Hank’s Balanced Salt Solution (HBSS). The cells were then suspended in a 1:1 (v/v) mixture of serum-free DMEM/F12 and Matrigel™, followed by subcutaneous injection of 1 × 105 cells into the right (differentiated cells) or left (tumorosphere cells) mid-abdominal area using a 23-gauge needle. Animals underwent autopsy at 28 days post-cell implantation and a straight ruler was used to assess tumor growth. The tumor volume (V) was calculated as:
V = 0.5 (L × W2)
where L is length and W is width. Visible tumors were excised for histological examination. Harvested tissues were fixed in phosphate-buffered 10% formalin (pH 7.2), embedded in paraffin, cut into 4 μm thick sections and stained with Nanog-specific antibodies as described above.
Cancer cell apoptosis assay
For quantitative analysis of apoptosis, xenograft tumors were trypsinized as described above, washed once in ice-cold PBS, and at least 400 cells were incubated with annexin-V-fluoroescein/ PI (Boehringer Mannheim, USA) in calcium-containing Hepes buffer (pH 7.0); then analyzed immediately with a FACScan machine (BD, USA).
Statistical analysis
We used independent-sample t-test to identify significant changes in transcription factor expression between the ESCC and EM samples. We used the χ2 test determine whether there was significant variation in the frequency of transcription factor expression between the ESCC and EM samples. The level of statistically significant difference was set at P ≤ 0.05. SPSS software for PC (version 13.0 for Windows, SPSS, Inc., USA) was used for all statistical analysis.
RESULTS
Oct4 protein expression in ESCC and esophageal mucosa tissue array
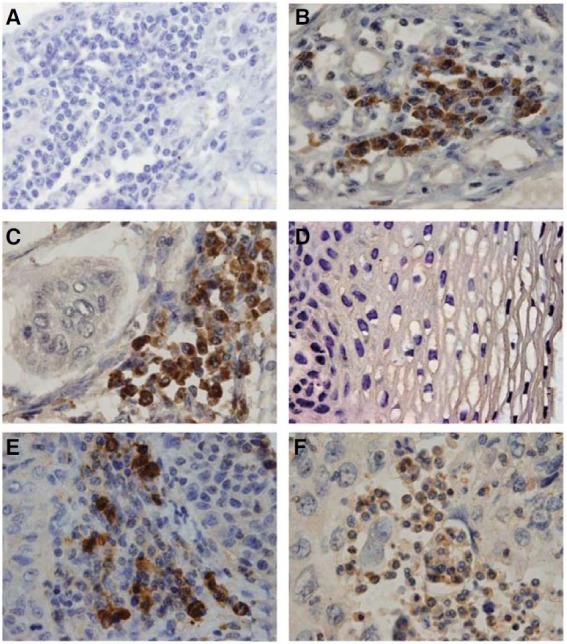
Immunohistochemical staining of Oct4 was done on a tissue array containing ESCC samples. We found that most (93.7%) ESCC samples expressed Oct4 in the tissue array. Although punctate Oct4 expression was observed in cells within the cancer nests, Oct4 was not detected in every cancer cell. Oct4 was located in both the cell cytoplasm and nucleus (Fig. 1C). Oct4 was not detected in the esophageal mucosa (Fig. 1D) (P < 0.001, comparison with ESCC). Sox2 (74.8%) and Nanog (71.6%) were also expressed in ESCCs, with an expression pattern similar to that of Oct4 (Figs. 1E and 1F).
Fig. 1. Immunohistochemical analysis of Oct4, Nanog and Sox2 expression in ESCC and esophageal mucosa. (A) An ESCC tissue section stained with an irrelevant antibody used as a negative control. (B) Oral squamous cell carcinoma stained with antihuman Oct4 used as a positive control. (C) Oct4 is expressed in ESCCs. (D) Oct4 is not expressed in esophageal mucosa. (E) Nanog expression in a representative ESCC sample. (F) Sox2 expression in a representative ESCC sample. Magnification 1000×.
Tumorosphere formation of cancer cells in ESCC
Single-cell suspensions of ESCC cells were prepared in non-serum medium discription before, then cultured in stem cell medium at cloning density for 15 days. We found that a few ESCC cells formed individual colonies in the stem cell medium and that all of the tissues contained these cells. Some of the cell colonies were comprised of small, densely packed cells of high proliferative potential, possibly initiated by the ESCC stem/progenitor cells (Fig. 2A). The more frequent, small colonies were composed of large, loosely arranged cells of low proliferative potential (Fig. 2A) initiated by more mature cells, which we propose were transit amplifying cells (Fig. 2A). Immunohistochemical analysis of the dissociated tumorospheres showed that Oct4 was expressed in the nucleus and cytoplasm of some of the tumorosphere cells (Fig. 2B). Differentiated cells within the tumorosphere were obtained by addition of serum to the stem cell medium after nonadherent tumorospheres were detached from the coverslips (Fig. 2C). As shown in Fig. 2D, only a few of the “differentiated” tumor cells from the tumorospheres expressed Oct4. The tumorospheres were found to have a higher level of proliferation (P = 0.014, Fig. 2E) and expressed higher levels of the mRNA of stem cell markers Oct4, Nanog and Sox2 compared to that of differentiated cells (P = 0.019, Fig. 2F).
Fig. 2. Tumorosphere formation of ESCCs and Oct4 expression within the tumorosphere and its differentiated cells. (A) Tumorosphere formation. (B) Oct4 expression in the tumor cells derived from the tumorosphere. (C) Differentiated cells derived from the tumorosphere. (D) Oct4 expression in the differentiated cells. Magnification 400×. Oct4 expression in representative differentiated cells derived from the tumorospheres. Magnification 400×. (E) Comparison of the proliferative potential between the tumorosphere cells (TM) and differentiated cells (DC) derived from the tumorosphere (independent-sample t-test). (F) Nanog, Oct4 and Sox2 mRNA were detected in tumorosphere cells and differentiated cells by RT-PCR. Control: without primers (independent-sample t-test).

The tumorigenic cancer cell population generated the phenotypic diversity of the initial tumor
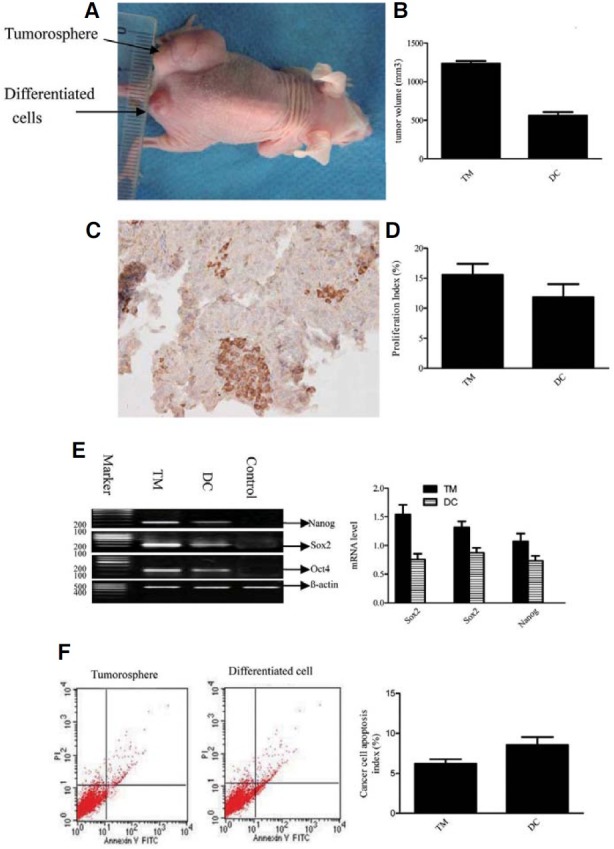
To determine whether tumorospheres have highly tumorigenic properties, the tumorospheres were dissociated, then injected into nude mice and the resultant tumors were analyzed. All of the tumorospheres derived from 21 ESCC patient samples formed tumors in mice, whereas only five of the 21 differentiated cells derived from the tumorospheres (P < 0.001, Fig. 3A) formed small tumors in mice (P = 0.028, Fig. 3B). The pattern of Oct4 expression evident in the secondary tumors (Fig. 3C) was similar to that observed in the patients’ tumors. The tumorospheres consisting of Oct4+-rich cells were highly tumorigenic compared to its differentiated cells (P = 0.018, Fig. 3D). Furthermore, the xenograft tumors showed high levels of proliferation (P = 0.021, Fig. 3D). Oct4, Nanog and Sox2 mRNA expression was observed in the xenograft tumors derived from the tumorospheres with higher levels of expression than those observed in the xenograft tumors derived from the differentiated cells (P = 0.020, Fig. 3E). The levels of apoptosis observed in the tumor cells were significantly lower than those in the differentiated cells (P = 0.013, Fig. 3F).
Fig. 3. Tumor formation in nude mice injected with dissociated tumorosphere cells or differentiated cells derived from the tumorosphere. (A) A representative experiment depicting large tumor formation in a mouse at the injection site of the tumorosphere, and small tumor formation at the injection site of its differentiated cells. (B) Comparison of the volume of tumors derived from tumorosphere cells (TM) or its differentiated cells (DC). (C) Expression of Oct4 in xenograft tumors derived from tumorosphere cells (independent-sample t-test). Magnification 400×. (D) Comparison of the proliferative potential of xenograft tumors derived from tumorosphere cells (TM) or its differentiated cells (DC) (independent- sample t-test). (E) Nanog, Oct4 and Sox2 mRNA were detected in xenograft tumors derived from tumorosphere cells (TM) or its differentiated cells (DC). (F) Comparison of apoptosis in xenograft tumors derived from tumorosphere cells (TM) or its differentiated cells (DC) (independent-sample t-test).
DISCUSSION
Recently, a number of studies have reported that a few cancer cells within tumors have stem cell properties. These CSCs, which have been isolated from brain tumor (Singh et al., 2004), breast cancer (Al-Hajj et al., 2003), pancreatic cancer (Li et al., 2007) and prostate cancer (Collins et al., 2005), have been proposed to be the cancer initiating cells that are responsible for tumorigenesis and lead to cancer resistance in tumors (Ebben et al., 2010; Sell, 2010). Thus, CSCs are an ideal target for cancer therapy. However, the identification of CSCs in tumors has been limited by the lack of accessibility of cells within solid tumors, the absence of functional assays suitable for detecting and quantifying normal stem cells present in many organs and the lack of identification of cell surface markers required to isolate CSCs.
Self-renewal, an undifferentiated state and the ability to differentiate into heterogeneous mature cell types are the hallmarks of stem/progenitor cells (Morrison et al., 1997). Nanog, Sox2 and Oct3/4, transcription factors that form a core regulatory network that determines ESC self-renewal and differentiation, are recognized as stem cell markers (Okumura-Nakanishi et al., 2005). Nanog expression has been observed in human breast CSLCs, suggesting that its expression is involved in self-renewal and tumorigenesis via activation of downstream target genes (Ezeh et al., 2005). Sex-determining region Y (SRY)-box 2, Sox2, is a member of a superfamily of proteins that all possess a high mobility group (HMG) box DNA-binding domain (Adachi et al., 2010). Sox2 was recently identified as a novel major oncogene, recurrently amplified and activated in squamous cell carcinoma (Hussenet and du Manoir, 2010). Upregulation of Sox2 has been demonstrated in early pancreatic cancer lesions (Prasad et al., 2005) and in the human gastrointestinal tract (Long and Hornick, 2009). Sox2 has been associated with an immature phenotype in central nervous system teratomas (Phi et al., 2007) and glioblastomas (Schmitz et al., 2007). Furthermore, Sox2 knockdown arrested proliferation, resulting in a loss of tumorigenicity of glioblastoma CSCs (Gangemi et al., 2009). In summary, these data suggest that Oct4, Nanog and Sox2 have roles in carcinogenesis.
In this study, we examined the expression of Oct4, Nanog and Sox2 in ESCC biopsies as well as in tumor-derived cell cultures. Immunohistochemical staining of Oct4 in tumor tissue sections of ESCC revealed clear staining of Oct4 in most of the tumor sections analyzed, although expression of the stem cell markers was detected in only a few cancer cells within each section. Using a novel cell culture approach, we successfully cultured primary cell cultures derived from clinical samples of ESCCs. We found that a few of the cancer cells formed tumorospheres, which expressed high levels of Oct4, Sox2 and Nanog. Moreover, Oct4 was expressed in some of the cells within the tumorospheres. The in vitro culture under unattached conditions where cells grow in round balls called spheres is used routinely for the enrichment and propagation of stem cells (Jensen and Parmar, 2006). Our findings indicated that Oct4+ cells are possibly esophageal cancer stem-like cells (ECSLCs). Oct4 expression in the tumorospheres and in secondary tumors indicated that Oct4+ cells are the initiating cells responsible for tumor regrowth. The xenograft tumors had high proliferative potential and low levels of apoptosis, which might explain why the xenograft tumors derived from the tumorospheres were larger than those derived from the differentiated cells.
Recently, a small subset of cancer cells, termed side population cells, that possess properties of CSCs were found within esophageal carcinomas (ECs) (Haraguchi et al., 2006a; 2006b; Loebinger et al., 2008). In the present study, we detected the presence of the stem cell markers Nanog and Sox2 in ESCCs, providing further evidence for the existence of ECSCs. The presence of Oct4 in ESCC suggests a potential role for Oct4 in the tumorigenesis of ESCC. Our results support earlier findings that implicate Oct4 as a multifunctional factor involved in stem cell self-renewal and differentiation as well as carcinogenesis (Tysnes, 2010). Because of the shortage of material available for sorting live cells by nuclear protein markers, whether Oct4 expression in ESCC provides sufficient evidence for the existence of ECSC requires further assessment. However, given its potential role in tumorigenesis and maintenance of CSC properties, Oct4 expressing cells might represent a specific subset of target cells for the development of effective treatment strategy.
Acknowledgments
We thank the Pathology Department of Renmin Hospital for assistance with the histological staining. We are grateful to Yun Zhao for his assistance in the writing of this manuscript. This work was supported by the Shiyan Biologic Research Foundation (no. 045D).
References
- 1.Adachi K., Suemori H., Yasuda S.Y., Nakatsuji N., Kawase E. Role of SOX2 in maintaining pluripotency of human embryonic stem cells. Genes Cells. (2010);15:455–470. doi: 10.1111/j.1365-2443.2010.01400.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. (2003);100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atlasi Y., Mowla S.J., Ziaee S.A., Bahrami A.R. OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int. J. Cancer. (2007);120:1598–1602. doi: 10.1002/ijc.22508. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z., Xu W.R., Qian H., Zhu W., Bu X.F., Wang S., Yan Y.M., Mao F., Gu H.B., Cao H.L., et al. Oct4, a novel marker for human gastric cancer. J. Surg. Oncol. (2009);99:414–419. doi: 10.1002/jso.21270. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L., Thomas A., Roth L.M., Zheng W., Michael H., Karim F.W. OCT4: a novel biomarker for dysgerminoma of the ovary. Am. J. Surg. Pathol. (2004);28:1341–1346. doi: 10.1097/01.pas.0000135528.03942.1f. [DOI] [PubMed] [Google Scholar]
- 6.Cheng L., Sung M.T., Cossu-Rocca P., Jones T.D., MacLennan G.T., De Jong J., Lopez-Beltran A., Montironi R., Looijenga L.H. OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J. Pathol. (2007);211:1–9. doi: 10.1002/path.2105. [DOI] [PubMed] [Google Scholar]
- 7.Chiou S.H., Yu C.C., Huang C.Y., Lin S.C., Liu C.J., Tsai T.H., Chou S.H., Chien C.S., Ku H.H., Lo J.F. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. (2008);14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 8.Collins A.T., Berry P.A., Hyde C., Stower M.J., Maitland N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. (2005);65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 9.Ebben J.D., Treisman D.M., Zorniak M., Kutty R.G., Clark P.A., Kuo J.S. The cancer stem cell paradigm: a new understanding of tumor development and treatment. Exp. Opin. Ther. Targets. (2010);14:621–632. doi: 10.1517/14712598.2010.485186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enzinger P.C., Mayer R.J. Esophageal cancer. N. Engl. J. Med. (2003);349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 11.Ezeh U.I., Turek P.J., Reijo R.A., Clark A.T. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. (2005);104:2255–2265. doi: 10.1002/cncr.21432. [DOI] [PubMed] [Google Scholar]
- 12.Gangemi R.M., Griffero F., Marubbi D., Perera M., Capra M.C., Malatesta P., Ravetti G.L., Zona G.L., Daga A., Corte G. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. (2009);27:40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 13.Gao J.X. Cancer stem cells: the lessons from pre-cancerous stem cells. J. Cell. Mol. Med. (2008);12:67–96. doi: 10.1111/j.1582-4934.2007.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haraguchi N., Inoue H., Tanaka F., Mimori K., Utsunomiya T., Sasaki A., Mori M. Cancer stem cells in human gastrointestinal cancers. Hum. Cell. (2006a);19:24–29. doi: 10.1111/j.1749-0774.2005.00004.x. [DOI] [PubMed] [Google Scholar]
- 15.Haraguchi N., Utsunomiya T., Inoue H., Tanaka F., Mimori K., Barnard G.F., Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. (2006b);24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 16.Hu T., Liu S., Breiter D.R., Wang F., Tang Y., Sun S. Octamer 4 small interfering RNA results in cancer stem cell-like cell apoptosis. Cancer Res. (2008);68:6533–6540. doi: 10.1158/0008-5472.CAN-07-6642. [DOI] [PubMed] [Google Scholar]
- 17.Hussenet T., du Manoir S. SOX2 in squamous cell carcinoma: Amplifying a pleiotropic oncogene along carcinogenesis. Cell Cycle. (2010);9:1480–1486. doi: 10.4161/cc.9.8.11203. [DOI] [PubMed] [Google Scholar]
- 18.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T., Thun M.J. Cancer statistics, 2008. CA Cancer J. Clin. (2008);58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 19.Jensen J.B., Parmar M. Strengths and limitations of the neurosphere culture system. Mol. Neurobiol. (2006);34:153–161. doi: 10.1385/MN:34:3:153. [DOI] [PubMed] [Google Scholar]
- 20.Jones T., Ulbright T., Eble J., Baldridge L., Cheng L. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am. J. Surg. Pathol. (2004);28:935–940. doi: 10.1097/00000478-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Li C., Heidt D.G., Dalerba P., Burant C.F., Zhang L., Adsay V., Wicha M., Clarke M.F., Simeone D.M. Identification of pancreatic cancer stem cells. Cancer Res. (2007);67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 22.Loebinger M.R., Giangreco A., Groot K.R., Prichard L., Allen K., Simpson C., Bazley L., Navani N., Tibrewal S., Davies D., et al. Squamous cell cancers contain a side population of stem-like cells that are made chemosensitive by ABC transporter blockade. Br. J. Cancer. (2008);98:380–387. doi: 10.1038/sj.bjc.6604185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long K.B., Hornick J.L. SOX2 is highly expressed in squamous cell carcinomas of the gastrointestinal tract. Hum. Pathol. (2009);40:1768–1773. doi: 10.1016/j.humpath.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Looijenga L.H., Stoop H., de Leeuw H.P., de Gouveia Brazao C.A., Gillis A.J., van Roozendaal K.E., van Zoelen E.J., Weber R.F., Wolffenbuttel K.P., van Dekken H., et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. (2003);63:2244–2250. [PubMed] [Google Scholar]
- 25.Morrison S.J., Shah N.M., Anderson D.J. Regulatory mechanisms in stem cell biology. Cell. (1997);88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 26.Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., Scholer H., Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. (1998);95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 27.Okumura-Nakanishi S., Saito M., Niwa H., Ishikawa F. Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. J. Biol. Chem. (2005);280:5307–5317. doi: 10.1074/jbc.M410015200. [DOI] [PubMed] [Google Scholar]
- 28.Phi J.H., Park S.H., Paek S.H., Kim S.K., Lee Y.J., Park C.K., Cho B.K., Lee D.H., Wang K.C. Expression of Sox2 in mature and immature teratomas of central nervous system. Mod. Pathol. (2007);20:742–748. doi: 10.1038/modpathol.3800793. [DOI] [PubMed] [Google Scholar]
- 29.Prasad N.B., Biankin A.V., Fukushima N., Maitra A., Dhara S., Elkahloun A.G., Hruban R.H., Goggins M., Leach S.D. Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res. (2005);65:1619–1626. doi: 10.1158/0008-5472.CAN-04-1413. [DOI] [PubMed] [Google Scholar]
- 30.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. (2001);414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz M., Temme A., Senner V., Ebner R., Schwind S., Stevanovic S., Wehner R., Schackert G., Schackert H.K., Fussel M., et al. Identification of SOX2 as a novel gliomaassociated antigen and potential target for T cell-based immunotherapy. Br. J. Cancer. (2007);96:1293–1301. doi: 10.1038/sj.bjc.6603696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sell S. On the stem cell origin of cancer. Am. J. Pathol. (2010);176:2584–494. doi: 10.2353/ajpath.2010.091064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh S.K., Hawkins C., Clarke I.D., Squire J.A., Bayani J., Hide T., Henkelman R.M., Cusimano M.D., Dirks P.B. Identification of human brain tumour initiating cells. Nature. (2004);432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 34.Tysnes B.B. Tumor-initiating and -propagating cells: cells that we would like to identify and control. Neoplasia. (2010);12:506–515. doi: 10.1593/neo.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., Komaki R., Wang L., Fang B., Chang J.Y. Treatment of radioresistant stem-like esophageal cancer cells by an apoptotic gene-armed, telomerase-specific oncolytic adenovirus. Clin. Cancer Res. (2008);14:2813–2823. doi: 10.1158/1078-0432.CCR-07-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]


