Abstract
Acquired resistance to tamoxifen (TAM) is a serious therapeutic problem in breast cancer patients. We have shown that Pin1, a peptidyl prolyl isomerase, is consistently overexpressed in TAM-resistant MCF-7 cells (TAMR-MCF-7 cells) and plays a key role in the enhanced angiogenic potential of TAMR-MCF-7 cells. In the present study, we focused on signaling pathways for Pin1 up-regulation in TAMR-MCF-7 cells. Relative to MCF-7 cells, Pin1 gene transcription and E2 transcription factor1 (E2F1) expression were enhanced in TAMR-MCF-7 cells. E2F1 siRNA significantly reduced both the protein expression and the promoter transcriptional activity of Pin1. Activities of phosphatidylinositol 3-kinase (PI3K), extracellular signalregulated kinase (ERK) and p38 kinase were all higher in TAMR-MCF-7 cells than in control MCF-7 cells and the enhanced Pin1 and E2F1 expression in TAMR-MCF-7 cells was reversed by inhibition of PI3K or p38 kinase. Moreover, the higher production of vascular endothelial growth factor (VEGF) in TAMR-MCF-7 cells was significantly diminished by suppression of PI3K or p38 kinase. These results suggest that Pin1 overexpression and subsequent VEGF production in TAMR-MCF-7 cells are mediated through PI3-kinase or p38 kinase-dependent E2F1 activation.
Keywords: E2F1, p38 kinase, PI3-kinase, Pin1, tamoxifen-resistant breast cancer, VEGF
INTRODUCTION
Pin1, a peptidyl prolyl isomerase, specifically recognizes phosphorylated serine or threonine immediately preceding proline (pSer/Thr-Pro) and then facilitates isomerization of the peptide bond (Bayer et al., 2003; Lu, 2004). Because cellular enzymes such as kinases and protein phosphatases react with substrate proteins in a manner that is dependent on the conformation of substrate protein, Pin1 could be important for the activities of diverse enzymes (Weiwad et al., 2000; Zhou et al., 2000). Pin1 is frequently overexpressed in cancer tissues as well as in human breast and prostate cancer cell lines (Bao et al., 2004). The pathophysiological roles of Pin1 were originally identified in cancer cells such as breast cancer cells (Wulf et al., 2001). Pin1 overexpression stimulates neoplastic transformation of non-tumorigenic hepatocyte and epidermal cell lines (Lee et al., 2009; Pang et al., 2007). Furthermore, Pin1 ablation is highly effective in preventing Neu- or HBx-enhanced tumor growth in mouse tumor models (Pang et al., 2007; Wulf et al., 2004). Hence, Pin1 may be an important mediator of the development of several types of cancers.
The growth of estrogen receptor (ER)-positive breast cancers is generally under the control of ovarian hormones. Hence, antiestrogens such as tamoxifen (TAM) are used as first-line preventive or therapeutic tools (Petrangeli et al., 1994; Rose et al., 1985). A major obstacle for anti-estrogen therapy is the development of resistance to TAM during long-term treatment (Clemons et al., 2002). We previously established TAM-resistant breast cancer cells using an ER-positive MCF-7 cell line (TAMR-MCF- 7 cells) by long-term culture of MCF-7 cells in hormone-free culture medium that contained 4-hydroxytamoxifen (Choi et al., 2007). A series of our studies revealed that Pin1 was highly expressed in TAMR-MCF-7 cells and that it was required for both enhanced vascular endothelial growth factor (VEGF)- mediated angiogenesis and the Snail-dependent epithelial mesenchymal transition in the cell type (Kim et al., 2009a; 2009b). These results suggested that Pin1 is a potential therapeutic target for the treatment of TAM-resistant breast cancer. Although the pathological function of Pin1 during the acquisition of TAM-resistance was studied, the molecular mechanism of Pin1 induction in TAM-resistant breast cancer has not been elucidated. In the present study, we showed that Pin1 expression is dependent on phosphatidyl inositol 3-kinase (PI3K) dependent or p38 kinase-dependent E2 transcription factor1 (E2F1) activation in TAMR-MCF-7 cells. Moreover, the exaggerated VEGF production in TAMR-MCF-7 cells was suppressed by blocking both kinases.
MATERIALS AND METHODS
Materials
Antibodies against Pin1, E2F1 and retinoblastoma (Rb), and siRNA targeting E2F1 were obtained from Santa Cruz Biotechnology (USA). Antibodies that recognize phosphorylated or total Akt, extracellular signal regulated kinase (ERK), p38 kinase, c- Jun N-terminal kinase (JNK) and phosphorylated Rb (pRb) were obtained from Cell Signaling Technology (USA). Horseradish peroxidase-conjugated donkey anti-rabbit IgG, anti-goat IgG, and alkaline phosphatase-conjugated donkey anti-mouse IgG were purchased from Jackson Immunoresearch Laboratories (USA). The anti-actin antibody and most reagents used for molecular studies were purchased from Sigma (USA).
Cell culture and establishment of TAMR-MCF-7 cells
MCF-7 cells were cultured at 37℃ in 5% CO2/95% air in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin. TAMR-MCF-7 cells were established using the methods previously reported (Choi et al., 2007).
Immunoblot analysis
After washing the cells with sterile PBS, they were lysed in buffer containing 20 mM Tris·Cl (pH 7.5), 1% Triton X-100, 137 mM sodium chloride, 10% glycerol, 2 mM EDTA, 1 mM sodium orthovanadate, 25 mM β-glycerophosphate, 2 mM sodium pyrophosphate, 1 mM phenylmethylsulfonylfluoride, and 1 μg/ ml leupeptin. Total cell lysates were centrifuged at 10,000× g for 10 min to remove cell debris, and proteins were fractionated on a 10% SDS-PAGE gel. The fractionated proteins were then transferred electrophoretically to nitrocellulose paper and immunoblotted with specific antibodies. Nuclear extracts were prepared as previously described (Kim et al., 2009a).
VEGF enzyme-linked immunosorbent assay (ELISA)
A commercial ELISA kit (Biosource Diagnostics, Belgium) was used to determine VEGF concentrations in media, according to the manufacturer’s protocol. Briefly, cells were plated in 6-well culture plates, incubated with or without kinase inhibitors in serum-free medium for 24 h, and then the culture medium was measured by ELISA. VEGF concentrations were determined by measuring their absorbance at 420 nm and were normalized to total protein concentration in each well.
Reporter gene analysis
A dual-luciferase reporter assay system (Promega, USA) was used to determine promoter activity. Briefly, cells were plated in 12-well plates and transiently transfected with reporter and phRL-SV plasmids (hRenilla luciferase expression for normalization) (Promega, USA) using Hillymax® reagent (Dojindo Molecular Technologies, USA). Cells were then incubated in culture medium without serum for 18 h. Firefly and hRenilla luciferase activities in the cell lysates were measured using a luminometer (LB941, Berthold Technologies, Bad Wildbad, Germany). Relative luciferase activity was calculated by normalizing the promoter-driven firefly luciferase activity to the hRenilla luciferase activity.
Statistical analysis
Student’s t-test was used to determine the significance of differences between treatment groups. Statistical significance was accepted for p < 0.05.
RESULTS
Transcriptional activation of the Pin1 gene in TAMR-MCF-7 cells
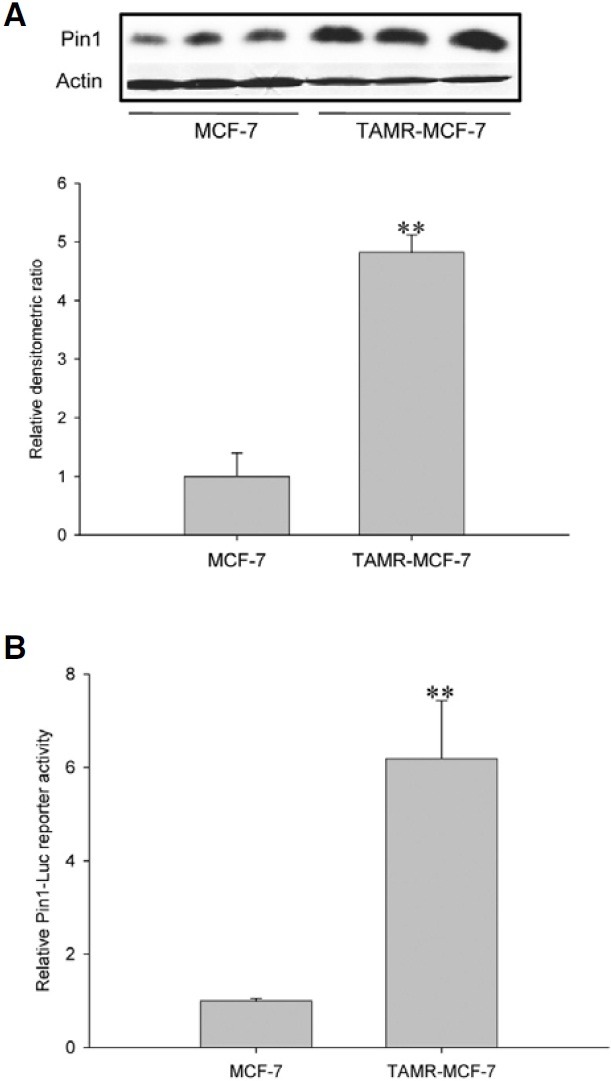
We showed that the Pin1 protein level was consistently increased in TAMR-MCF-7 cells compared to control MCF-7 cells (Kim et al., 2009a; 2009b) (Fig. 1A). We then assessed whether Pin1 gene transcription was enhanced in TAMRMCF- 7 cells. Reporter gene analysis using a Pin1-Luc reporter plasmid containing the luciferase structural gene and the human Pin1 promoter showed that Pin1-Luc reporter activity was increased 6.1 fold in TAMR-MCF-7 cells versus MCF-7 cells (Fig. 1B). These results demonstrate that the Pin1 increase in TAMR-MCF-7 cells is mainly due to transcriptional activation of the Pin1 gene.
Fig. 1. Transcriptional activation of Pin1 gene in TAMR-MCF-7 cells. (A) Immunoblot analysis of Pin1 in MCF-7 and TAMR-MCF-7 cells. Relative changes in the Pin1 protein levels were assessed by scanning densitometry. Data represent the mean ± SD (n = 3) (significant as compared to MCF-7 cells, **p < 0.01). (B) Enhanced Pin1 gene transcription in TAMR-MCF-7 cells. MCF-7 and TAMRMCF- 7 cells were transiently transfected with Pin1-Luc and phRL-SV plasmids and incubated in serum-free medium for 18 h. Data represent the mean ± SD (n = 6) (significant as compared to MCF-7 cells, **p < 0.01).
Involvement of E2F1 in enhanced Pin1 gene transcription
The family of E2F proteins has been shown to play a key role in the transcriptional regulation of the Pin1 gene (Ryo et al., 2002; Shimizu et al., 2006). Three E2F binding sites have been identified in the promoter region of the human Pin1 gene and recombinant E2F1 binds to these sites (Ryo et al., 2002). E2F1 functions as a downstream effector of pRB and plays a key role in regulating cell cycle progression (Polager et al., 2008). Thus, we first determined E2F1 and pRb levels. As shown in Fig. 2A, E2F1 and pRb were increased in TAMR-MCF-7 cells compared to control MCF-7 cells. The data suggest that the Rb/ E2F1 pathway is continuously activated in TAMR-MCF-7 cells.
Fig. 2. Involvement of E2F1 in enhanced Pin1 gene transcription. (A) The protein levels of nuclear E2F1 and P-Rb in MCF-7 and TAMR-MCF-7 cells. Western blot analyses were performed using nuclear fractions or total cell lysates from serum-deprived MCF-7 and TAMR-MCF-7 cells. (B) Effect of E2F1 siRNA on the Pin1-Luc reporter activity. MCF-7 and TAMR-MCF-7 cells were co-transfected with Pin1-Luc in combination with 20 pmole E2F1 siRNA or control siRNA. Data represents means ± SD (n = 4) (significant as compared to MCF-7 cells, **p < 0.01; significant as compared to control siRNA group, ##p < 0.01). (C) Effect of E2F1 siRNA on the Pin1 expression in TAMR-MCF-7 cells. TAMR-MCF-7 cells were transfected with E2F1 siRNA (60 pmole, E2F1siR) or control siRNA (60 pmole, ConsiR) and then total cell lysates were subjected to Pin1 immunoblotting.
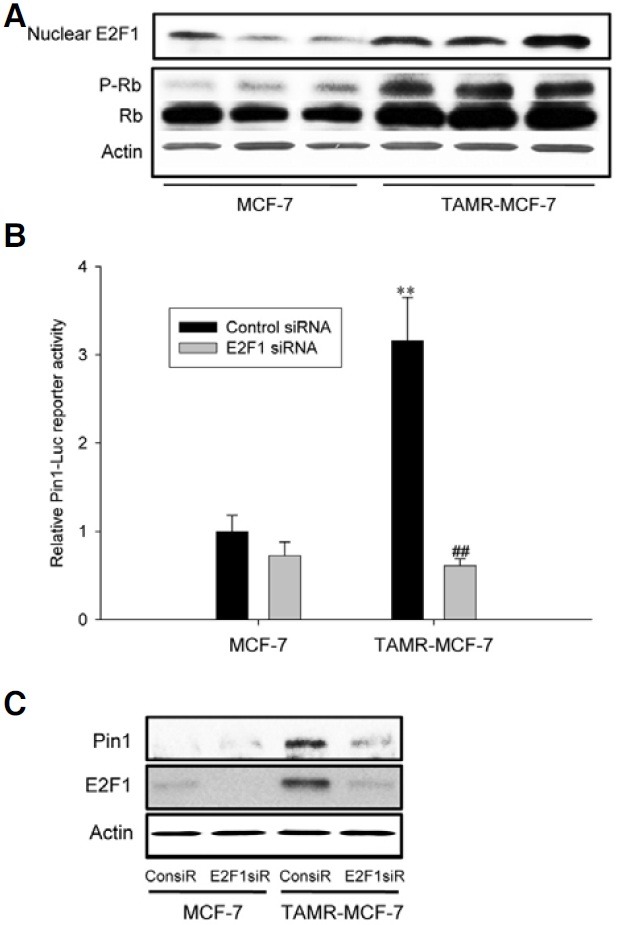
To verify whether E2F1 activation is required for transcriptional activation of the Pin1 gene, we did reporter gene analyses using siRNA against E2F1 and the Pin1-Luc reporter gene. Co-transfection of TAMR-MCF-7 cells with E2F1 siRNA significantly inhibited the increase of Pin1-Luc reporter activity in TAMR-MCF-7 cells (Fig. 2B). Furthermore, the enhanced Pin1 expression in TAMR-MCF-7 cells was significantly reversed by E2F1 siRNA (Fig. 2C). These results provide strong evidence that E2F1 activation is required for enhanced Pin1 gene expression in TAMR-MCF-7 cells.
Role of PI3K and p38 kinase in E2F1-dependent Pin1 expression in TAMR-MCF-7 cells
Since Pin1 expression is under the control of growth factors and serum (Ryo et al., 2002; You et al., 2002), it is possible that cell proliferation signaling, including PI3K/Akt and ERK, regulates Pin1 induction in TAMR-MCF-7 cells. Moreover, hepatitis B virus X protein-stimulated p38 kinase activation led to phosphorylation of Rb and release of E2F1 in hepatocytes (Wang et al., 2008). Hence, we assessed basal activities of PI3K, ERK and p38 kinase. Western blot analyses using phosphorylated form-specific antibodies showed that the basal activities of PI3K, ERK and p38 kinase were all increased in TAMR-MCF-7 cells compared to MCF-7 cells (Fig. 3A). We then assessed the effect of specific kinase inhibitors on protein levels of Pin1 and nuclear E2F1. Figure 3B shows that a PI3-kinase inhibitor, LY294002 potently suppresses protein levels of Pin1 and E2F1 in TAMR-MCF-7 cells; the ERK inhibitor PD98059 did not (Fig. 3B, left). Blocking of p38 kinase activity by SB203580 also decreased expression levels of Pin1 and E2F1 in TAMR-MCF-7 cells, but the degree of inhibition was not as great as for blocking of PI3K (Fig. 3B, right).
Fig. 3. Role of PI3K and p38 kinase in E2F1-dependent Pin1 expression in TAMR-MCF-7 cells. (A) Activities of PI3K, ERK and p38 kinase in MCF-7 and TAMR-MCF-7 cells. Western blot analyses were performed using nuclear fractions or total cell lysates from serum-deprived MCF-7 and TAMR-MCF-7 cells. Each kinase activity was determined by using phosphorylated form-specific antibodies against Akt (PI3K activity), ERK and p38 kinase. (B) Role of PI3K and p38 kinase in E2F1-dependent Pin1 expression. The levels of nuclear E2F1 and Pin1 were determined by Western blot analyses in TAMR-MCF-7 cells cultured in the presence or absence of LY294002 (LY, 20 μM), PD98059 (PD, 30 μM) or SB203580 (SB, 10 μM). (C) Effects of PI3K and p38 kinase inhibitors on VEGF production in TAMR-MCF-7 cells. TAMR-MCF-7 cells were incubated with or without LY294002 (LY, 20 μM) or SB203580 (SB, 10 μM) for 24 h and the culture media (50 μl) were subjected to VEGF ELISA. Data represent the mean ± SD (n = 4) (significant as compared to control MCF-7 cells, **p < 0.01; significant as compared to TAMR-MCF-7 cells, ##p < 0.01).
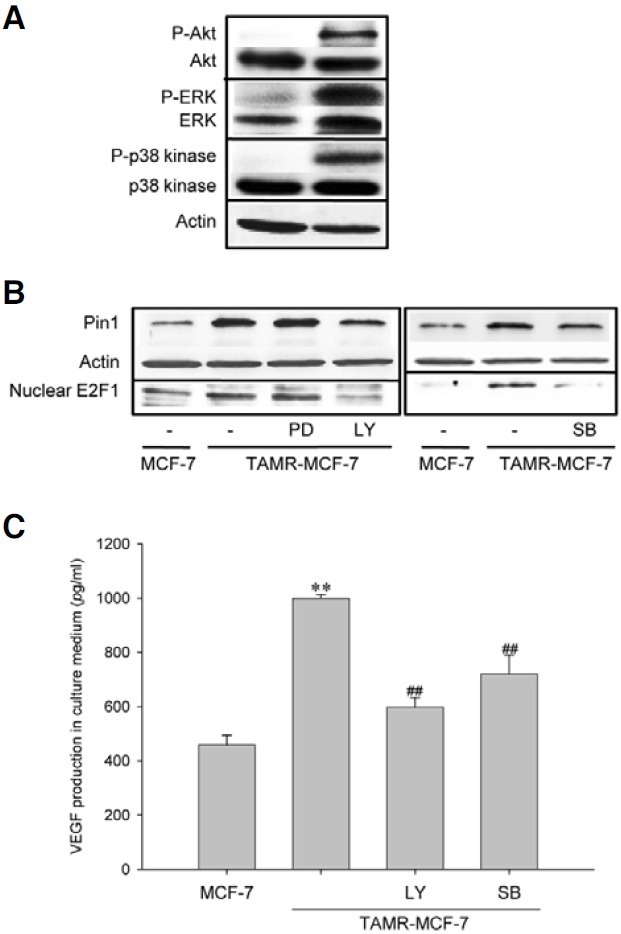
We previously demonstrated that VEGF production was increased via Pin1 up-regulation in TAMR-MCF-7 cells (Kim et al., 2009a). Hence, we determined the effect of LY294002 and SB203580 in VEGF secretion of TAMR-MCF-7 cells using VEGF-specific ELISA. As shown in Fig. 3C, enhanced VEGF secretion in TAMR-MCF-7 cells was significantly diminished by the blocking of either PI3K or p38 kinase. These results imply that activation of E2F1/pRb by PI3K or p38 kinase is essential for Pin1-mediated VEGF production in TAMR-MCF-7 cells.
DISCUSSION
A majority (67%) of breast tumor tissues express ER; so endocrine therapy is popular for treating ER-positive breast cancers. Unfortunately, resistance to hormonal therapy is a major clinical problem -most cancer cells can utilize alternative mechanisms for growth and survival after initiation of first-line endocrine therapy (Ali et al., 2002). A series of our studies revealed that both VEGF-mediated angiogenesis and snail-dependent epithelial mesenchymal transitions were stimulated in TAMresistant breast cancer cells, and Pin1 overexpression in this cell type is a critical event for both processes (Kim et al., 2008; 2009a; 2009b). In the present study, we demonstrated that Pin1 overexpression in TAMR-MCF-7 cells was dependent on transcriptional activation of the Pin1 gene. Although many studies have revealed that Pin1 expression is enhanced in diverse tumor types, and there has been speculation regarding possible functions of Pin1 (Finn et al., 2008), studies focusing on transcriptional regulation of the Pin1 gene are still limited. Wulf et al. showed that Pin1 transcription was triggered by oncogenic Neu/Ras signaling via E2F activation (Ryo et al., 2002). Although a recent study reported the mutant form (C/EBPα- p30) of the CCAAT enhancer-binding proteinα has a positive effect on Pin1 gene transcription in leukemia cells, C/EBPα-p30 indirectly stimulated Pin1 transcription via an increase in E2F1 recruitment in the gene promoter (Pulikkan et al., 2010).
Initial studies suggested that E2F regulates time-dependent expression of genes required for entry into the cell cycle and progression to S phase. It is now generally accepted that E2F is involved in a variety of processes in cellular physiology, including DNA replication and repair, mitotic and DNA damage checkpoints, development and apoptosis (Bracken et al., 2004; Ren et al., 2002). Though E2F-mediated transcriptional activity can be controlled in multiple ways, the best known one is interaction with the Rb protein, a tumor-suppressor gene product. This interaction results in the inhibition of E2F1’s transactivator function in the promoters of E2F-responsive genes. Rb phosphorylation disrupts the formation of the Rb/E2F1 complex, leading to cell cycle progression (Polager et al., 2008). Here, we tested possible involvement of E2F1 in enhanced Pin1 expression in TAMR-MCF-7 cells. As expected, E2F1 expression and pRb levels were increased in TAMR-MCF-7 cells compared to MCF-7 cells and E2F1 suppression by siRNA reduced both the protein expression and promoter transcription of the Pin1 gene in TAMR-MCF-7 cells. Hence, it can be concluded that E2F1 activation is crucial for the up-regulation of Pin1 expression in TAMR-MCF-7 cells. Given that deregulation of E2F is found in a majority of human cancers (Johnson et al., 2006), the E2F1/Rb pathway could be affected by long-term exposure of MCF-7 cells to TAM.
Pin1 expression is up-regulated by cell proliferation conditions such as exposure to serum or growth factors (Ryo et al., 2002; You et al., 2002). Moreover, it has been reported that E2F1 activity is controlled by PI3K, ERK and p38 kinase (Pintus et al., 2003; Reichert et al., 2007; Wang et al., 2008). In the present study, we showed that the activities of all these kinases were enhanced in TAMR-MCF-7 cells. When we used specific kinase inhibitors, we also found that PI3K and p38 kinase pathways were required for both the Pin1 expression and the E2F1 increase in TAMR-MCF-7 cells. Although inhibition of p38 kinase caused a marginal decrease in Pin1 and E2F1 expression, inhibition of PI3K distinctly suppressed E2F1-mediated Pin1 expression. Hence, PI3-kinase seems to mainly control Pin1 gene transcription in TAMR-MCF-7 cells. Although E2F1 activity can be regulated by PI3K and p38 kinase, E2F1 itself positively modulates PI3K and p38 kinase pathways through the induction of the Gab2 adaptor protein and apoptosis signalregulating kinase1, respectively (Chaussepied et al., 2004; Hershko et al., 2006). Therefore, it is plausible that positive cross-talk between (a) PI3K and p38 kinase and (b) E2F1 guarantees sustained Pin1 induction in TAMR-MCF-7 cells.
In conclusion, our studies reveal that overexpression of Pin1 in TAM-resistant breast cancer cells results from PI3K and p38 kinase-dependent E2F1 activation. As suggested in our previous studies (Kim et al., 2008; 2009a), Pin1 functions as a master regulator for VEGF-mediated angiogenesis. Therefore, PI3K or p38 kinase could be useful as therapeutic targets for the treatment of TAM-resistant breast cancer. In this study, we demonstrated that enhancement of VEGF production in TAMRMCF- 7 cells is significantly suppressed by inhibition of PI3K or p38 kinase.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Education, Science and Technology) (No. 2009-0070587) and a grant of the Korea Healthcare technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A090470).
References
- 1.Ali S., Coombes R.C. Endocrine-responsive breast cancer and strategies for combating resistance. Nat. Rev. Cancer. (2002);2:101–112. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 2.Bao L., Sauter G., Sowadski J., Lu K.P., Wang D. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am. J. Pathol. (2004);164:1727–1737. doi: 10.1016/S0002-9440(10)63731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer E., Goettsch S., Mueller J.W., Griewel B., Guiberman E., Mayr L.M., Bayer P. Structural analysis of the mitotic regulator hPin1 in solution: insights into domain architecture and substrate binding. J. Biol. Chem. (2003);278:26183–26193. doi: 10.1074/jbc.M300721200. [DOI] [PubMed] [Google Scholar]
- 4.Bracken A.P., Ciro M., Cocito A., Helin K. E2F target genes: unraveling the biology. Trends Biochem. Sci. (2004);29:409–417. doi: 10.1016/j.tibs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Chaussepied M., Ginsberg D. Transcriptional regulation of AKT activation by E2F. Mol. Cell. (2004);16:831–837. doi: 10.1016/j.molcel.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Choi H.K., Yang J.W., Roh S.H., Han C.Y., Kang K.W. Induction of multidrug resistance associated protein 2 in tamoxifen-resistant breast cancer cells. Endocr. Relat. Cancer. (2007);14:293–303. doi: 10.1677/ERC-06-0016. [DOI] [PubMed] [Google Scholar]
- 7.Clemons M., Danson S., Howell A. Tamoxifen (‘Nolvadex’):a review, Cancer Treat. Rev. (2002);28:165–180. doi: 10.1016/s0305-7372(02)00036-1. [DOI] [PubMed] [Google Scholar]
- 8.Finn G., Lu K.P. Phosphorylation-specific prolyl isomerase Pin1 as a new diagnostic and therapeutic target for cancer. Curr. Cancer Drug Targets. (2008);8:223–229. doi: 10.2174/156800908784293622. [DOI] [PubMed] [Google Scholar]
- 9.Hershko T., Korotayev K., Polager S., Ginsberg D. E2F1 modulates p38 MAPK phosphorylation via transcriptional regulation of ASK1 and Wip1. J. Biol. Chem. (2006);281:31309–31316. doi: 10.1074/jbc.M601758200. [DOI] [PubMed] [Google Scholar]
- 10.Johnson D.G., Degregori J. Putting the oncogenic and tumor suppressive activities of E2F into context. Curr. Mol. Med. (2006);6:731–738. doi: 10.2174/1566524010606070731. [DOI] [PubMed] [Google Scholar]
- 11.Kim M.R., Choi H.S., Heo T.H., Hwang S.W., Kang K.W. Induction of vascular endothelial growth factor by peptidyl- prolyl isomerase Pin1 in breast cancer cells. Biochem. Biophys. Res. Commun. (2008);369:547–553. doi: 10.1016/j.bbrc.2008.02.045. [DOI] [PubMed] [Google Scholar]
- 12.Kim M.R., Choi H.S., Yang J.W., Park B.C., Kim J.A., Kang K.W. Enhancement of vascular endothelial growth factor- mediated angiogenesis in tamoxifen-resistant breast cancer cells: role of Pin1 overexpression. Mol. Cancer Ther. (2009a);8:2163–2171. doi: 10.1158/1535-7163.MCT-08-1061. [DOI] [PubMed] [Google Scholar]
- 13.Kim M.R., Choi H.K., Cho K.B., Kim H.S., Kang K.W. Involvement of Pin1 induction in epithelial-mesenchymal transition of tamoxifen-resistant breast cancer cells. Cancer Sci. (2009b);100:1834–1841. doi: 10.1111/j.1349-7006.2009.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee N.Y., Choi H.K., Shim J.H., Kang K.W., Dong Z., Choi H.S. The prolyl isomerase Pin1 interacts with a ribosomal protein S6 kinase to enhance insulin-induced AP-1 activity and cellular transformation. Carcinogenesis. (2009);30:671–681. doi: 10.1093/carcin/bgp027. [DOI] [PubMed] [Google Scholar]
- 15.Lu K.P. Pinning down cell signaling, cancer and Alzheimer’s disease. Trends Biochem. Sci. (2004);29:200–209. doi: 10.1016/j.tibs.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Pang R., Lee T.K., Poon R.T., Fan S.T., Wong K.B., Kwong Y.L., Tse E. Pin1 interacts with a specific serineproline motif of hepatitis B virus X-protein to enhance hepatocarcinogenesis. Gastroenterology. (2007);132:1088–1103. doi: 10.1053/j.gastro.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Petrangeli E., Lubrano C., Ortolani F., Ravenna L., Vacca A., Sciacchitano S., Frati L., Gulino A. Estrogen receptors: new perspectives in breast cancer management. J. Steroid Biochem. Mol. Biol. (1994);49:327–331. doi: 10.1016/0960-0760(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 18.Pintus G., Tadolini B., Posadino A.M., Sanna B., Debidda M., Carru C., Deiana L., Ventura C. PKC/Raf/MEK/ ERK signaling pathway modulates native-LDL-induced E2F-1 gene expression and endothelial cell proliferation. Cardiovasc. Res. (2003);59:934–944. doi: 10.1016/s0008-6363(03)00526-1. [DOI] [PubMed] [Google Scholar]
- 19.Polager S., Ginsberg D. E2F-at the crossroads of life and death. Trends Cell Biol. (2008);18:528–535. doi: 10.1016/j.tcb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Pulikkan J.A., Dengler V., Peer Zada A.A., Kawasaki A., Geletu M., Pasalic Z., Bohlander S.K., Ryo A., Tenen D.G., Behre G. Elevated PIN1 expression by C/EBPalpha-p30 blocks C/EBPalpha-induced granulocytic differentiation through c-Jun in AML. Leukemia. (2010);24:914–923. doi: 10.1038/leu.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichert M., Saur D., Hamacher R., Schmid R.M., Schneider G. Phosphoinositide-3-kinase signaling controls S-phase kinase-associated protein 2 transcription via E2F1 in pancreatic ductal adenocarcinoma cells. Cancer Res. (2007);67:4149–4156. doi: 10.1158/0008-5472.CAN-06-4484. [DOI] [PubMed] [Google Scholar]
- 22.Ren B., Cam H., Takahashi Y., Volkert T., Terragni J., Young R.A., Dynlacht B.D. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. (2002);16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose C., Thorpe S.M., Andersen K.W., Pedersen B.V., Mouridsen H.T., Blichert-Toft M., Rasmussen B.B. Beneficial effect of adjuvant tamoxifen therapy in primary breast cancer patients with high oestrogen receptor values. Lancet. (1985);1:16–19. doi: 10.1016/s0140-6736(85)90966-3. [DOI] [PubMed] [Google Scholar]
- 24.Ryo A., Liou Y.C., Wulf G., Nakamura M., Lee S.W., Lu K.P. PIN1 is an E2F target gene essential for Neu/Rasinduced transformation of mammary epithelial cells. Mol. Cell. Biol. (2002);22:5281–5295. doi: 10.1128/MCB.22.15.5281-5295.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu T., Akiyama H., Abe Y., Sasada H., Sato E., Miyamoto A., Uchida T. Expression of Pin1, a peptidyl-prolyl isomerase, in the ovaries of eCG/hCG-treated immature female mice. J. Reprod. Dev. (2006);52:287–291. doi: 10.1262/jrd.17057. [DOI] [PubMed] [Google Scholar]
- 26.Wang W.H., Hullinger R.L., Andrisani O.M. Hepatitis B virus X protein via the p38MAPK pathway induces E2F1 release and ATR kinase activation mediating p53 apoptosis. J. Biol. Chem. (2008);283:25455–25467. doi: 10.1074/jbc.M801934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiwad M., Küllertz G., Schutkowski M., Fischer G. Evidence that the substrate backbone conformation is critical to phosphorylation by p42 MAP kinase. FEBS Lett. (2000);478:39–42. doi: 10.1016/s0014-5793(00)01794-4. [DOI] [PubMed] [Google Scholar]
- 28.Wulf G.M., Ryo A., Wulf G.G., Lee S.W., Niu T., Petkova V., Lu K.P. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. EMBO J. (2001);20:3459–3472. doi: 10.1093/emboj/20.13.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wulf G., Garg P., Liou Y.C., Iglehart D., Lu K.P. Modeling breast cancer in vivo and ex vivo reveals an essential role of Pin1 in tumorigenesis. EMBO J. (2004);23:3397–3407. doi: 10.1038/sj.emboj.7600323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You H., Zheng H., Murray S.A., Yu Q., Uchida T., Fan D., Xiao Z.X. IGF-1 induces Pin1 expression in promoting cell cycle S-phase entry. J. Cell. Biochem. (2002);84:211–216. doi: 10.1002/jcb.10037. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X.Z., Kops O., Werner A., Lu P.J., Shen M., Stoller G., Küllertz G., Stark M., Fischer G., Lu K.P. Pin1- dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol. Cell. (2000);6:873–883. doi: 10.1016/s1097-2765(05)00083-3. [DOI] [PubMed] [Google Scholar]


