Abstract
Vascular endothelial growth factor (VEGF) signaling plays an important role in angiogenesis. In the VEGF signaling pathway, the key components are VEGF and its receptors, Flt-1 and KDR. In this study, we show that transfection of synthetic miR-200b reduced protein levels of VEGF, Flt-1, and KDR. In A549 cells, miR-200b targeted the predicted binding sites in the 3′-untranslated region (3′-UTR) of VEGF, Flt-1, and KDR as revealed by a luciferase reporter assay. When transfected with miR-200b, the ability of HUVECs to form a capillary tube on Matrigel and VEGF-induced phosphorylation of ERK1/2 were significantly reduced. Taken together, these results suggest that miR- 200b negatively regulates VEGF signaling by targeting VEGF a nd i ts r eceptors a nd t hat miR- 200b may have therapeutic potential as an angiogenesis inhibitor.
Keywords: Flt-1, KDR, microRNA, miR-200b, VEGF, VEGF signaling
INTRODUCTION
Angiogenesis is the formation of new blood vessels from preexisting vasculature in physiological and pathological conditions (Carmeliet, 2005; Folkman, 1995). When tumors are formed, angiogenesis plays a crucial role in tumor growth, invasion, and metastasis (Folkman, 1971). In tumor angiogenesis, one of the most important regulators is vascular endothelial growth factor (VEGF), which stimulates endothelial cell proliferation, migration, and survival through its receptors, Flt-1 (fms-like tyrosine kinase, or VEGFR-1) and KDR (kinase-insert domain containing receptor, or VEGFR-2) (Ferrara, 1999; 2004; Ferrara et al., 2003). VEGF expression is induced by hypoxia, and its high level in a hypoxic area promotes tumor angiogenesis. Since angiogenesis is essential for tumor growth, inhibition of VEGF signaling using several strategies such as small molecule inhibitors, antibodies, VEGF-trap, and siRNA is a promising therapeutic approach for the treatment of cancer (Eskens, 2004; Ferrara and Kerbel, 2005).
MicroRNAs (miRNAs) are short (~22 nucleotides long) and endogenous RNAs which post-transcriptionally regulate a large number of eukaryotic genes (Bartel, 2004; He and Hannon, 2004; Kim, 2005). Increasing evidence indicates that miRNAs are involved in a variety of fundamental biological processes including cell proliferation, apoptosis, and differentiation. Recently, studies have shown that miRNAs are involved in angiogenesis, either as pro-angiogenic miRNA or as anti-angiogenic miRNA (Suárez and Sessa, 2009; Wang and Olson, 2009). Currently, miR-126, the miR-17-92 cluster, let-7b and -7f, miR- 130a, miR-210, miR-378, and miR-296 are known as proangiogenic miRNAs, while anti-angiogenic miRNAs include miR-221/miR-222, miR-328, miR-15b, miR-16, miR-20a, and miR-200b (Chan et al., 2011; Wu et al., 2009). Among proangiogenic miRNAs, miR-126 is highly expressed in human endothelial cells (van Solingen et al., 2009; Wang et al., 2008). Recent studies showed that pro-angiogenic function of miR-126 is in part mediated by down-regulating Sprouty-related protein SPRED1 and phosphoinositol-3 kinase regulatory subunit 2 (PIK3R2/p85-β), which are inhibitors of angiogenic signaling (Fish et al., 2008; Wang et al., 2008). In vivo, knockdown of miR-126 in mice resulted in leaky vessels, hemorrhaging, and partial embryonic lethality, suggesting that miR-126 functions as a positive regulator of angiogenesis. In the case of antiangiogenic miRNAs, miR-200b targets v-ets erythroblastosis virus E26 oncogene homolog 1 (Ets-1), thereby inhibiting the angiogenic response of human microvascular endothelial cells (HMECs) (Chan et al., 2011). When transfected with miR-200b, the ability of HMECs to migrate and to form tubes on Matrigel was inhibited.
In this study, we show that key players in VEGF signaling, VEGF-A (hereafter, VEGF) and its receptors Flt-1 and KDR were negatively regulated by miR-200b. Luciferase reporter assay revealed that miR-200b directly targets the 3′-UTR of those genes. Transfection of human umbilical vein endothelial cells (HUVECs) with miR-200b resulted in inhibition of tube formation and ERK1/2 phosphorylation, suggesting anti-angiogenic activity of miR-200b. Since the inhibition of VEGF signaling interferes with angiogenesis, miR-200b may provide a potential anti-angiogenesis therapy for the treatment of cancer and other diseases dependent on angiogenesis.
MATERIALS AND METHODS
RNA oligonucleotides
MicroRNA mimics and a negative control (CN-001000-01) were purchased from Dharmacon (USA). KDR siRNA (siGENOME SMARTpool siRNA reagent) was also obtained from Dharmacon. All siRNA and miRNA mimics were resuspended in DEPC-treated water to a final concentration of 20 μM.
Cell lines and transfection
Human cell lines A549 (lung carcinoma) and HeLa (cervix adenocarcinoma) were obtained from Korean Cell Line Bank (Korea) and human umbilical vein endothelial cells (HUVECs) were purchased from BioBud (Korea). A549 and HeLa cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. HUVECs were cultured on gelatinized dishes in EGM2 medium (Clonetics, USA) and were used between passages 5 and 7. Both cells were maintained at 37℃ in a humidified 5% CO2 incubator. Transfection was carried out using Lipofectamine RNAiMAX (Invitrogen, USA) as described previously with 20 nM of miRNA or siRNA (Kim et al., 2008).
Western blot analysis
Total protein extracts (40 μg) were electrophoresed on a 4-12% precast protein gel (Komabiotech, Korea) and transferred to nitrocellulose membrane (Komabiotech). After incubating with antibodies, immunoreactive protein bands were detected by using the ECL plus Western blotting reagent (Amersham Biosciences, USA). Primary antibodies specific for Flt-1 (EWC), KDR (A-3), and anti-β-actin (C-11) were purchased from Santa Cruz Biotechnology (USA). Primary antibodies for ERK1/2 (137F5) and phospho-ERK1/2 (D13.14.4E) were obtained from Cell Signaling Technology (USA).
Construction of 3′-UTR reporter plasmids and luciferase assay
The full-length cDNA clone containing the 3′-UTR of VEGF gene (NM_001025366) was obtained from Open Biosystems (USA). The 3′-UTR of Flt-1 (NM_002019) and KDR (NM_ 002253) genes was synthesized by GenScript (USA). To construct 3′-UTR-luciferase reporter plasmids, 3′-UTR sequences were amplified by PCR and cloned into XhoI/NotI sites of a psiCHECK-2 vector (Promega, USA). The following 3′-UTR sequences of VEGF, Flt-1, and KDR genes were used for cloning: (1) The sequence between 1738 and 3659 nt of VEGF amplified using primers VEGF-1738L and VEGF-3659R, (2) the sequence between 4301 and 5217 nt of Flt-1 amplified using primers Flt-4301L and Flt-5217R, and (3) the sequence between 4374 and 6055 nt of KDR amplified using primers KDR- 4374L and KDR-6055R. To delete the predicted target site of miR-200b from 3′-UTR-luciferase reporter plasmids, PCR was performed using the following primer pairs as described previously (Kim et al., 2008): (1) VEGF-1738L/3022R and VEGF- 3043L/-3659R for VEGF, (2) FLT-4301L/-4357R and FLT- 4378L/-5217R for Flt-1, (3) KDR-4374L/-5388R and KDR- 5410L/-6055R for the 5′ target site of KDR; KDR-4374L/-5738R and KDR-5768L/-6055R for the 3′ target site of KDR. The DNA fragments amplified using the above primer pairs were digested with XbaI (VEGF), EcoRI (Flt-1), HindIII (5′ target site of KDR), and XbaI (3′ target site of KDR). The digested fragments were then ligated at 4℃ overnight, digested with XhoI and NotI, and cloned into psiCHECK-2 vector. Primer sequences were as follows: VEGF-1738L; 5′-CATCTCGAGGCCGGGCAGGAGGA AGGAGCCTCCCT-3′, VEGF-3022R; 5′-CATTCTAGAACTCT TTAATTAAATTAACTGTTTTAA-3′, VEGF-3043L; 5′-CATTCT AGATTGGTTAATATTTAATTTCAACTATTT-3′, VEGF-3659R; 5′-CATGCGGCCGCGAGATCAGAATTAAATTCTTTAAT-3′, Flt- 4301L; 5′-CATCTCGAGAGAGTTTGACACGAAGCCTTATTT CT-3′, Flt-4357R; 5′-CATGAATTCCTGGGGGTATAAATACAC ATGTG-3′, Flt-4378L; 5′-CATGAATTCTGCATATATAAGTTT ACACCTTTA-3′, Flt-5217R; 5′- CATGCGGCCGCAAATAGAA TGTGACATTTTCAGTGT-3′, KDR-4374L; 5′-GATCTCGAGAA GGAAGCATCCACACCCCCAACT-3′, KDR-6055R; 5′-GATGCGGCCGCTCTGATTCCTTCTTCTCCATTTT-3′, KDR-5388R; 5′-CATAAGCTTAGAGTGGGTTGGGGACAGGGGGA-3′, KDR- 5410L; 5′-CATAAGCTTTAGTTATTTGGCCTCTACTCCAGTA- 3′, KDR-5738R; 5′-CATTCTAGAAGTACAAAGTTCATTATATA TAAGG-3′, KDR-5768L; 5′-CATTCTAGATAACAAAGGTCATA ATGCTTTCAGC-3′. For the luciferase assay, 8 × 104 A549 cells in a 12-well plate were cotransfected with 250 ng of luciferase vector and miRNA mimics (10 nM) using Lipofectamine 2000 (Invitrogen, USA). At 48 h post-transfection, firefly and Renilla luciferase activities were measured using Dualluciferase assay (Promega, USA) following the manufacturer’s protocol.
VEGF ELISA
One day before transfection, 7 × 104 HeLa cells were seeded in 6-well plates. The following day, cells were transfected with 20 nM miRNA mimics using Lipofectamine RNAiMAX. At 24 h post-transfection, HeLa cells were treated with 400 μM desferrioxamine (DFX) to induce hypoxic conditions. After 24 h incubation, VEGF concentration in supernatants was measured in triplicate using an ELISA kit (R&D Systems, USA).
Tube formation assay
For the tube formation assay, 5 × 105 HUVECs were seeded on 100 mm dishes, and the following day, cells were transfected with miRNA mimics (20 nM) using Lipofectamine RNAiMAX reagent. At 48 h post-transfection, HUVECs were serum-starved for 20 h by incubating in endothelial basal medium (EBM; Clonetics) with 0.2% BSA. After serum starvation, HUVECs were harvested and 8 × 104 HUVECs were seeded on a 12-well plate coated with Matrigel basement membrane matrix (BD Biosciences, USA). After 6 h cultivation in EBM containing VEGF (25 ng/ml), tube-like structures were photographed using Leica DMI6000B microscope, and cumulative tube length was measured in three photographic fields using LAS software (Leica).
ERK phosphorylation assay
At 48 h after transfection, HUVECs were serum-starved for 20 h by incubating in EBM (Clonetics, USA) with 1% BSA. After serum starvation, cells were stimulated for 5 min by adding EBM containing 25 ng/ml VEGF. Whole cell lysates were then prepared and subjected to Western blot analysis with phosphospecific ERK1/2 antibodies.
RESULTS
Identification of miRNAs targeting Flt-1 using 3′-UTR-luciferase reporter assay
To identify miRNAs that target the 3′-UTR of Flt-1, we used a 3′-UTR-luciferase reporter assay. Since the 3′-UTR of Flt-1 is relatively long, only the 5′-proximal part of the 3′-UTR (915 bp) was cloned downstream of the luciferase gene in a psiCHECK- 2 vector. To search for potential miRNA candidates that target Flt-1, we used a target prediction program called TargetScan. A total of 7 miRNAs were selected, including miR-200b and miR- 181a, 2 miRNAs whose sites are conserved among vertebrates and 5 miRNAs, miR-142-3p, miR-199a-3p, miR-587, miR-222, and miR-24, whose sites are poorly conserved. Each miRNA was cotransfected into A549 cells with a 3′-UTR-luciferase reporter plasmid, and luciferase activity was assayed at 48 h after transfection. Successful transfection of most miRNAs except miR-587 resulted in down-regulation of luciferase activity (Fig. 1). Among them, miR-200b and miR-24 reduced luciferase activity below 50% of that in NC-transfected cells. Since KDR and VEGF play a key role in VEGF signaling in addition to Flt-1, we examined whether there is a potential miRNA which down-regulates all three of these genes. Using TargetScan, we found that the predicted binding site for miR-200b/-200c/-429, three members of the miR-200 family sharing the same seed sequence, is present in all three genes. One binding site was found in Flt-1 and VEGF genes, and two were found in the KDR gene (Fig. 2C). Therefore, we next examined whether miR-200b down-regulates all three genes through binding to the predicted sites in their 3′-UTRs.
Fig. 1. Screen for miRNAs that target the 3′-UTR of Flt-1. (A) A schematic diagram of binding sites for miRNAs predicted by TargetScan in the 3′-UTR of Flt-1. Only the 5′-proximal part of the 3′- UTR (915 bp) was cloned into a psiCHECK-2 vector. Numbers in parentheses represent the location of miRNA binding sites in the Flt-1 gene. (B) Luciferase reporter assay. At 48 h after cotransfection with miRNAs and a luciferase reporter plasmid harboring 3′- UTR of Flt-1, luciferase activity was measured using Dual-luciferase assay. Luciferase activity in NC-transfected cells was set at 100%. Results represent the mean ± SD from three independent experiments. **P < 0.01, ***P < 0.05.
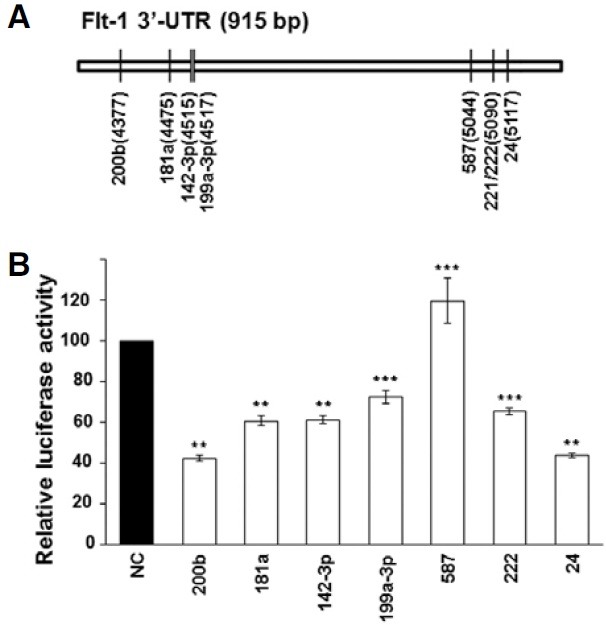
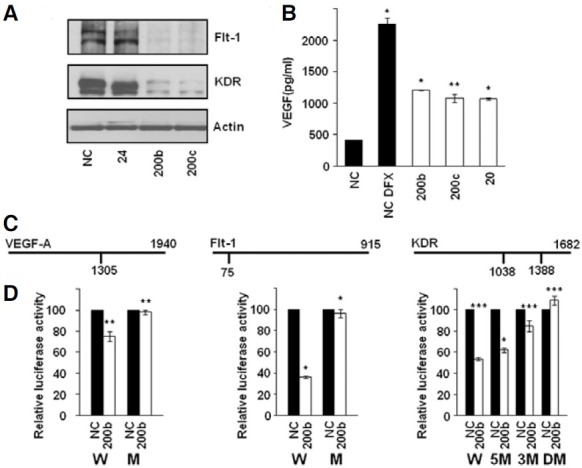
Fig. 2. Down-regulation of VEGF, Flt-1, and KDR by miR-200b through direct targeting of 3′-UTRs. (A) Western blots of lysates from HUVECs transfected with miRNA mimics for 48 h. (B) At 48 h after transfection of HeLa cells with miRNA mimics, VEGF concentration in the culture medium was measured by ELISA. Hypoxia was induced by DFX treatment for 24 h before harvesting the culture medium. Results represent the mean ± SD from three independent experiments. *P < 0.001, **P < 0.01. (C) Schematic diagrams of binding sites for miR-200b in the 3′- UTRs of VEGF, Flt-1, and KDR. The number below the vertical bar indicates the nucleotide position of the binding site for miR-200b. (D) Direct targeting of 3′-UTRs of VEGF, Flt-1, and KDR by miR-200b as revealed by luciferase reporter assay. At 48 h after transfection with miRNA, luciferase activity was measured in A549 cells. Normalized Renilla luciferase activity in cells transfected with NC was set at 100%. Luciferase activity of cells transfected with NC and miR-200b is shown as filled and open bars, respectively. Results represent the mean ± SD from three independent experiments. *P < 0.001, **P < 0.01, ***P < 0.05. W, wild-type 3′-UTR; M, mutated 3′-UTR; 5M, mutated at 5′ site in 3′-UTR of KDR, 3M, mutated at 3′ site in 3′-UTR of KDR; DM, mutated at both 5′ and 3′ sites in 3′-UTR of KDR.
VEGF, Flt-1, and KDR are direct targets of miR-200b
To validate VEGF, Flt-1, and KDR as targets of miR-200b, down-regulation of three genes at the protein level by miR-200b was examined by Western blot and ELISA analyses. Additionally included in these analyses was miR-200c, which shares the same seed sequence with miR-200b. As can be seen in Fig. 2A, Flt-1 and KDR proteins in miR-200b- and miR-200ctransfected HUVECs were significantly decreased compared to NC-transfected cells. Although luciferase activity was significantly decreased when A549 cells were cotransfected with miR-24 and luciferase reporter plasmid harboring the 3′-UTR of Flt-1 (Fig. 1), Western blot analysis revealed that Flt-1 protein was not decreased after transfection of HUVECs with miR-24. Since VEGF is up-regulated and secreted under hypoxic conditions, miRNA-transfected HeLa cells were treated with DFX for 24 h to mimic hypoxia, and VEGF concentrations in culture supernatants were measured by ELISA. We observed that the VEGF level in miR-200b- and miR-200c-transfected HeLa cells was decreased to ~50% of that in NC-transfected cells under DFX-treated conditions (Fig. 2B). When transfected with miR- 20, which is known to target VEGF, secreted VEGF level was similar to that in miR-200b- and miR-200c-transfected cells (Hua et al., 2006). Together, these results show that miR-200b and miR-200c down-regulate Flt-1, KDR, and VEGF at the protein level.
To demonstrate direct interaction between miR-200b and the 3′-UTRs of these 3 genes, we investigated the effect of miR- 200b on the activity of 3′-UTR-luciferase reporter plasmids. As can be seen in Fig. 2D, cotransfection of miR-200b with a luciferase reporter plasmid bearing the 3′-UTR of VEGF, Flt-1, or KDR resulted in significant decreases in luciferase activity compared to that in NC-transfected cells. Although luciferase activity after transfection with miR-200b was somewhat high in the case of VEGF, a similar level of luciferase activity was observed in cells transfected with miR-20 (data not shown). To verify that miR-200b interacts with the predicted binding sites in the 3′-UTRs, these sites were deleted from 3′-UTR-luciferase reporter plasmids, and the resulting plasmids were cotransfected with miR-200b. We observed that luciferase activity was not decreased when predicted binding sites were deleted from the 3′-UTRs of VEGF, Flt-1, and KDR (Fig. 2D). In the case of KDR, the 2 binding sites in the 3′-UTR appeared to function synergistically, with the downstream 3′ binding site being more effective. In addition to miR-200b, cotransfection with miR-200c yielded essentially the same results in luciferase reporter assays using wild-type and mutant 3′-UTR-luciferase reporter plasmids (data not shown). Taken together, these data indicate that miR-200b directly regulates VEGF, Flt-1, and KDR through interaction with predicted binding sites in their 3′-UTRs.
Tube formation is inhibited by miR-200b/-200c
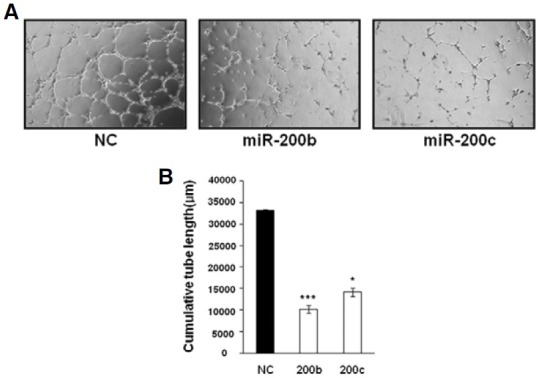
Since capillary tube formation on Matrigel is an essential angiogenic property of HUVECs, we investigated whether downregulation of KDR and Flt-1 by miR-200b and miR-200c affects tube formation. At 48 h after transfection, HUVECs were serum- starved overnight and the following day, HUVECs were cultured on a Matrigel-coated 12-well plate for 6 h. As can be seen in Fig. 3, NC-transfected HUVECs formed well-organized capillary-like structures. However, transfection with miR-200b and miR-200c resulted in significant impairment of tube-forming activity. Thus, these results indicate that miR-200b and miR- 200c have an adverse effect on angiogenic activity of HUVECs
Fig. 3. Tube-formation ability of HUVECs was inhibited by miR-200b/-200c. At 48 h post-transfection with miRNA mimics, HUVECs were serumstarved for 20 h and then cultured on Matrigel in EBM containing VEGF for 6 h. (A) Micrographs of capillary-like structures formed by HUVECs transfected with NC, miR-200b, and miR-200c. (B) Cumulative tube length was measured in three photographic fields using LAS software (Leica). *P < 0.001, ***P < 0.05.
ERK phosphorylation is inhibited by miR-200b
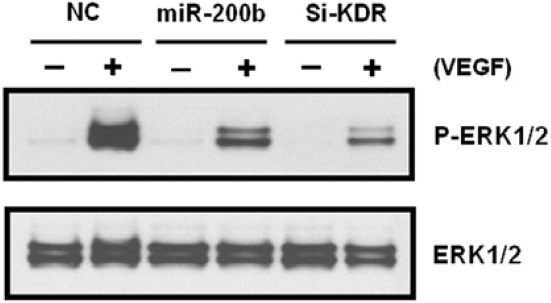
Since ERK1/2 phosphorylation is one of the downstream pathways of VEGF-KDR signaling, we examined whether downregulation of KDR by miR-200b affects ERK1/2 phosphorylation. At 48 h post-transfection, HUVECs were serum-starved for 20 h, stimulated by VEGF for 5 min, and subsequently subjected to Western blot analysis. As can be seen in Fig. 4, ERK phosphorylation was strongly induced by VEGF in NC-transfected cells. However, VEGF-inducedhorylation was significantly reduced in miR-200b-transfected cells. A weak induction ofhorylation by VEGF was also observed in HUVECs transfected with KDR siRNA. These data indicate that a decrease in KDR protein by miR-200b suppresses VEGFinduced activation of the ERK pathway as evident by reduction inhorylation.
Fig. 4. Inhibitory effects of miR-200b on VEGF-induced ERK phosphorylation. Western blots of HUVEC lysates. After transfection with miRNA mimics and subsequent serum starvation, cells were stimulated for 5 min by VEGF. Phosphorylation of ERK1/2 was detected by anti-phospho-Thr202/Tyr204 ERK antibody. Si-KDR; KDR siRNA.
DISCUSSION
In this study, we identified miR-200b as a regulator of VEGF signaling. Intriguingly, both VEGF and its receptors (Flt-1 and KDR) were negatively regulated by miR-200b. Previously, it was reported that the miR-200 family, which comprises 5 members (miR-200a, -200b, -200c, miR-429, and miR-141), regulates the epithelial-mesenchymal transition (EMT) (Gregory et al., 2008; Park et al., 2008). Direct targets of the miR-200 family are E-cadherin transcriptional repressors ZEB1 and ZEB2. Thus, loss of miR-200 expression during EMT leads to up-regulation of ZEB1 and ZEB2, which represses the expression of E-cadherin, a mediator of cell-cell adhesion, in mesenchymal cells. Transfection with a miR-200b inhibitor promoted EMT and conversely, mesenchymal-epithelial transition (MET) was induced by ectopic expression of a synthetic miR-200b precursor, indicating the direct effect of miR-200b on EMT or MET. In our study, we found that E-cadherin was not expressed in HUVECs even after transfection with a miR-200b mimic, suggesting that DNA methylation may play a role in the inactivation of E-cadherin gene in HUVECs (Graff et al., 1995; Yoshiura et al., 1995). Thus, the possibility that E-cadherin reexpression after ectopic expression of miR-200b affects angiogenic phenotype can be ruled out in the case of HUVECs.
Recently, Liu et al. (2010) reported that miRNA targets can be identified by analyzing expression levels of mRNA and miRNA in tumor and normal samples. They selected mRNAs containing a conserved seed sequence in their 3′-UTRs for miRNAs which are differentially expressed between tumor and normal kidney and identified target mRNAs whose expression level has an inverse correlation with that of miRNA. Using clear cell Renal Cell Carcinoma and matched normal kidney samples, they found that a strong negative correlation is observed between VEGF and the miR-200 family. Thus, this result lends support to our conclusion that VEGF is a direct target of miR- 200b as revealed by ELISA and luciferase reporter assays.
Recent studies showed that epigenetic mechanisms are involved in the regulation of miR-200 expression (Vrba et al., 2010; Wiklund et al., 2010). Since an inverse correlation exists between DNA methylation and miR-200 expression in tumor and normal cells, we investigated the methylation status of 2 regions, including 1-200 bp downstream of miR-200b and 234- 371 bp upstream of miR-200c. Bisulfite sequencing revealed that CpG dinucleotides in the analyzed regions are heavily methylated, suggesting transcriptional repression of miR-200 family members by DNA methylation in HUVECs (82.5% and 81.8% methylation in miR-200b and miR-200c loci, respectively). Considering the inhibitory effect of miR-200 on VEGF signaling, it is likely that the miR-200 family is silenced or expressed at low levels in HUVECs.
Two recent papers reported that miR-200b is involved in angiogenesis. As stated earlier, miR-200b targets Ets-1 and inhibits angiogenic activity of HMECs (Chan et al., 2011). Another paper reported that miR-200b targets murine Flt-1 (Roybal et al., 2011). Down-regulation of Flt-1 by miR-200b suppressed invasion and metastasis of lung adenocarcinoma cells. Thus, these studies and our current results indicate that miR-200b plays an important role in angiogenesis by targeting multiple angiogenesis- related genes. Blocking VEGF signaling by targeting both the ligand and its receptors may be an effective means of inhibiting angiogenesis.
In summary, we identified a novel role for miR-200b in the regulation of VEGF signaling. We also demonstrated that direct targets of miR-200b are VEGF and its receptors, Flt1 and KDR, which play a pivotal role in VEGF signaling. Since tumor angiogenesis depends on VEGF signaling, miR-200b can function as an angiogenesis inhibitor. In addition, miR-200b targets the Ets- 1 transcription factor, whose down-regulation inhibits angiogenic activity of HMECs (Chan et al., 2011). Ets-1 targeting by miR-200b results in down-regulation of multiple target genes including KDR. Thus, in the case of KDR, miR-200b downregulates it both directly and indirectly via Ets-1. In addition to its role in angiogenesis, miR-200b functions as a regulator of EMT in cancer cells. Thus, introduction of miR-200b into cancer cells has a potential to reverse the process of tumor metastasis. Restoration of miR-200b in cancer and endothelial cells to inhibit multiple targets involved in angiogenesis and tumor metastasis may provide a new therapy to treat cancer.
Acknowledgments
This work was supported by the Kyung Hee University Research Fund in 2009 (KHU-20090718).
References
- 1.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. (2004);116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. (2005);438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 3.Chan Y.C., Khanna S., Roy S., Sen C.K. miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J. Biol. Chem. (2011);286:2047–2056. doi: 10.1074/jbc.M110.158790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eskens F.A. Angiogenesis inhibitors in clinical development; where are we now and where are we going? Br.J. Cancer. (2004);90:1–7. doi: 10.1038/sj.bjc.6601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. (1999);77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr. Rev. (2004);25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N., Kerbel R.S. Angiogenesis as a therapeutic target. Nature. (2005);438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N., Gerber H.P., LeCouter J. The biology of VEGF and its receptors. Nat. Med. (2003);9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 9.Fish J.E., Santoro M.M., Morton S.U., Yu S., Yeh R.F., Wythe J.D., Ivey K.N., Bruneau B.G., Stainier D.Y., Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell. (2008);15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. (1971);285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. (1995);1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 12.Graff J.R., Herman J.G., Lapidus R.G., Chopra H., Xu R., Jarrard D.F., Isaacs W.B., Pitha P.M., Davidson N.E., Baylin S.B. E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res. (1995);55:5195–5199. [PubMed] [Google Scholar]
- 13.Gregory P.A., Bert A.G., Paterson E.L., Barry S.C., Tsykin A., Farshid G., Vadas M.A., Khew-Goodall Y., Goodall G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. (2008);10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 14.He L., Hannon G.J. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. (2004);5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 15.Hua Z., Lv Q., Ye W., Wong C.K., Cai G., Gu D., Ji Y., Zhao C., Wang J., Yang B.B., et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One. (2006);1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim V.N. Small RNAs: classification, biogenesis, and function. Mol. Cells. (2005);19:1–15. [PubMed] [Google Scholar]
- 17.Kim S., Lee U.J., Kim M.N., Lee E.J., Kim J.Y., Lee M.Y., Choung S., Kim Y.J., Choi Y.C. MicroRNA miR- 199a* regulates the MET proto-oncogene and the downstream extracellularsignal-regulated kinase 2 (ERK2). J. Biol. Chem. (2008);283:18158–18166. doi: 10.1074/jbc.M800186200. [DOI] [PubMed] [Google Scholar]
- 18.Liu H., Brannon A.R., Reddy A.R., Alexe G., Seiler M.W., Arreola A., Oza J.H., Yao M., Juan D., Liou L.S., et al. Identifying mRNA targets of microRNA dysregulated in cancer: with application to clear cell renal cell carcinoma. BMC Syst. Biol. (2010);4:51. doi: 10.1186/1752-0509-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S.M., Gaur A.B., Lengyel E., Peter M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. (2008);22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roybal J.D., Zang Y., Ahn Y.H., Yang Y., Gibbons D.L., Baird B.N., Alvarez C.A., Thilaganathan N., Saintigny P., Liu D., et al. miR-200 inhibits lung adenocarcinoma cell invasion and metastasis by targeting Flt1/VEGFR1. Mol. Cancer Res. (2011);9:25–35. doi: 10.1158/1541-7786.MCR-10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suárez Y., Sessa W.C. MicroRNAs as novel regulators of angiogenesis. Circ. Res. (2009);104:442–454. doi: 10.1161/CIRCRESAHA.108.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Solingen C., Seghers L., Bijkerk R., Duijs J.M., Roeten M.K., van Oeveren-Rietdijk A.M., Baelde H.J., Monge M., Vos J.B., de Boer H.C., et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J. Cell. Mol. Med. (2009);13:1577–1585. doi: 10.1111/j.1582-4934.2008.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrba L., Jensen T.J., Garbe J.C., Heimark R.L., Cress A.E., Dickinson S., Stampfer M.R., Futscher B.W. Role for DNA methylation in the regulation of miR-200c and miR-141 expression in normal and cancer cells. PLoS One. (2010);5:e8697. doi: 10.1371/journal.pone.0008697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S., Olson E.N. AngiomiRs--key regulators of angiogenesis. Curr. Opin. Genet. Dev. (2009);19:205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A., Richardson J.A., Bassel-Duby R., Olson E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell. (2008);15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiklund E.D., Bramsen J.B., Hulf T., Dyrskjøt L., Ramanathan R., Hansen T.B., Villadsen S.B., Gao S., Ostenfeld M.S., Borre M., et al. Coordinated epigenetic repression of the miR- 200 family and miR-205 in invasive bladder cancer. Int. J. Cancer. (2010);128:1327–1334. doi: 10.1002/ijc.25461. [DOI] [PubMed] [Google Scholar]
- 27.Wu F., Yang Z., Li G. Role of specific microRNAs for endothelial function and angiogenesis. Biochem. Biophys. Res. Commun. (2009);386:549–553. doi: 10.1016/j.bbrc.2009.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshiura K., Kanai Y., Ochiai A., Shimoyama Y., Sugimura T., Hirohashi S. Silencing of the E-cadherin invasionsuppressor gene by CpG methylation in human carcinomas. Proc. Natl. Acad. Sci. USA. (1995);92:7416–7419. doi: 10.1073/pnas.92.16.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]


