Abstract
Orthodontic force causes gradual compression of the periodontal ligament tissues, which leads to local hypoxia in the compression side of the tissues. In this study, we investigated whether antioxidants exert a regulatory effect on two factors: the expression of pro-inflammatory cytokines in human periodontal ligament fibroblasts (PDLFs) that were exposed to mechanical compression and hypoxia and the rate of orthodontic tooth movement in rats. Exposure of PDLFs to mechanical compression (0.5-3.0 g/cm2) or hypoxic conditions increased the production of intracellular reactive oxygen species. Hypoxic treatment for 24 h increased the mRNA levels of IL-1β, IL-6 and IL-8 as well as vascular endothelial growth factor (VEGF) in PDLFs. Resveratrol (10 nM) or N-acetylcysteine (NAC, 20 mM) diminished the transcriptional activity of hypoxiainducible factor-1 and hypoxia-induced expression of VEGF. Combined treatment with mechanical compression and hypoxia significantly increased the expression levels of IL-1β, IL-6, IL-8, TNF-α and VEGF in PDLFs. These levels were suppressed by NAC and resveratrol. The maxillary first molars of rats were moved mesially for seven days using an orthodontic appliance. NAC decreased the amount of orthodontic tooth movement compared to the vehicletreated group. The results from immunohistochemical staining demonstrated that NAC suppressed the expression of IL-1β and TNF-α in the periodontal ligament tissues compared to the vehicle-treated group. These results suggest that antioxidants have the potential to negatively regulate the rate of orthodontic tooth movement through the down-regulation of pro-inflammatory cytokines in the compression sides of periodontal ligament tissues.
Keywords: antioxidant, hypoxia, mechanical compression, orthodontic tooth movement, pro-inflammatory cytokine
INTRODUCTION
Classically, orthodontic tooth movement has been explained by the “pressure-tension” hypothesis. Bone resorption is dominant in the compression side of the periodontal ligament (PDL) tissues, whereas placing the PDL tissues under tensile force leads to bone deposition. The recruitment and differentiation of osteoclasts in the compression side of PDL tissues is essential for bone resorption and tooth movement. Numerous studies have shown that pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6 and IL-8, play an important role in orthodontic tooth movement through the regulation of osteoclast differentiation and matrix metalloproteinase expression (Jager et al., 2005; Meier et al., 1989). In addition to the pro-inflammatory cytokines, vascular endothelial growth factor (VEGF) is also involved in orthodontic tooth movement. VEGF is detected within the periodontal tissues during orthodontic tooth movement, and local administration of recombinant VEGF enhances the extent of tooth movement (Kaku et al., 2001; Kohno et al., 2003). Factors that affect the production and/or function of these cytokines exert regulatory effects on the rate of orthodontic tooth movement. Local administration of soluble receptors to IL-1 or TNF-α or neutralizing antibodies to VEGF blocks the function of IL-1, TNF-α or VEGF, respectively, and retards orthodontic tooth movement, whereas stimulation of the expression of pro-inflammatory cytokines by osteoperforation near the moving teeth increases the rate of tooth movement (Jager et al., 2005; Kohno et al., 2005; Teixeira et al., 2010).
Orthodontic force causes gradual compression of the PDL, which leads to circulatory disturbances, such as ischemia and hypoxia, in the compression side of the PDL tissues at the early stages of orthodontic tooth movement (Arai et al., 2010). Both hypoxia and compression stimulate the production of reactive oxygen species (ROS) (Chandel et al., 1998; Xu et al., 2005). Several lines of evidence indicate that activation of NF-κB can be controlled by ROS, and antioxidants block pro-inflammatory cytokine transcription by preventing NF-κB migration to the nucleus (Janssen-Heininger et al., 2000; Schubert et al., 2002). Furthermore, previous studies have demonstrated that Nacetylcysteine (NAC), an antioxidant, decreases lipopolysaccharde- induced production of pro-inflammatory cytokines in gingival fibroblasts, and NAC decreases alveolar bone loss in experimental periodontitis (Kim et al., 2007; Toker et al., 2009). These reports suggest that antioxidants may exert a regulatory effect on the rate of orthodontic tooth movement by regulating the production of pro-inflammatory cytokines. The consumption of antioxidant supplements in developed countries has become widespread, and about one-third of adults are addicted to these supplements (Millen et al., 2004). Their widespread use has created a need to address the possibility that antioxidants might delay orthodontic tooth movement. Therefore, in this study, we investigated whether antioxidants exert a regulatory effect on two factors: the expression of pro-inflammatory cytokines in human periodontal ligament fibroblasts (PDLFs) that were exposed to mechanical compression and hypoxia and the rate of orthodontic tooth movement in rats.
MATERIALS AND METHODS
Materials
Alpha-modified Eagles medium (α-MEM), fetal bovine serum (FBS) and other cultural reagents were obtained from Hyclone (USA). The 2′,7′-dichlorofluorescein diacetate (DCFH-DA), NAC, N-acetylalanine (NAA) and resveratrol were purchased from Sigma (USA). GasPak™ EZ CO2 Pouch System was purchased from BD (USA). The easy-BLUE™ was purchased from iNtRON Biotechnology (Korea), the AccuPower RT-PreMix was purchased from Bioneer (Korea), and the SYBR Premix Ex Taq™ was purchased from TaKaRa (Japan). The PCR primers were synthesized by CosmoGenetech (Korea). Human IL-1β, IL-6, IL-8, TNF-α and VEGF ELISA kit pink-ONE were obtained from Koma Biotech (Korea). Anti-IL-1β and anti-TNF-α antibodies were purchased from Bioworld Technology (USA), and the Rabbit Vectastatin Elite ABC kit was obtained from Vector Laboratories (USA). LipofectAMINE 2000 reagent was purchased from Invitrogen (USA). The Bright-Glo™ luciferase assay system was ordered from Promega (USA).
Cell culture and exposure to mechanical compression and hypoxia
Human PDLFs (ScienCell™ research laboratories, USA) were maintained in α-MEM supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. PDLFs were used between passages four and six. To examine the effect of antioxidants, the PDLFs were exposed to 10 nM resveratrol or 20 mM NAC.
Mechanical compression was applied to the PDLFs using the following procedure. PDLFs were seeded in six-well culture plates and grown to confluence. Then, a cover glass (diameter, 25 mm; Marienfeld, Germany) was put on the confluent cell layer, and a plastic cylinder was placed over the cover glass in the wells. The compressive force was adjusted by adding metal slices to the cylinder. PDLFs were subjected to 0.5, 1.0, 2.0 or 3.0 g/cm2 of compressive force for the indicated period of time.
To induce hypoxia, the PDLFs were transferred to a GasPak Pouch, where the total oxygen concentration was reduced to less than 1%, and the PDLFs were incubated for the indicated period of time (Steinbach et al., 2004). To induce hypoxia inducible factor (HIF), the PDLFs were incubated for 24 h in the ducible factor (HIF), the PDLFs were incubated for 24 h in the presence of desferoxamine (200 μM), a high affinity iron chelator (Hamrick et al., 2005).
Measurement of intracellular ROS
Intracellular ROS were measured using DCFH-DA. DCFH-DA diffuses into cells and is hydrolyzed to DCFH. DCFH is then converted by ROS-mediated oxidation to the highly fluorescent derivative, 2′,7′-dichlorofluorescein (DCF) (Bass et al., 1983). To examine the effect of mechanical compression on ROS production, the PDLFs were seeded onto cover glasses (diameter, 25 mm) at a density of 8 × 104 cells/cover glass, incubated in six-well culture plates and grown to confluence. PDLFs were serum-starved for 16-18 h, pre-incubated for 30 min in serum-free medium containing 50 μM DCFH-DA and transferred to fresh serum-free medium. Then, mechanical compression was applied to the PDLFs as described above for 4 h. At the end of incubation period, the cells were washed and immediately observed using the OLYMPUS FV300 (OLYMPUS, Japan) at an excitation wavelength of 488 nm. About 30 cells were randomly selected from three independent experiments, and DCF fluorescence intensities were quantified using Flou- View 4.3 (OLYMPUS). To examine the effect of hypoxia on ROS production, the PDLFs were seeded into 96-well culture plates, incubated overnight and serum-starved for 16-18 h. Cells were then pre-incubated for 30 min in serum-free medium containing 50 μM DCFH-DA, transferred to fresh serum-free medium and incubated for 4 h in a GasPak Pouch. DCF fluorescence was measured using a FLUOstar OPTIMA (BMG Lab-Technologies, Germany).
Quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis
Quantitative RT-PCR was performed to evaluate mRNA expression. Total RNA was isolated using easy-BLUE™ RNA Extraction Reagents. Complementary DNA was synthesized from 1 μg of total RNA using the AccuPower RT-PreMix and subsequently used for PCR amplification. Real-time PCR was performed using the SYBR Premix Ex Taq™ and AB 7500 Fast Real-Time system (Applied Biosystems, USA). Each sample was analyzed in triplicate, and target genes were normalized to the reference housekeeping gene, GAPDH. Fold differences were calculated for each treatment group using normalized CT values for the control. Primer sequences used for real-time PCR were as follows: IL-1β-forward (f) 5′-GTCATTCGCTCCC ACATTCT-3′, IL-1β-reverse (r) 5′-ACTTCTTGCCCCCTTTGA AT-3′; IL-6-f 5’-ATGCAATAACCACCCCTGAC-3′, IL-6-r 5′- AAAGCTGCGCAGAATGAGAT-3′; IL-8-f 5′-CAGGAATTGAAT GGGTTTGC-3′, IL-8-r 5′-AGCAGACTAGGGTTGCCAGA-3′; TNF-α-f 5′-TAGGCTGTTCCCATGTAGCC-3′, TNF-α-r 5′-CA GAGGCTCAGCAATGAGTG-3′; VEGF-f 5′-GCTGTCTTGGG TGCATTGGA-3′, VEGF-r 5′-ATGATTCTGCCCTCCTCCTTCT -3′; and GAPDH-f 5′-CCATCTTCCAGGAGCGAGATC-3′, GAPDH-r 5′-GCCTTCTCCATGGTGGTGAA-3′.
Enzyme-linked immunosorbent assay (ELISA)
PDLFs were exposed to mechanical compression and hypoxia in the presence or absence of resveratrol (10 nM) for 48 h. The concentrations of IL-6, IL-8, TNF-α and VEGF that were secreted by PDLFs into culture medium were analyzed using the respective ELISA kits according to the manufacturer’s instructions.
Luciferase reporter assay
The erythropoietin enhancer-driven luciferase reporter gene (EPO-luc) was a generous gift from Prof. J.-W. Park at Seoul National University (Chun et al., 2002). PDLFs were seeded in a 96-well plate at a density of 5 × 103 cells/well. After overnight culture, the cells were transiently transfected with EPO-luc using the LipofectAMINE™ reagent. After 24 h incubation in the presence or absence of desferoxamine and antioxidants, the cells were harvested, and the luciferase activity was measured using the Bright-Glo luciferase assay kit.
Induction of tooth movement in rats
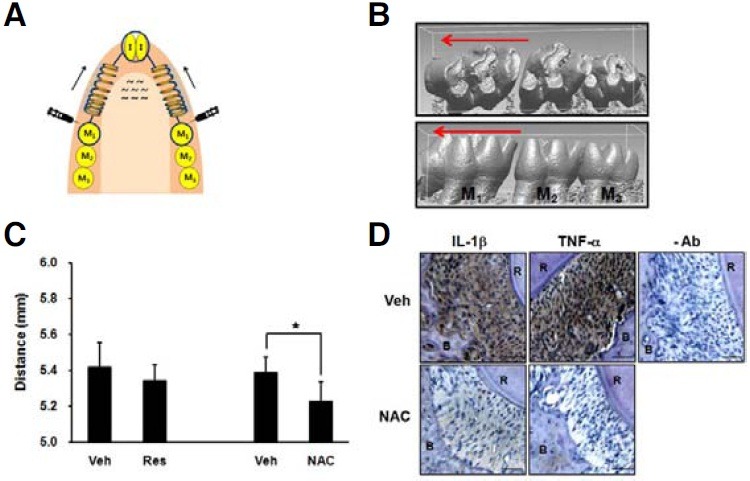
All experimental procedures were approved by the Institute of Laboratory Animal Resources at Seoul National University. Sixweek- old male Sprague Dawley rats (body weight around 200 g) were used in this study. The rats were anesthetized with a peritoneal injection of a mixture of Zoletil (Virbac, France) and Rompun (Bayer Korea, Korea). Twelve rats divided into groups of six were subjected to orthodontic forces. A closed coil nickeltitanium spring (0.009 × 0.036, Sentalloy, GAC International, USA) was connected between the maxillary first molar and maxillary central incisors with ligature wires (Fig. 6A). To prevent dislodgement of the spring, the maxillary first molar and maxillary central incisors were notched on the mesial and distal surfaces. After the ligatures were tied up, light-curing composite resin bonding material (3M Unitek, USA) was placed over the wire to prevent slipping. The appliance was activated immediately upon insertion with an initial force of 40 g, and the fit was checked daily. No reactivation was performed during the experimental period. To examine the effect of antioxidants on tooth movement, a split-mouth design was used. The experimental side was randomly chosen, and the contralateral was used as the vehicle. A daily injection of 50 μl of antioxidant or vehicle was performed in the buccal mucosa adjacent to the mesial surface of the first molars. The first injection was performed 10 min before the placement of the appliance. Resveratrol was solved in ethanol and diluted with phosphate-buffered saline immediately before injection (final concentration, 200 μg/50 μl). Ethanol of the same concentration that was diluted in phosphate-buffered saline was used as a vehicle for resveratrol. NAC was solved in distilled water, and the pH of the solution was adjusted to 7.4 (final concentration, 25 mg/50 μl). To minimize the effect of high osmolarity, NAA of the same concentration was used as a vehicle control for NAC. Seven days after appliance placement, the rats were sacrificed, and the maxilla was dissected, divided into two halves and prepared for micro CT examination (Skyscan 1172, Belgium). The micro CT images of the maxilla were reconstructed three dimensionally (Fig. 6B), and the amount of tooth movement was compared by measuring the distance between the mesial margin of the mesial fossa of the first molar and the distal margin of the distal fossa of the third molar with Rapidform2006 software (Inus Technology, Korea).
Fig. 6. NAC retards orthodontic tooth movement and suppresses the expression of IL-1β and TNF-α in rat PDL tissues. (A) Illustration for the design of orthodontic tooth movement. The mesial movement of the maxillary first molars was induced by applying closed nickel-titanium spring. To examine the effect of antioxidants on tooth movement, a split-mouth design was used. Daily injection of 50 μl of antioxidant (Res 200 μg, NAC 25 mg) or vehicle (Veh) was performed in the buccal mucosa adjacent to the mesial surface of the first molar. Seven days after appliance placement, the rats were sacrificed. Arrow shows the direction of tooth movement. I; incisor, M; molar. (B) Representative micro CT images of vehicle-treated group: (upper) occlusal view, (lower) buccal view. The micro CT images of maxilla were obtained and reconstructed three dimensionally. (C) The distance between the mesial fossa of first molar and the distal fossa of the third molar was measured. The data represent the mean ± S.D. of five independent experiments. *p < 0.05. (D) Horizontal slices of the decalcified specimens were prepared, and immunohistochemical staining for IL- 1β and TNF-α was performed. Scale bar = 50 μm. -Ab, staining without primary antibody; R, root; B, alveolar bone.
Immunohistochemical staining
The animals were sacrificed, and the maxillae were dissected, fixed in 10% buffered neutral formalin for 48 h and decalcified in 10% EDTA for 3 weeks. The decalcified specimens were dehydrated and embedded in paraffin. Each sample was sliced into 5 μm continuous sections in the horizontal direction and prepared for immunohistochemical staining. Deparaffinized sections were immersed in 0.3% H2O2/methanol for 20 min to quench the endogenous peroxidase activity. The sections were pre-incubated with 1% goat serum in phosphate-buffered saline for 30 min and incubated overnight at 4℃ with rabbit anti-IL-1β or rabbit anti-TNF-α antibodies at a 1:1000 dilution. After washing, these antibodies were detected using the Rabbit Vectastatin Elite ABC kit. Antigen-antibody complexes were visualized using 3′,3′-diaminobenzidine and counterstaining with hematoxylin. Staining specificity was tested by the omission of primary antibody.
Statistic analysis
The data were expressed as the mean ± S.D., and the statistical significance was analyzed by Student’s t-test. Because the distribution of the data from the animal experiments had a Gaussian distribution (Kolmogorov-Smirnov test; p > 0.05), the paired t-test was used to compare the amount of tooth movement. A p value less than 0.05 was considered to be statistically significant.
RESULTS
Both mechanical compression and hypoxic treatment increases ROS production
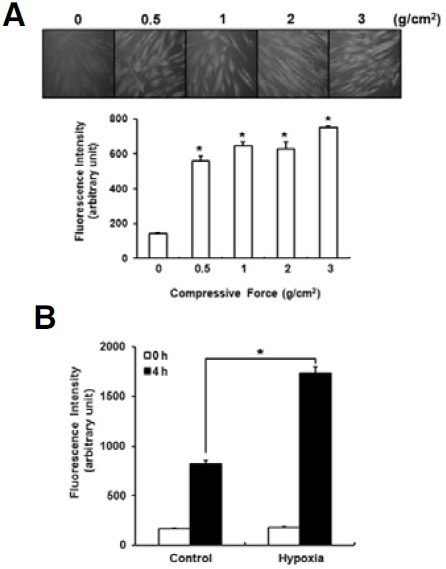
PDL tissues on the compression sides are exposed to mechanical compression and hypoxia during orthodontic tooth movement. Because previous studies have demonstrated that hypoxic condition or mechanical compression stimulates ROS generation, we first examined whether the intracellular ROS level is increased by mechanical compression or hypoxic treatment in human PDLFs cultured in vitro (Chandel et al., 1998; Xu et al., 2005). The levels of intracellular ROS were significantly increased when PDLFs were exposed to a mechanical compression of 0.5 to 3 g/cm2 for 4 h (Fig. 1A). When the PDLFs were incubated in hypoxic conditions for 4 h, intracellular ROS levels were much higher than levels in the PDLFs incubated in normoxic conditions (Fig. 1B).
Fig. 1. Mechanical compression and hypoxia increase intracellular ROS production in human PDLFs. Human periodontal ligament fibroblasts (PDLFs) were exposed to mechanical compression (A) or hypoxic conditions (B) for 4 h, and intracellular reactive oxygen species (ROS) were measured using DCFH-DA, which is converted to fluorescence emitting DCF by ROS. *p < 0.05.
Hypoxic treatment increases the expression of pro-inflammatory cytokines
Although the stimulatory effects of mechanical compression on the expression of pro-inflammatory cytokines have been well known, there are only a few reports demonstrating the effect of hypoxia on the expression of pro-inflammatory cytokines in human PDLFs (Motohira et al., 2007). Therefore, we examined whether hypoxic treatment induces the expression of proinflammatory cytokines in PDLFs. When the PDLFs were incubated in the hypoxic conditions for 24 h, the mRNA levels of IL- 1β, IL-6 and IL-8 were significantly increased, and the induction folds were similar to or higher than VEGF (Fig. 2). Although not statistically significant, the level of TNF-α mRNA was also increased (Fig. 2).
Fig. 2. Hypoxic treatment induces the expression of pro-inflammatory cytokines and VEGF in human PDLFs. PDLFs were incubated in GasPak pouches for 24 h to induce hypoxia. The levels of pro-inflammatory cytokines and vascular endothelial growth factor (VEGF) were determined by quantitative RT-PCR and normalized to GAPDH. The data represent the mean ± S.D. of three independent experiments. *p < 0.05, compared to control (normoxia).
Antioxidants decrease the transcriptional activity of HIF-1
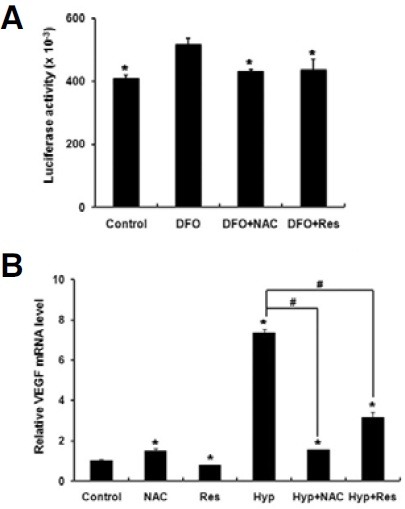
Recent reports have shown that antioxidants diminish the protein level of hypoxia-induced HIF-1 by destabilization (Gao et al., 2007; Vissers et al., 2007). Because HIF-1 is an important transcription factor for the expression of hypoxia-induced target genes, we examined whether antioxidants decrease the transcriptional activity of HIF-1 in PDLFs (Loboda et al., 2010; Storz and Imlay, 1999). Desferoxamine, a well-known inducer of HIF- 1, significantly increased the HIF-dependent reporter activity of EPO-luc (Fig. 3A). Furthermore, NAC and resveratrol blocked desferoxamine-induced increases in reporter activity (Fig. 3A). Because VEGF is a well-known target gene of HIF-1, we also examined whether antioxidants exert a regulatory effect on VEGF expression. Incubation of PDLFs under hypoxic conditions increased the level of VEGF mRNA, whereas NAC and resveratrol diminished hypoxia-induced VEGF expression (Fig. 3B).
Fig. 3. Antioxidants decrease the transcriptional activity of hypoxia inducible factor-1 and the expression of VEGF in human PDLFs. (A) PDLFs were transiently transfected with EPO-luc, an erythropoietin enhancer-driven luciferase reporter gene and incubated for 24 h in the presence or absence of desferoxamine (200 μM, DFO), resveratrol (10 nM, Res) or N-acetylcysteine (20 mM, NAC). Next, the luciferase reporter assay was performed. Data represent the mean ± S.D. of six independent experiments. *p < 0.05, compared to DFO treatment alone. (B) PDLFs were incubated for 24 h in the presence or absence of antioxidants under the normoxic or hypoxic conditions (Hyp), and quantitative RT-PCR was performed to determine the levels of VEGF mRNA. The data represent the mean ± S.D. of three independent experiments. *p < 0.05, compared to control (normoxia). #p < 0.05.
Antioxidants decrease the expression of pro-inflammatory cytokines induced by combined treatment with mechanical compression and hypoxia
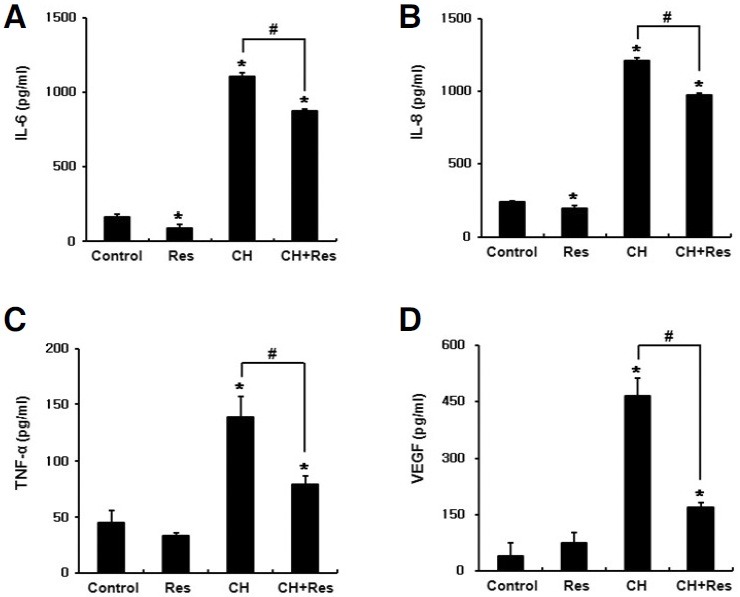
Because PDL tissues on the compression side are exposed to both mechanical compression and hypoxia in vivo, we examined the effect of antioxidants on the expression of proinflammatory cytokines in PDLFs incubated under these conditions. Combined treatment with mechanical compression and hypoxia significantly increased the mRNA levels of IL-1β, IL-6, IL-8 and TNF-α whereas the addition of resveratrol significantly diminished the induction folds of these cytokines (Fig. 4). For NAC, there was a trend of decreasing induction folds, but a statistically significant difference was only observed in IL-1β (Fig. 4). To further confirm the effect of resveratrol on the protein level, the levels of secreted cytokines were assessed by ELISA after 48 h treatment. Consistent with the results from RT-PCR analysis, combined treatment with mechanical compression and hypoxia significantly increased the levels of IL-6, IL-8 and TNF-α secretion, whereas these levels were significantly diminished by resveratrol (Fig. 5). VEGF secretion was also increased by this combined treatment, whereas secretion was significantly decreased by resveratrol (Fig. 5D). IL-1β was not detected by ELISA kit used in this experiment.
Fig. 4. Antioxidants suppress the mRNA expression of proinflammatory cytokines, which were induced by combined treatment with mechanical compression and hypoxia. PDLFs were exposed to mechanical compression of 3 g/cm2 and hypoxic conditions (CH) in the presence or absence of antioxidants for 24 h, and quantitative RT-PCR was performed to determine the levels of IL-1β (A), IL-6 (B), IL-8 (C) and TNF-α (D) mRNA. The data represent the mean ± S.D. of three independent experiments. *p < 0.05, compared to control (normoxia). #p < 0.05.
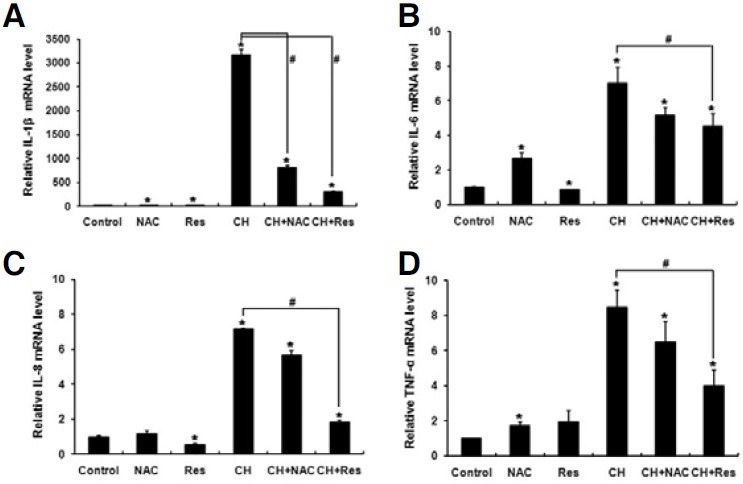
Fig. 5. Resveratrol suppresses the secretion of proinflammatory cytokines and VEGF, which were induced by combined treatment with mechanical compression and hypoxia. PDLFs were exposed to mechanical compression of 3 g/cm2 and hypoxic conditions (CH) in the presence or absence of resveratrol for 48 h, and ELISA was performed using the conditioned medium to determine the levels of IL- 6 (A), IL-8 (B), TNF-α (C) and VEGF (D). The data represent the mean ± S.D. of three independent experiments. *p < 0.05, compared to control (normoxia). #p < 0.05.
NAC decreases orthodontic tooth movement in rats
Based on the in vitro results, it can be assumed that antioxidants may affect orthodontic tooth movement by decreasing the expression of pro-inflammatory cytokines. Therefore, we examined whether local application of antioxidants delay orthodontic tooth movement in rats. Because we used 3D-reconstructed micro CT images to evaluate tooth movement, we had to sacrifice the subjects to obtain the images. Therefore, instead of measuring the actual amount of tooth movement in each subject (differences in measurement before and after orthodontic tooth movement), we measured the distance from the first molar to the third molar in each subject and compared the values of the vehicle control group to those of the antioxidant treatment group. When compared with the vehicle-treated group, the NAC-treated group showed a significant decrease in the distance between the first molar and third molar (Fig. 6C). However, there was no significant difference between the vehicle and resveratrol treatment (Fig. 6C). These results suggest that NAC delayed orthodontic tooth movement.
To verify whether antioxidants diminish the expression of proinflammatory cytokines in vivo, we performed immunohistochemical staining for IL-1β and TNF-α using the tissue sections from the vehicle- and NAC-treated rats. There was no positive staining in the absence of primary antibody (Fig. 6D). The immunoreactivity of IL-1β and TNF-α was strongly localized in the PDL tissues of the vehicle-treated group, whereas the immunoreactivity was very weak in the PDL tissues of the NAC-treated group (Fig. 6D). Furthermore, inflammatory cell infiltration was more severe in the vehicle-treated group compared to the NACtreated group. These results suggest that NAC exhibits antiinflammatory activity in PDL tissues subjected to orthodontic force.
DISCUSSION
In this study, we evaluated the effects of antioxidants on the compression and hypoxia-induced production of pro-inflammatory cytokines and orthodontic tooth movement in rats. We aimed to determine the potential influence of antioxidants on the rate of orthodontic tooth movement in humans. We have shown that NAC and resveratrol suppressed the expression of pro-inflammatory cytokines and VEGF, which were induced by combined treatment with mechanical compression and hypoxia in human PDLFs. Furthermore, in vivo experiments demonstrated that NAC delayed orthodontic tooth movement in rats.
NAC is a precursor of glutathione and functions as an ROS scavenger (Aruoma et al., 1989). Interest in NAC has increased because of its ability to inhibit synthesis of pro-inflammatory molecules and to enhance the immune functions of phagocytes and lymphocytes (Victor et al., 2003; Winyard and Blake, 1997). Studies have also demonstrated that NAC suppresses osteoclast differentiation and stimulates osteoblast differentiation (Jun et al., 2008; Lee et al., 2005). Furthermore, NAC has been shown to inhibit the expression of lipopolysaccharide-induced inflammatory mediators in gingival fibroblasts and suppress alveolar bone loss in an experimental periodontitis model in rats (Kim et al., 2007; Toker et al., 2009). Resveratrol is a naturally occurring phytoalexin present in grapes and has attracted considerable interest because of its anti-cancer effects (Jang et al., 1997). Resveratrol has been also shown to suppress osteoclast differentiation and promote osteoblast differentiation (Kupisiewicz et al., 2010). Orthodontic tooth movement is largely achieved by remodeling changes in dental and paradental tissues, especially in alveolar bone. Given that remodeling changes in alveolar bone include bone resorption primarily in the compression side and new bone formation primarily in the tension side, there is a possibility that antioxidants may exert a negative effect on orthodontic tooth movement by inhibiting osteoclastic bone resorption and/or stimulating osteoblastic bone formation in the compression side. Therefore, in this study, we examined the effect of antioxidants using NAC and resveratrol.
In this study, combined treatment of PDLFs in vitro with mechanical compression and hypoxia was employed to reproduce the conditions of compression and hypoxia during orthodontic tooth movement. Previous studies have shown that mechanical compression of cartilage or the spinal nerve increases the recruitment of inflammatory cells to these tissues, resulting in ROS production, inflammatory responses and subsequent tissue damage (Burkhardt et al., 1986; Xu et al., 2005). However, it is not clear whether the mechanical compression of cells induces ROS production in vitro. In the present study, we demonstrated that mechanical compression increases ROS production in human PDLFs per se. Furthermore, consistent with previous reports (Chandel et al., 1998), hypoxic treatment increased ROS production in human PDLFs. These results suggest that in addition to ROS of inflammatory cell-origin, PDLFsderived ROS may play a role in inflammatory responses in the compression side of PDL tissues.
Although it is well known that hypoxia induces VEGF expresexpression, the effect of hypoxia on the expression of pro-inflammatory cytokines in PDLFs is unclear. A previous report demonstrated that hypoxia stimulates the PDLFs to produce VEGF, IL-1β, IL-6 and prostaglandin E2 (Motohira et al., 2007). Consistent with this report, incubation of PDLFs under hypoxic conditions induced the expression of VEGF, IL-1β, IL-6 and IL-8 in the present study. As a mechanism for the induction of these cytokines by hypoxia, it has been suggested that hypoxia induces the activation of HIF-1 and NF-κB, promoting the expression of VEGF and pro-inflammatory cytokines, respectively (Storz and Imlay, 1999). ROS activates NF-κB, and antioxidants reduce the NF-κB-dependent transcription of pro-inflammatory cytokines (Janssen-Heininger et al., 2000; Schubert et al., 2002). Furthermore, antioxidants destabilize HIF-1 and reduce the expression of HIF-1 target genes (Gao et al., 2007; Vissers et al., 2007). In the present study, we confirmed that hypoxia increased ROS production and the expression of proinflammatory cytokines. Furthermore, antioxidants suppressed ROS production and the expression of IL-6 and IL-8 that were induced by hypoxia (data not shown). As for HIF-1, both NAC and resveratrol significantly diminished the transcriptional activity of HIF-1 and the mRNA level of VEGF, a target gene of HIF- 1. These results suggest that antioxidants may reduce the hypoxia- induced expression of pro-inflammatory cytokines and VEGF through the down-regulation of HIF-1- and NF-κBdependent transcription.
In this study, NAC and resveratrol decreased the expression of pro-inflammatory cytokines, which were induced by a combined treatment of mechanical compression and hypoxia. Immunohistochemical staining of IL-1β and TNF-α confirmed that NAC blocked the expression of these cytokines. Furthermore, the infiltration of inflammatory cells in PDL tissues was also diminished by NAC. Although the inhibitory effect of NAC on the combined treatment-induced expression of TNF-α in human PDLFs was not statistically significant in vitro, NAC clearly suppressed TNF-α expression in vivo. Given that NAC strongly inhibits the synthesis of pro-inflammatory molecules in leukocytes (Victor et al., 2003; Winyard and Blake, 1997), it is likely that NAC exerts a stronger inhibitory effect on the expression of IL-1β and TNF-α through the inhibition of inflammatory cells in vivo. Because the functional blocking of IL-1 or TNF-α by local administration of soluble receptors is known to retard orthodontic tooth movement (Jager et al., 2005), NAC may delay orthodontic tooth movement through the inhibition of these proinflammatory cytokines. Although resveratrol showed stronger inhibitory effect than NAC in vitro, there was no statistically significant difference in tooth movement between the vehicletreated group and the resveratrol-treated group. The reason for this discrepancy is not clear. But resveratrol showed delayed tooth movement at the fourth day samples (data not shown) and there was a trend of decrease in mean distance of the resveratrol-treated group compared to the vehicle-treated group at the seventh day samples even if not statistically significant. Therefore, there is a possibility that resveratrol might delay tooth movement if sample size increased.
Hypoxia is known to stimulate bone resorption by promoting the osteoclastic differentiation of hematopoietic precursor cells and the bone-resorbing activity of osteoclasts in a HIF-1-dependent manner (Arnett et al., 2003). In addition to direct regulation of osteoclast precursor cells, hypoxia enhances osteoclastogenesis indirectly by increasing the secretion of VEGF from non-osteoclastic bone marrow cells (Knowles and Athanasou, 2008). Local administration of neutralizing antibody to VEGF delays the orthodontic tooth movement (Kohno et al., 2005). In the present study, hypoxic treatment increased the expression of VEGF in PDLFs, which were suppressed by NAC and resveratrol. Furthermore, antioxidants prevented des-feroxamineinduced transcriptional activation of HIF-1. These results suggest that the inhibition of HIF-1 transcriptional activity and VEGF expression is also involved in antioxidant-induced retardation of orthodontic tooth movement through the down-regulation of osteoclastogenesis and subsequent bone resorption. Receptor activator of nuclear factor-κB ligand (RANKL) is an essential factor for osteoclastogenesis, and RANKL expression is up-regulated in the compression side of PDL tissues (Ogasawara et al., 2004; Wada et al., 2006). RANKL expression was also up-regulated by hypoxia in PDLFs, and antioxidants diminished hypoxia-induced RANKL expression in PDLFs in vitro and in the rat PDL tissues in vivo [data not shown, Park et al. (in press)]. These results further support the hypothesis that antioxidants may exert a negative effect on the orthodontic tooth movement through the down-regulation of bone resorption.
Based on the in vitro data, the animal study was designed to examine whether orthodontic tooth movement is delayed by NAC and resveratrol. We used a nickel-titanium spring to provide a relatively constant force of 40 g over the course of the experiment. Previous studies have shown that a 40-60 g of force stimulated substantial molar tooth movement in rats (Dunn et al., 2007). Although no significant decrease was observed in the resveratrol group, NAC significantly decreased the mesial movement of the maxillary first molar compared to the vehicle control. The mean difference between the vehicle and NAC group was 161 μm, and this value is comparable to the effect of locally delivered osteoprotegerin, a decoy receptor of RANKL (Dunn et al., 2007). These results further support the hypothesis that antioxidants might delay orthodontic tooth movement. The drawbacks of our animal study are small sample size, short experimental period and local administration of antioxidants. Despite the popularity of antioxidants, there is little information available on the effect of antioxidants, especially in the field of dentistry. Further studies based on the larger animals, such as beagles, with a long-term follow-up and systemic administration of antioxidants, need to be conducted to verify the findings of this study.
In summary, this study demonstrates that antioxidants decrease the expression of pro-inflammatory cytokines and VEGF in human PDLFs, which were induced by combined treatment with mechanical compression and hypoxia in vitro, and NAC delays orthodontic tooth movement in rats. These results suggest that antioxidants have the potential to retard orthodontic tooth movement.
Acknowledgments
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090531).
References
- 1.Arai C., Nomura Y., Ishikawa M., Noda K., Choi J.W., Yashiro Y., Hanada N., Nakamura Y. HSPA1A is upregulated in periodontal ligament at early stage of tooth movement in rats. Histochem. Cell Biol. (2010);134:337–343. doi: 10.1007/s00418-010-0737-3. [DOI] [PubMed] [Google Scholar]
- 2.Arnett T.R., Gibbons D.C., Utting J.C., Orriss I.R., Hoebertz A., Rosendaal M., Meghji S. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J. Cell. Physiol. (2003);196:2–8. doi: 10.1002/jcp.10321. [DOI] [PubMed] [Google Scholar]
- 3.Aruoma O.I., Halliwell B., Hoey B.M., Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. (1989);6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 4.Bass D.A., Parce J.W., Dechatelet L.R., Szejda P., Seeds M.C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J. Immunol. (1983);130:1910–1917. [PubMed] [Google Scholar]
- 5.Burkhardt H., Schwingel M., Menninger H., Macartney H.W., Tschesche H. Oxygen radicals as effectors of cartilage destruction. Direct degradative effect on matrix components and indirect action via activation of latent collagenase from polymorphonuclear leukocytes. Arthritis Rheum. (1986);29:379–387. doi: 10.1002/art.1780290311. [DOI] [PubMed] [Google Scholar]
- 6.Chandel N.S., Maltepe E., Goldwasser E., Mathieu C.E., Simon M.C., Schumacker P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA. (1998);95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun Y.S., Choi E., Kim T.Y., Kim M.S., Park J.W. A dominant-negative isoform lacking exons 11 and 12 of the human hypoxia-inducible factor-1alpha gene. Biochem. J. (2002);362:71–79. doi: 10.1042/0264-6021:3620071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn M.D., Park C.H., Kostenuik P.J., Kapila S., Giannobile W.V. Local delivery of osteoprotegerin inhibits mechanically mediated bone modeling in orthodontic tooth movement. Bone. (2007);41:446–455. doi: 10.1016/j.bone.2007.04.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao P., Zhang H., Dinavahi R., Li F., Xiang Y., Raman V., Bhujwalla Z.M., Felsher D.W., Cheng L., Pevsner J., et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. (2007);12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamrick S.E., McQuillen P.S., Jiang X., Mu D., Madan A., Ferriero D.M. A role for hypoxia-inducible factor-1alpha in desferoxamine neuroprotection. Neurosci. Lett. (2005);379:96–100. doi: 10.1016/j.neulet.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 11.Jager A., Zhang D., Kawarizadeh A., Tolba R., Braumann B., Lossdorfer S., Gotz W. Soluble cytokine receptor treatment in experimental orthodontic tooth movement in the rat. Eur. J. Orthod. (2005);27:1–11. doi: 10.1093/ejo/cjh089. [DOI] [PubMed] [Google Scholar]
- 12.Jang M., Cai L., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W., Fong H.H., Farnsworth N.R., Kinghorn A.D., Mehta R.G., et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. (1997);275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 13.Janssen-Heininger Y.M., Poynter M.E., Baeuerle P.A. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic. Biol. Med. (2000);28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 14.Jun J.H., Lee S.H., Kwak H.B., Lee Z.H., Seo S.B., Woo K.M., Ryoo H.M., Kim G.S., Baek J.H. N-acetylcysteine stimulates osteoblastic differentiation of mouse calvarial cells. J. Cell. Biochem. (2008);103:1246–1255. doi: 10.1002/jcb.21508. [DOI] [PubMed] [Google Scholar]
- 15.Kaku M., Kohno S., Kawata T., Fujita I., Tokimasa C., Tsutsui K., Tanne K. Effects of vascular endothelial growth factor on osteoclast induction during tooth movement in mice. J. Dent Res. (2001);80:1880–1883. doi: 10.1177/00220345010800100401. [DOI] [PubMed] [Google Scholar]
- 16.Kim D.Y., Jun J.H., Lee H.L., Woo K.M., Ryoo H.M., Kim G.S., Baek J.H., Han S.B. N-acetylcysteine prevents LPS-induced pro-inflammatory cytokines and MMP2 production in gingival fibroblasts. Arch. Pharm. Res. (2007);30:1283–1292. doi: 10.1007/BF02980269. [DOI] [PubMed] [Google Scholar]
- 17.Knowles H.J., Athanasou N.A. Hypoxia-inducible factor is expressed in giant cell tumour of bone and mediates paracrine effects of hypoxia on monocyte-osteoclast differentiation via induction of VEGF. J. Pathol. (2008);215:56–66. doi: 10.1002/path.2319. [DOI] [PubMed] [Google Scholar]
- 18.Kohno S., Kaku M., Tsutsui K., Motokawa M., Ohtani J., Tenjo K., Tohma Y., Tokimasa C., Fujita T., Kawata T., et al. Expression of vascular endothelial growth factor and the effects on bone remodeling during experimental tooth movement. J. Dent. Res. (2003);82:177–182. doi: 10.1177/154405910308200306. [DOI] [PubMed] [Google Scholar]
- 19.Kohno S., Kaku M., Kawata T., Fujita T., Tsutsui K., Ohtani J., Tenjo K., Tohma Y., Motokawa M., Shigekawa M., et al. Neutralizing effects of an anti-vascular endothelial growth factor antibody on tooth movement. Angle Orthod. (2005);75:797–804. doi: 10.1043/0003-3219(2005)75[797:NEOAAE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Kupisiewicz K., Boissy P., Abdallah B.M., Hansen F.D., Erben R.G., Savouret J.F., Soe K., Andersen T.L., Plesner T., Delaisse J.M. Potential of resveratrol analogues as antagonists of osteoclasts and promoters of osteoblasts. Calcif. Tissue Int. (2010);87:437–449. doi: 10.1007/s00223-010-9399-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee N.K., Choi Y.G., Baik J.Y., Han S.Y., Jeong D.W., Bae Y.S., Kim N., Lee S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. (2005);106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 22.Loboda A., Jozkowicz A., Dulak J. HIF-1 and HIF-2 transcription factors - similar but not identical. Mol. Cells. (2010);29:435–442. doi: 10.1007/s10059-010-0067-2. [DOI] [PubMed] [Google Scholar]
- 23.Meier B., Radeke H.H., Selle S., Younes M., Sies H., Resch K., Habermehl G.G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem. J. (1989);263:539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millen A.E., Dodd K.W., Subar A.F. Use of vitamin, mineral, nonvitamin, and nonmineral supplements in the United States: The 1987, 1992, and 2000 National Health Interview Survey results. J. Am. Diet Assoc. (2004);104:942–950. doi: 10.1016/j.jada.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Motohira H., Hayashi J., Tatsumi J., Tajima M., Sakagami H., Shin K. Hypoxia and reoxygenation augment boneresorbing factor production from human periodontal ligament cells. J. Periodontol. (2007);78:1803–1809. doi: 10.1902/jop.2007.060519. [DOI] [PubMed] [Google Scholar]
- 26.Ogasawara T., Yoshimine Y., Kiyoshima T., Kobayashi I., Matsuo K., Akamine A., Sakai H. In situ expression of RANKL, RANK, osteoprotegerin and cytokines in osteoclasts of rat periodontal tissue. J. Periodontal Res. (2004);39:42–49. doi: 10.1111/j.1600-0765.2004.00699.x. [DOI] [PubMed] [Google Scholar]
- 27.Park H.J., Baek K.H., Lee H.L., Kwon A., Hwang H.R., Qadir A.S., Woo K.M., Ryoo H.M., Baek J.H. Hypoxia inducible factor-1α directly induces the expression of receptor activator of nuclear factor-κB ligand in periodontal ligament fibroblasts. Mol. Cell. (2011) doi: 10.1007/s10059-011-1055-x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schubert S.Y., Neeman I., Resnick N. A novel mechanism for the inhibition of NF-kappaB activation in vascular endothelial cells by natural antioxidants. FASEB J. (2002);16:1931–1933. doi: 10.1096/fj.02-0147fje. [DOI] [PubMed] [Google Scholar]
- 29.Steinbach J.P., Klumpp A., Wolburg H., Weller M. Inhibition of epidermal growth factor receptor signaling protects human malignant glioma cells from hypoxia-induced cell death. Cancer Res. (2004);64:1575–1578. doi: 10.1158/0008-5472.can-03-3775. [DOI] [PubMed] [Google Scholar]
- 30.Storz G., Imlay J.A. Oxidative stress. Curr. Opin. Microbiol. (1999);2:188–194. doi: 10.1016/s1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 31.Teixeira C.C., Khoo E., Tran J., Chartres I., Liu Y., Thant L.M., Khabensky I., Gart L.P., Cisneros G., Alikhani M. Cytokine expression and accelerated tooth movement. J. Dent. Res. (2010);89:1135–1141. doi: 10.1177/0022034510373764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toker H., Ozdemir H., Eren K., Ozer H., Sahin G. Nacetylcysteine, a thiol antioxidant, decreases alveolar bone loss in experimental periodontitis in rats. J. Periodontol. (2009);80:672–678. doi: 10.1902/jop.2009.080509. [DOI] [PubMed] [Google Scholar]
- 33.Victor V.M., Rocha M., De la Fuente M. N-acetylcysteine protects mice from lethal endotoxemia by regulating the redox state of immune cells. Free Radic. Res. (2003);37:919–929. doi: 10.1080/1071576031000148727. [DOI] [PubMed] [Google Scholar]
- 34.Vissers M.C., Gunningham S.P., Morrison M.J., Dachs G.U., Currie M.J. Modulation of hypoxia-inducible factor-1 alpha in cultured primary cells by intracellular ascorbate. Free Radic. Biol. Med. (2007);42:765–772. doi: 10.1016/j.freeradbiomed.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Wada T., Nakashima T., Hiroshi N., Penninger J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. (2006);12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Winyard P.G., Blake D.R. Antioxidants, redox-regulated transcription factors, and inflammation. Adv. Pharmacol. (1997);38:403–421. doi: 10.1016/s1054-3589(08)60993-x. [DOI] [PubMed] [Google Scholar]
- 37.Xu W., Chi L., Xu R., Ke Y., Luo C., Cai J., Qiu M., Gozal D., Liu R. Increased production of reactive oxygen species contributes to motor neuron death in a compression mouse model of spinal cord injury. Spinal Cord. (2005);43:204–213. doi: 10.1038/sj.sc.3101674. [DOI] [PubMed] [Google Scholar]


