Abstract
Epstein-Barr virus (EBV) is associated with human cancers such as nasopharyngeal carcinoma, Burkitt’s lymphoma, Hodgkin’s disease, and gastric carcinoma (GC). EBV is associated with about 10% of all GC cases globally. EBV-associated GC has distinct features from EBV-negative GC. However, it is still unclear if EBV infection has any effect on GC chemoresistance. Cell proliferation assay, cell cycle analysis, and active caspase Western blot revealed that the EBV-positive GC cell line (AGS-EBV) showed chemoresistance to docetaxel compared to the EBV-negative GC cell line (AGS). Docetaxel treatment increased expression of Bax similarly in AGS and AGS-EBV cell lines. However, Bcl-2 induction was markedly higher in AGS-EBV cells, after docetaxel treatment. Although docetaxel increased the expression of p53 to a similar extent in both cell lines, induction of p21 in AGS-EBV cells was lower than in AGS cells. Furthermore, expression of survivin was higher in AGS-EBV cells than in AGS cells following docetaxel treatment as well as at basal state. EBVlytic gene expression was induced by docetaxel treatment in AGS-EBV cells. The results suggest that EBV infection and lytic induction confers chemoresistance to GC, possibly by regulating cellular and EBV latent and lytic gene expression.
Keywords: apoptosis, cell cycle related genes, chemoresistance, docetaxel, EBV-positive gastric carcinoma
INTRODUCTION
Epstein-Barr virus (EBV) is associated with human cancers such as nasopharyngeal carcinoma, Burkitt’s lymphoma, Hodgkin’s disease, and gastric carcinoma (GC) (Rickinson and Kieff, 2001; Wolf et al., 1993). EBV shows three distinct latency types depending on the expression of EBV latent genes. EBV latency II and III tumors express well-known EBV oncogenes, such as latent membrane protein 1 (LMP1), Epstein-Barr virus nuclear antigen (EBNA) 2, EBNA3A, and EBNA3C (Bornkamm and Hammerschmidt, 2001). LMP1 behaves like an activated tumor necrosis factor (TNF) receptor and interacts with TNF receptor associated factors (TRAFs). LMP1 is essential for EBV-induced immortalization of B cells (Jeon et al., 2009; Peng and Lundgren, 1992), and inhibits apoptosis by inducing Bcl-2 expression. EBNA2 is a transcriptional activator of viral and cellular genes. It is targeted to DNA by cellular J kappa recombination signal sequence binding protein. EBNA3A contributes to the initiation of cellular proliferation in EBV-infected B cells, while EBNA3C functions as a transcriptional activator of CD21 and LMP1, and modulates EBNA2 (Speck and Ganem, 2010).
Many investigators have tried to find efficient methods to treat EBV-positive tumors. The presence of the EBV genome in EBV-associated tumors offers the potential for novel EBVtargeted therapies. In one study, EBV-positive lymphoma was efficiently treated with ganciclovir (GCV) or azidothymidine (AZT) in vitro and in vivo following radiation-mediated lytic induction (Westphal et al., 2000). The EBV lytic cycle can be induced in EBV-infected cells by treatment with a variety of drugs, such as gemcitabine, doxorubicin, dexamethasone/rituximab, and valporic acid; subsequent treatment with GCV effectively induces apoptosis (Daibata et al., 2005; Feng et al., 2004; Jones et al., 2010). The therapeutic efficacy can be increased by controlling the Akt or MEKK1 signaling pathway in addition to treatment with the aforementioned drugs (He et al., 2008).
Even though the proliferation of EBV-positive C666-1 and C15 nasopharyngeal carcinoma (NPC) tumor cells can be effectively suppressed by doxorubicin, taxol, or cis-platinum treatment, effective induction of apoptosis is not achieved, while apoptosis is efficiently induced in C15 cells treated with doxorubicin in combination with farnesyl-transferase inhibitor (Vicat et al., 2003). Other studies have described successful induction of the lytic cycle in EBV-positive GC cells by treatment with cisplatinum, 5-fluorouracil (5-FU), trichostatin A (TSA), or 5-aza-2′- deoxycytidine (5-aza-CdR), followed by the addition of GCV to kill the lytically activated cells (Feng et al., 2002; Jung et al., 2007a). These studies highlight the importance of altered cellular and EBV protein expression after anti-cancer drug treatment in providing more effective therapy for EBV-associated GC.
GC is one of the most common carcinomas globally, and recent studies revealed the association of EBV with about 10% of GC cases worldwide (Burke et al., 1990; Shibata and Weiss, 1992; Takada, 2000; van Beek et al., 2002). However, the influence of EBV infection on GC development and treatment is still unclear. EBV-positive GC presents histologically and pathologically different features from EBV-negative GC (Akiba et al., 2008; Lee et al., 2004). EBV-positive GC exerts modulated latency 1, expressing latent genes such as EBNA1, LMP2A, BamHI-A rightward frame 1 (BARF1), and EBV-encoded RNAs (EBERs) (Imai et al., 1994; Oh et al., 2004). EBV lytic genes such as BamHI-Z leftward and rightward reading frame 1 (BZLF1 and BRLF1, respectively) are also expressed in EBVpositive GC following anti-cancer drug treatment (Feng et al., 2002; 2004; Jung et al., 2007b). Thus, EBV genes possibly play roles in conferring chemoresistance to EBV-associated GC.
Docetaxel (DOC) is one of a widly used anti-mitotic chemotherapy medications classified as taxane. It inactivates antiapoptotic function of Bcl-2 by phosphorylating it, in addition to inhibiting mitosis by disrupting assembly and disassembly of microtubules. (Lyseng-Williamson and Fenton, 2005; Pathan et al., 2001; Yvon et al., 1999). We compared chemoresistance of EBV-positive and EBV-negative GC cells to docetaxel. Expressions of apoptosis-related genes and cell cycle regulating genes as well as EBV latent and lytic genes were also analyzed after docetaxel treatment of the cells.
MATERIALS AND METHODS
Cell lines and anti-cancer drug
AGS is an EBV-negative GC cell line, while AGS-EBV is an AGS cell line infected with a recombinant Akata virus (Shimizu et al., 1996). AGS was maintained in RPMI-1640 medium (Gibco BRL, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, USA) and antibiotics (penicillin 100 units/ml and streptomycin 100 μg/ml; Gibco BRL). AGS-EBV was cultured in RPMI-1640 supplemented with 10% FBS, antibiotics, and 400 μg/ml G418 (Gibco BRL). Docetaxel (Aventis, France) was used as an anti-cancer drug.
Cell viability assay
Cell viability was analyzed using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Japan). Each cell line (5 × 104 cells/ml) was plated in a 96-well plate. After incubation for 24 h, cells were treated with the indicated concentrations of docetaxel for 72 h. This was followed by addition of 10 μl of CCK- 8 solution to each well. After 3 h incubation, the absorbance was measured using a SoftMax apparatus (Molecular Devices, USA) at wavelength of 450 nm.
Analysis of apoptosis
Each cell line was treated with 30 nM docetaxel. After incubating for 72 h, cells were harvested, washed with cold phosphate buffered saline (PBS), and fixed in 70% ethanol at -20℃ overnight. The cells were then washed twice with PBS, and resuspended in PBS containing 10 μg/ml RNase A (Invitrogen, USA) and 50 μg/ml propidium iodide (Sigma-Aldrich, USA). The distribution of cells in each phase of the cell cycle was analyzed using a FACScaliber apparatus (Becton Dickinson, USA).
Western blot
To detect the expression of apoptosis-related genes and cell cycle regulating genes, anti-Bcl-2 (Santa Cruz Biotechnology, USA; 1:500), anti-Bax (Santa Cruz Biotechnology; 1:2000), anti-p53 (Santa Cruz Biotechnology; 1:1000), anti-p21 (Santa Cruz Biotechnology; 1:500), anti-poly ADP ribose polymerase (anti-PARP; Becton Dickinson; 1:500), anti-survivin (Santa Cruz Biotechnology; 1:1000), and anti-cleaved caspase-3 (Cell Signaling Technologies, USA, 1:500) antibodies were used. To detect the expression of EBV lytic genes, anti-BZLF1 (Dako, Glostrup, Denmark, 1:500), anti-BRLF1 (Argene, France; 1:500), anti-BMRF1 (Novocastra, UK; 1:500), and anti-BHRF1 (3E8, 1:250) (Chou et al., 2004) antibodies were used. To detect the expression of EBV latent genes, anti-LMP2A (Abcam, UK; 1:1000) and anti-EBNA1 (a gift from Dr. Jaap Middeldorp, Department of Pathology, VU University Medical Center, Amsterdam, The Netherlands; 1:1000) antibodies were used. Anti-β- actin (Sigma-Aldrich, 1:2000) and anti-α-tubulin (Calbiochem, USA; 1:1000) antibodies were also used to confirm comparable loading.
Quantitative RT-PCR
AGS and AGS-EBV cells were harvested and total RNA was extracted using RNAzol™ B reagent (Tel-Test, USA) according to the manufacturer’s instructions. cDNA was synthesized using 1 μg total RNA, an oligo(dT) (Ahram Biosystems, Korea), and M-MLV reverse transcriptase (Invitrogen). Real-time PCRs for indicated genes were carried out using SYBR green qPCR kit (Takara, Japan) by a Mx3000P™ Real-Time PCR System (Stratagene, USA). Sequences of the primers were as follows: Bcl-2 5′-GAACTGGGGGAGGATTGTGG-3′ a n d 5′-CCGGTT CAGGTACTCAGTCA-3′, β-Actin: 5′-ATCATGTTTGAGACCTT CAA-3′ and 5′-CATCTCTTGCTCGAAGTCCA-3′. The PCR conditions were 95℃ for 10 s, followed by 45 cycles at 95℃ for 5 s, 60℃ for 20 s with a single fluorescence measurement. For the dissociation curve, reactions were incubated at 95℃ for 10 s, and ramped up from 55℃ to 95℃ with a heating rate of 0.1℃/s and fluorescence was measured continuously. Relative gene expression was calculated according to the comparative Ct method using β-actin as an internal standard.
Immunofluorescence assay
To detect the expression of EBV lytic genes, anti-BZLF1 (Dako, 1:50), anti-BRLF1 (Argene, USA; 1:100), and anti-BMRF1 (Novocastra; 1:200) antibodies were used. To detect the expression of EBV latent genes, anti-LMP2A (Abcam; 1:300) and anti- EBNA1 (1:300) antibodies were used. Nuclei were stained with Prolong Gold Anti-fade Reagent (Invitrogen) containing 4′, 6- diamino-2-phenylindole (DAPI).
RESULTS
Docetaxel chemoresistance of EBV-positive and EBV-negative GC cells
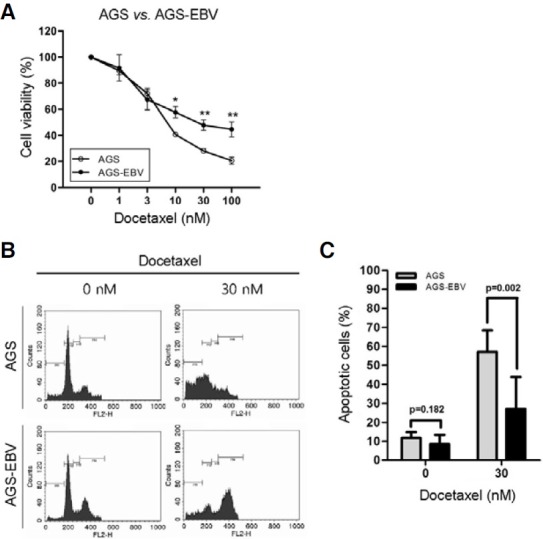
To analyze the effect of EBV infection on chemoresistance, AGS cells (EBV-negative GC cells) and AGS-EBV cells (EBVpositive GC cells) were treated with docetaxel, and cell viability and cell cycle phases were compared. Cell viability of AGSEBV cells were significantly higher than that of AGS cells 72 h after treatment with over 10 nM docetaxel (Fig. 1A). Cell cycle distribution of the cells treated with 30 nM docetaxel was accessed by fluorescent-activated cell sorting (FACS) after staining with propidium iodide (Fig. 1B). Following docetaxel treatment, the sub-G1 peak of AGS cells was increased more than that of AGS-EBV cells. Figure 1C shows the average of the sub-G1 ratio of the two cell lines obtained from three independent experiments. When treated with docetaxel, the sub-G1 population of AGS cells was significantly higher than that of AGS-EBV cells.
Fig. 1. Docetaxel chemosensitivity of AGS and AGS-EBV cells. The cells were treated with the indicated concentrations of docetaxel for 72 h. (A) Cell number was estimated using CCK-8 reagent. The results are expressed as percentages of cell viability relative to that of the untreated control cells. The results are presented as mean ± SD of three independent experiments with triplicate samples. *p < 0.05; **p < 0.01. (B) Detection of apoptosis in AGS and AGS-EBV cells after docetaxel treatment. The cells were treated with 30 nM docetaxel for 72 h, stained with propidium iodide, and analyzed by FACS. (C) The ratio of sub-G1 population of AGS and AGS-EBV cells following docetaxel treatment. The graph represents mean ± SD of three independent experiments.
Expression of apoptosis-related genes in AGS and AGS-EBV cells
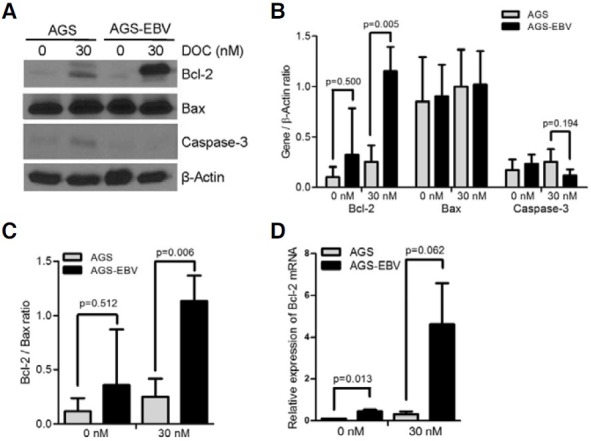
To check the relationship between apoptosis-related gene expression and chemoresistance to docetaxel, Western blots were performed for AGS and AGS-EBV cells. At the basal state, there was no noticeable difference between the two cells in the level of Bax (Fig. 2A). Bax expression was not affected by docetaxel in both cell lines, either. In contrast, Bcl-2 showed slightly higher expression in AGS-EBV cells than in AGS cells at the basal state. Furthermore, Bcl-2 expression was increased further in AGS-EBV cells than in AGS cells following docetaxel treatment. Cleaved caspase-3, which is an active form, was detected at a similar level in AGS and AGS-EBV cells at the basal state, but was increased more in AGS cells following docetaxel treatment. Expression levels of each gene from three independent experiments were normalized using the expression level of β-actin and mean values are shown in Fig. 2B. The expression ratio of Bcl-2 to Bax showed higher tendency in AGS-EBV cells than in AGS cells at both basal state and following docetaxel treatment (Fig. 2C). In addition, the level of Bcl-2 mRNA determined by real-time RT-PCR was higher in AGS-EBV cells than in AGS cells at basal state and following docetaxel treatment (Fig. 2D).
Fig. 2. Effect of docetaxel on the expression of apoptosis-related genes in AGS and AGS-EBV cells. Cells were treated with 30 nM docetaxel for 72 h. (A) Cell lysate (50 μg protein/lane) was analyzed by Western blot using anti-Bcl-2 (1:500), anti-Bax (1:2000), and anti-cleaved caspase- 3 (1:500) antibodies. Anti-β-actin antibody (1:2000) was used to check the loading amount. The expression ratio of Bcl-2, Bax, or caspase-3 relative to that of β-actin (B) and the expression ratio of Bcl- 2 to Bax (C) are also shown. The data are presented as mean ± SD of three independent experiments. (D) The level of Bcl- 2 mRNA expression was determined by real-time RT-PCR following treatment with 30 nM docetaxel for 72 h. Data are shown as mean ± SD of triplicate experiments.
Expression of cell cycle-related proteins in AGS and AGS-EBV cells
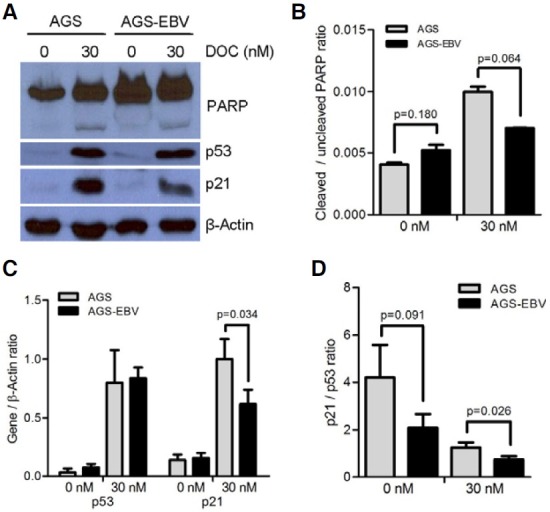
The effect of docetaxel treatment on the expression of proteins involved in cell cycle regulation was investigated by Western blot. First, the expression of PARP, which functions in DNA repair, was analyzed. At the basal level, the level of the uncleaved form of PARP was higher in AGS-EBV cells than in AGS cells, while the cleaved form of PARP was undetectable in both cell lines (Fig. 3A). When the cells were treated with docetaxel, the cleaved form of PARP became detectable in both cells. The ratio of the cleaved to uncleaved forms of PARP was higher in AGS cells than in AGS-EBV cells but the difference was not statistically significant (Fig. 3B). The expression levels of p53 at the basal state as well as following docetaxel treatment did not show significant differences between AGS and AGS-EBV cells. p21, which is induced by p53, was rarely detectable at the basal state, but was induced following docetaxel treatment in both cell lines. Interestingly, the level of p21 induction in AGS-EBV cells was lower than in AGS cells. Band densities of p53 and p21 obtained in three independent Western blot experiments were normalized using β-actin; the average values are plotted in Fig. 3C. The expression of p53 and p21 increased significantly by docetaxel treatment in both cells, but the induction of p21 was less in AGS-EBV cells than in AGS cells (Fig. 3C). The ratio of p21 to p53 in AGS-EBV cells was lower than in AGS cells following 30 nM docetaxel treatment (Fig. 3D).
Fig. 3. Expression of cell cycle-related genes in AGS and AGS-EBV cells. Cells were treated with 30 nM docetaxel for 72 h. (A) Cell lysate (50 μg protein/lane) was analyzed by Western blot. Anti-p53 (1:1000), anti-p21 (1:500), and anti-PARP (1:500) antibodies were used as primary antibodies. Anti-β-actin antibody (1:2000) was used to check the loading amount. (B) The expression ratios of cleaved PARP relative to uncleaved PARP are shown. (C) The expression ratios of p53 and p21, and cleaved PARP relative to that of β-actin are shown. (D) The expression ratios of p21 relative to p53 are shown. The data are presented as mean ± SD of three independent experiments.
EBV lytic gene induction by docetaxel treatment in AGS-EBV cells
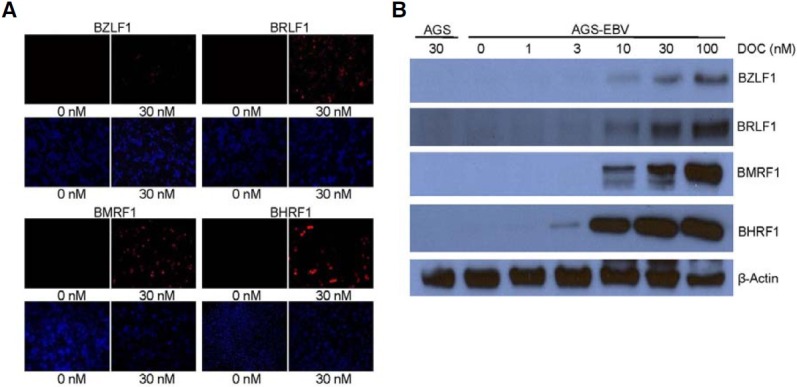
As several anti-cancer drugs have been reported to induce EBV lytic cycle, the effect of docetaxel on EBV lytic gene expression was analyzed in AGS-EBV. EBV lytic genes were undetectable at the basal state, but were readily detected following docetaxel treatment by immunofluorescence assay (Fig. 4A). When the cells were exposed to docetaxel, dose-dependent induction of BZLF1, BRLF1, BMRF1, and BHRF1 production was confirmed by Western blot (Fig. 4B).
Fig. 4. Induction of EBV lytic gene by docetaxel treatment. Cells were treated with indicated concentrations of docetaxel for 72 h. (A) Expression of EBV lytic genes was analyzed by immunofluorescence assay. Anti-BZLF1 (1:50), anti-BRLF1 (1:100), anti-BMRF1 (1:200), anti- BHRF1 (1:100) antibodies, and Alexa Fluor 555-conjugated goat anti-mouse IgG (1:500, red) were used. Nuclei were stained with DAPI (blue). (B) Western blot was performed using anti-BZLF1 (1:500), anti-BRLF1 (1:500), anti-BMRF1 (1:500), anti-BHRF1 (1:250), and anti-β-actin (1:2000) antibodies.
Effect of docetaxel on the expression of EBV latent genes in AGS-EBV cells
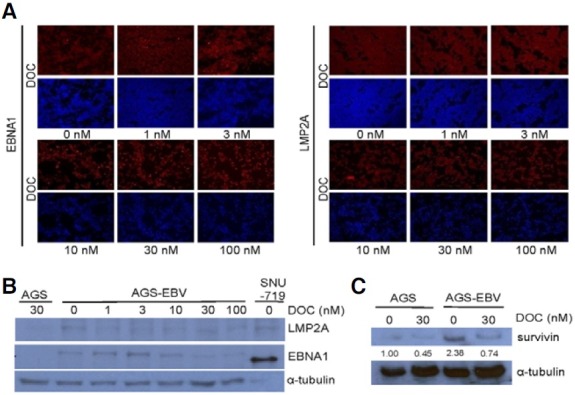
To determine whether EBV latent genes were also affected by anti-cancer drug treatment, the expression of EBNA1 and LMP2A was evaluated in AGS-EBV cells by immunofluorescence assay following docetaxel treatment. The expression of EBNA1 and LMP2A was always detected in AGS-EBV cells before and after docetaxel treatment (Fig. 5A). In addition, the expression level of LMP2A analyzed by Western blot was constant regardless the concentration of treated docetaxel. In contrast, the level of EBNA1 expression decreased slightly at docetaxel concentrations exceeding 30 nM (Fig. 5B). Next, the expression of survivin was assessed by Western blot following docetaxel treatment, as survivin is known to be up-regulated by EBNA1 and LMP2A (Fig. 5C). At the basal state, the expression level of survivin was noticeably higher in AGS-EBV cells than in AGS cells. The expression of survivin decreased following 30 nM docetaxel treatment in both cells, but the expression of survivin in AGS-EBV cells remained higher than in AGS cells.
Fig. 5. Effect of docetaxel on the expression of EBV latent genes in AGS-EBV cells. Cells were treated with indicated concentrations of docetaxel for 72 h. (A) Immunofluorescence assay was performed using anti-LMP2A (1:300), anti-EBNA1 (1:300), Cy-3-conjugated goat anti-rat IgG (1:2000, red), and Cy-3-conjugated goat anti-rabbit IgG (1:2000, red) antibodies. Nuclei were stained with DAPI (blue). (B) Western blot was performed using anti-LMP2A (1:1000), anti-EBNA1 (1:1000), and anti-α-tubulin (1:2000) antibodies. (C) Cells were treated with 30 nM docetaxel for 72 h. Cell lysate (50 μg protein/lane) was analyzed by Western blot. Anti-survivin antibody (1:1000) was used as a primary antibody and anti-α-tubulin (1:500) antibody was used to check loading amount.
DISCUSSION
Our data show that EBV-positive GC was more resistant to docetaxel than EBV-negative GC. This suggests that EBV confers chemoresistance to GC cells. At basal state, Bcl-2 expression was slightly higher in AGS-EBV cells than in AGS cells when both mRNA and protein levels were analyzed. We also found that Bcl-2 expression increased at both mRNA and protein levels following docetaxel treatment in both EBV-positive and EBV-negative GC cells. Interestingly the induction of Bcl-2 expression by docetaxel was more prominent in EBV-positive GC cells. Thus, some EBV lytic gene(s) expressed following docetaxel treatment may induce Bcl-2 expression to provide docetaxel chemoresistance to the cells.
To check whether other pro-apoptotic members of the Bcl-2 protein family that are regulated by p53 were involved in the chemoresistancy, we analyzed the expression of Noxa, Puma α, and Puma β. Our unpublished data showed higher expression of Noxa and Puma β in AGS-EBV cells than in AGS cells supporting that these proteins may also play a role in chemoresistancy (Hee Jong Shin et al., manuscript in preparation). However, their expression was not affected by docetaxel treatment (data not shown).
p21 functions in cell cycle arrest and is involves in apoptosis (Abbas and Dutta, 2009). The present data show comparable p53 induction following docetaxel treatment in the two cell lines, while p21 expression was lower in EBV-positive GC than in EBV-negative GC. In EBV-positive Hodgkin’s lymphoma, the expressions of p21 and caspase-3 were shown to be lower than in EBV-negative Hodgkin’s lymphoma due to EBER1 function, which resulted in decreased cell death (Liu et al., 2010). Thus, it is possibile that in EBV-positive GC cells, suppressed p21 expression by EBER1 might cause chemoresistance.
Following anti-cancer drug treatment, latent gene continued to be expressed and lytic gene expression was induced. These EBV genes may play roles in chemoresistance of EBV-positive GC. LMP2A activates signal transducer and activator of transcription 3 (STAT3), which is known to up-regulate cell proliferation and survival genes and decrease the expression of p53 (Hino et al., 2009; Yu et al., 2007). However, AGS-EBV, which expresses LMP2A, showed a similar expression of p53 with AGS.
In our experiment, LMP2A and EBNA1 might have induced chemoresistance through up-regulation of survivin in EBVpositive cells (Hino et al., 2008; Lu et al., 2011). Consistently, higher survivin expression was evident in EBV-positive GC cells than in EBV-negative GC cells, not only at basal level but also following 30 nM docetaxel treatment. As LMP2A expression was not significantly changed after docetaxel treatment, while EBNA1 expression decreased after 30 nM docetaxel treatment, we propose that EBNA1 is more closely associated with survivin expression than LMP2A in AGS-EBV cells.
BHRF1 is an EBV lytic gene, but is also expressed in EBVpositive GC cells (Luo et al., 2005). BHRF1 reduces apoptosis of primary T cell and B cell by inhibiting Bim (Desbien et al., 2009). BHRF1 also inhibits apoptosis of NPC cells induced by radiation (Huang et al., 1999). The present results show that expression of BHRF1 increased rapidly after docetaxel treatment, suggesting that BHRF1 may also confer chemoresistance to EBV-positive GC cells.
In summary, increased Bcl-2 and survivin as well as reduced p21 seem to play some role in docetaxel chemoresistance in EBV-positive GC cells. To accurately understand the chemoresistance mechanism of EBV-associated GC, further research is required. A recent report showed that when LMP1 was silenced in EBV-positive NPC cells, the cells became more sensitive to anti-cancer drugs (Mei et al., 2007). We may be able to improve the effect of anti-cancer drugs by clarifying the role of EBV or cellular genes involved in chemoresistance of EBV-associated GC.
Acknowledgments
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090577) and from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (0920210).
References
- 1.Abbas T., Dutta A. p21 in cancer: intricate networks and multiple activities. Nat. Rev. Cancer. (2009);9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiba S., Koriyama C., Herrera-Goepfert R., Eizuru Y. Epstein-Barr virus associated gastric carcinoma: epidemiological and clinicopathological features. Cancer Sci. (2008);99:195–201. doi: 10.1111/j.1349-7006.2007.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bornkamm G.W., Hammerschmidt W. Molecular virology of Epstein-Barr virus. Phil. Trans. R. Soc. B. (2001);356:437–459. doi: 10.1098/rstb.2000.0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke A.P., Yen T.S., Shekitka K.M., Sobin L.H. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod. Pathol. (1990);3:377–380. [PubMed] [Google Scholar]
- 5.Chou S.P., Tsai C.H., Li L.Y., Liu M.Y., Chen J.Y. Characterization of monoclonal antibody to the Epstein-Barr virus BHRF1 protein, a homologue of Bcl-2. Hybrid Hybridomics. (2004);23:29–37. doi: 10.1089/153685904322772006. [DOI] [PubMed] [Google Scholar]
- 6.Daibata M., Bandobashi K., Kuroda M., Imai S., Miyoshi I., Taguchi H. Induction of lytic Epstein-Barr virus (EBV) infection by synergistic action of rituximab and dexamethasone renders EBV-positive lymphoma cells more susceptible to ganciclovir cytotoxicity in vitro and in vivo. J. Virol. (2005);79:5875–5879. doi: 10.1128/JVI.79.9.5875-5879.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desbien A.L., Kappler J.W., Marrack P. The Epstein- Barr virus Bcl-2 homolog, BHRF1, blocks apoptosis by binding to a limited amount of Bim. Proc. Natl. Acad. Sci. USA. (2009);106:5663–5668. doi: 10.1073/pnas.0901036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng W.H., Israel B., Raab-Traub N., Busson P., Kenney S.C. Chemotherapy induces lytic EBV replication and confers ganciclovir susceptibility to EBV-positive epithelial cell tumors. Cancer Res. (2002);62:1920–1926. [PubMed] [Google Scholar]
- 9.Feng W.H., Hong G., Delecluse H.J., Kenney S.C. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J. Virol. (2004);78:1893–1902. doi: 10.1128/JVI.78.4.1893-1902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y., Cai S., Zhang G., Li X., Pan L., Du J. Interfering with cellular signaling pathways enhances sensitization to combined sodium butyrate and GCV treatment in EBV-positive tumor cells. Virus Res. (2008);135:175–180. doi: 10.1016/j.virusres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Hino R., Uozaki H., Inoue Y., Shintani Y., Ushiku T., Sakatani T., Takada K., Fukayama M. Survival advantage of EBV-associated gastric carcinoma: survivin up-regulation by viral latent membrane protein 2A. Cancer Res. (2008);68:1427–1435. doi: 10.1158/0008-5472.CAN-07-3027. [DOI] [PubMed] [Google Scholar]
- 12.Hino R., Uozaki H., Murakami N., Ushiku T., Shinozaki A., Ishikawa S., Morikawa T., Nakaya T., Sakatani T., Takada K., et al. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res. (2009);69:2766–2774. doi: 10.1158/0008-5472.CAN-08-3070. [DOI] [PubMed] [Google Scholar]
- 13.Huang H., Pan X., Zhou S., Li Z., Yu L., Kong X., Zheng Q. Flow cytometric analysis of BHRF1 expression prohibiting apoptosis induced by radiation. Ann. Otol. Rhinol. Laryngol. (1999);108:481–484. doi: 10.1177/000348949910800511. [DOI] [PubMed] [Google Scholar]
- 14.Imai S., Koizumi S., Sugiura M., Tokunaga M., Uemura Y., Yamamoto N., Tanaka S., Sato E., Osato T. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc. Natl. Acad. Sci. USA. (1994);91:9131–9135. doi: 10.1073/pnas.91.19.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon J.P., Nam H.Y., Shim S.M., Han B.G. Sustained viral activity of Epstein-Barr virus contributes to cellular immortalization of lymphoblastoid cell lines. Mol. Cells. (2009);27:143–148. doi: 10.1007/s10059-009-0018-y. [DOI] [PubMed] [Google Scholar]
- 16.Jones K., Nourse J., Corbett G., Gandhi M.K. Sodium valproate in combination with ganciclovir induces lysis of EBV-infected lymphoma cells without impairing EBV-specific Tcell immunity. Int. J. Lab. Hematol. (2010);32:e169–174. doi: 10.1111/j.1751-553X.2008.01130.x. [DOI] [PubMed] [Google Scholar]
- 17.Jung E.J., Lee Y.M., Lee B.L., Chang M.S., Kim W.H. Ganciclovir augments the lytic induction and apoptosis induced by chemotherapeutic agents in an Epstein-Barr virusinfected gastric carcinoma cell line. Anticancer Drugs. (2007a);18:79–85. doi: 10.1097/CAD.0b013e3280101006. [DOI] [PubMed] [Google Scholar]
- 18.Jung E.J., Lee Y.M., Lee B.L., Chang M.S., Kim W.H. Lytic induction and apoptosis of Epstein-Barr virusassociated gastric cancer cell line with epigenetic modifiers and ganciclovir. Cancer Lett. (2007b);247:77–83. doi: 10.1016/j.canlet.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Lee H.S., Chang M.S., Yang H.K., Lee B.L., Kim W.H. Epstein-barr virus-positive gastric carcinoma has a distinct protein expression profile in comparison with epsteinbarr virus-negative carcinoma. Clin. Cancer Res. (2004);10:1698–1705. doi: 10.1158/1078-0432.ccr-1122-3. [DOI] [PubMed] [Google Scholar]
- 20.Liu T.Y., Wu S.J., Huang M.H., Lo F.Y., Tsai M.H., Tsai C.H., Hsu S.M., Lin C.W. EBV-positive Hodgkin lymphoma is associated with suppression of p21cip1/waf1 and a worse prognosis. Mol. Cancer. (2010);9:32. doi: 10.1186/1476-4598-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu J., Murakami M., Verma S.C., Cai Q., Haldar S., Kaul R., Wasik M.A., Middeldorp J., Robertson E.S. Epstein- Barr Virus nuclear antigen 1 (EBNA1) confers resistance to apoptosis in EBV-positive B-lymphoma cells through up-regulation of survivin. Virology. (2011);410:64–75. doi: 10.1016/j.virol.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo B., Wang Y., Wang X.F., Liang H., Yan L.P., Huang B.H., Zhao P. Expression of Epstein-Barr virus genes in EBV-associated gastric carcinomas. World J. Gastroenterol. (2005);11:629–633. doi: 10.3748/wjg.v11.i5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyseng-Williamson K.A., Fenton C. Docetaxel: a review of its use in metastatic breast cancer. Drugs. (2005);65:2513–2531. doi: 10.2165/00003495-200565170-00007. [DOI] [PubMed] [Google Scholar]
- 24.Mei Y.P., Zhou J.M., Wang Y., Huang H., Deng R., Feng G.K., Zeng Y.X., Zhu X.F. Silencing of LMP1 induces cell cycle arrest and enhances chemosensitivity through inhibition of AKT signaling pathway in EBV-positive nasopharyngeal carcinoma cells. Cell Cycle. (2007);6:1379–1385. doi: 10.4161/cc.6.11.4274. [DOI] [PubMed] [Google Scholar]
- 25.Oh S.T., Seo J.S., Moon U.Y., Kang K.H., Shin D.J., Yoon S.K., Kim W.H., Park J.G., Lee S.K. A naturally derived gastric cancer cell line shows latency I Epstein-Barr virus infection closely resembling EBV-associated gastric cancer. Virology. (2004);320:330–336. doi: 10.1016/j.virol.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Pathan N., Aime-Sempe C., Kitada S., Basu A., Haldar S., Reed J.C. Microtubule-targeting drugs induce bcl-2 phosphorylation and association with Pin1. Neoplasia. (2001);3:550–559. doi: 10.1038/sj.neo.7900213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng M., Lundgren E. Transient expression of the Epstein-Barr virus LMP1 gene in human primary B cells induces cellular activation and DNA synthesis. Oncogene. (1992);7:1775–1782. [PubMed] [Google Scholar]
- 28.Rickinson A.B., Kieff E. Epstein-Barr virus. In Field’s Virology, B.M. Knipe, and P.M. Howley, eds. Lippincott Williams and Wilkins; Philadelphia, USA: (2001). pp. 2575–2627. [Google Scholar]
- 29.Shibata D., Weiss L.M. Epstein-Barr virus-associated gastric adenocarcinoma. Am. J. Pathol. (1992);140:769–774. [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu N., Yoshiyama H., Takada K. Clonal propagation of Epstein-Barr virus (EBV) recombinants in EBVnegative Akata cells. J. Virol. (1996);70:7260–7263. doi: 10.1128/jvi.70.10.7260-7263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speck S.H., Ganem D. Viral latency and its regulation: lessons from the gamma-herpesviruses. Cell Host Microbe. (2010);8:100–115. doi: 10.1016/j.chom.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada K. Epstein-Barr virus and gastric carcinoma. Mol. Pathol. (2000);53:255–261. doi: 10.1136/mp.53.5.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Beek J., zur Hausen A., Kranenbarg E.K., Warring R.J., Bloemena E., Craanen M.E., van de Velde C.J., Middeldorp J.M., Meijer C.J., van den Brule A.J. A rapid and reliable enzyme immunoassay PCR-based screening method to identify EBV-carrying gastric carcinomas. Mod. Pathol. (2002);15:870–877. doi: 10.1097/01.MP.0000024147.43288.B1. [DOI] [PubMed] [Google Scholar]
- 34.Vicat J.M., Ardila-Osorio H., Khabir A., Brezak M.C., Viossat I., Kasprzyk P., Jlidi R., Opolon P., Ooka T., Prevost G., et al. Apoptosis and TRAF-1 cleavage in Epstein-Barr viruspositive nasopharyngeal carcinoma cells treated with doxorubicin combined with a farnesyl-transferase inhibitor. Biochem. Pharmacol. (2003);65:423–433. doi: 10.1016/s0006-2952(02)01449-1. [DOI] [PubMed] [Google Scholar]
- 35.Westphal E.M., Blackstock W., Feng W., Israel B., Kenney S.C. Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV-positive malignancies. Cancer Res. (2000);60:5781–5788. [PubMed] [Google Scholar]
- 36.Wolf H., Bogedain C., Schwarzmann F. Epstein-Barr virus and its interaction with the host. Intervirology. (1993);35:26–39. doi: 10.1159/000150293. [DOI] [PubMed] [Google Scholar]
- 37.Yu H., Kortylewski M., Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. (2007);7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 38.Yvon A.M., Wadsworth P., Jordan M.A. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol. Biol. Cell. (1999);10:947–959. doi: 10.1091/mbc.10.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]


