Abstract
Hematopoietic stem cells (HSCs) are used therapeutically for hematological diseases and may also serve as a source for nonhematopoietic tissue engineering in the future. In other cell types, ion channels have been investigated as potential targets for the regulation of proliferation and differentiation. However, the ion channels of HSCs remain elusive. Here, we functionally characterized the ion channels of CD34+ cells from human peripheral blood. Using fluorescence-activated cell sorting, we confirmed that the CD34+ cells also express CD45 and CD133. In the CD34+/ CD45+/CD133high HSCs, RT-PCR of 58 ion channel mRNAs revealed the coexpression of Kv1.3, Kv7.1, Nav1.7, TASK2, TALK2, TWIK2, TRPC4, TRPC6, TRPM2, TRPM7, and TRPV2. Whole-cell patch clamp recordings identified voltage-gated K+ currents (putatively Kv1.3), pH-sensitive TASK2-like background K+ currents, ADP-ribose-activated TRPM2 currents, temperature-sensitive TRPV2-like currents, and diacylglycerol- analogue-activated TRPC6-like currents. Our results lend new insight into the physiological role of ion channels in HSCs, the specific implications of which require further investigation.
Keywords: CD34, hematopoietic stem cell, ion channel, potassium channel, TRP channel
INTRODUCTION
Hematopoiesis is characterized by the finely regulated selfrenewal, differentiation, and migration of pluripotent CD34+ hematopoietic stem cells (HSCs). CD34 originally was identified from the immature human myeloblast line, KG-1a, and was suggested to be a cell surface marker of hematopoietic cells (Civin et al., 1984). CD34+ HSCs maintain a lifelong supply of multilineage hematopoietic cells that can be reconstituted in the blood as needed. During steady-state hematopoiesis, CD34+ HSCs circulate at low numbers in the peripheral blood, suggesting continuous migration of these cells between the bone marrow and organs (Kronenwett et al., 2000). Despite the wellrecognized molecular phenotype of CD34+ HSCs, the regulatory mechanisms for proliferation and the molecular signals mediating mobilization, migration, and differentiation are only partially understood (Awong et al., 2009; Han et al., 2003; Nielsen and MaNagny, 2009; Steidl et al., 2002). Previous studies suggest that HSCs also are able to differentiate into nonhematopoietic cell types such as hepatocytes and cardiomyocytes (Lagasse et al., 2000; Orlic et al., 2001). Since CD34+ HSCs can be isolated from human peripheral blood, they have the potential to serve as therapeutic sources for a variety of degenerative diseases. Therefore, it is crucial that the physiological characteristics of CD34+ HSCs in primary culture are understood.
Ion channels are key players in the physiology of excitable cells composing neural tissue and muscle. However, a wide variety of ion channels also are expressed from nonexcitable cells and function in critical signaling pathways including electrolyte transport, cell volume regulation, differentiation, proliferation, and apoptosis. For example, the activity of K+ channels determines the negative membrane potential of the cell and is critical to the maintenance of cell volume and the electrical driving force facilitating the Ca2+ influx. Both membrane potential and cell volume change dynamically according to the cell cycle stage; oscillations of membrane voltage (Vm) in phase with cell cycle transitions have been observed in various cell types, particularly at the G1/S boundary (Shieh et al., 2007). Ca2+-permeable cation channels such as those from the mammalian homologues of transient receptor potential (TRP) protein family provide a direct pathway for the Ca2+ influx that is crucial for the initiation of an enormous variety of cellular signaling cascades (Nilius et al., 2007). For these reasons, the identification and characterization of ion channels expressed by CD34+ HSCs are essential prerequisites for biotechnological manipulation or physiological understanding of this cell type. However, direct investigations of ion channel function in CD34+ HSCs are rare and primarily focus on cancer cell lines (Korper et al., 2003; Pillozzi et al., 2002; Shirihai et al., 1998). One study using primary CD34+ HSCs demonstrated the inwardly rectifying K+ currents but did not systemically examine the other ion channels (Shirihai et al., 1998).
Our group has demonstrated that functional ion channels are heterogeneously expressed in various types of stem cells, and we have presented experimental evidence elucidating the roles of specific ion channels in cell signaling (Park et al., 2007; 2008; 2010). In the present study, using RT-PCR and patch clamp recording, we analyzed the expression and function of ion channels in CD34+ HSCs from human peripheral blood. We found that primary human CD34+ HSCs express voltage-gated K+ channels, two-pore domain background K+ channels, and TRP family nonselective cation channels.
MATERIALS AND METHODS
Purification and culture of human CD34+ cells
This study was performed according to the Declaration of Helsinki guidelines for biomedical research and was approved by the Seoul National University Hospital Institutional Review Board. Blood was collected from a voluntary donor from whom written informed consent was obtained. Peripheral blood mononuclear cells (PBMCs) were isolated by leukapheresis using a Cobe Spectra apheresis device (USA). The PBMCs were separated by density-gradient centrifugation using separation medium. CD34+ cells were positively selected from the PBMCs using a CD34+ cell isolation kit and a magnetic-activated cell sorting (MACS) column (Miltenyi Biotec, Germany) according to the manufacturer’s instructions. CD34+ cells then were cultured in StemSpan serum-free medium (STEMCELL Technologies, Canada) supplemented with cytokine cocktails consisting of stem cell factor, Flt-3 ligand, and thrombopoietin (100 ng/ml, respectively). For electrophysiological study, we used CD34+ cells cultured between 3-5 days which shows no significant morphological differences. Expression of other surface markers (CD133 and CD45) was investigated separately (Fig. 1).
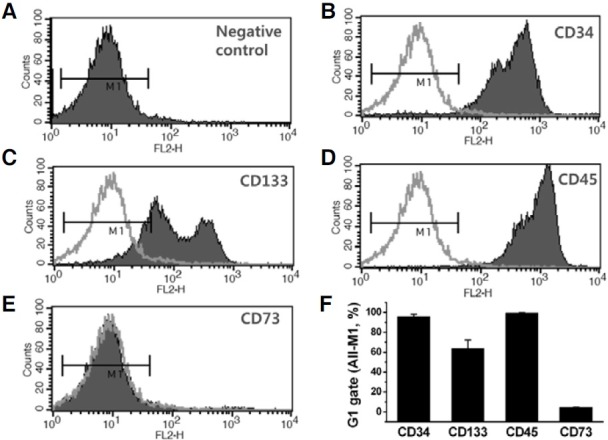
Fig. 1. Phenotype of surface markers in CD34+ HSCs. FACS analysis depicting the immunophenotype of CD34+ cells that were obtained by magnetic column separation of human peripheral blood. (A) IgG1 isotype control staining. Cells were stained with anti-CD34-PE (B); anti-CD133-PE (C); anti-CD45-PE (D); or anti-CD73-PE (E) human antibodies. (F) Summary of the FACS analysis. Each column gives the mean ± SD (n = 3) of the percentage of the markerpositive cells of the total number of cells analyzed.
Fluorescence-activated cell sorting
Cells were analyzed by fluorescence-activated cell sorting (FACS) u sing t he FACSCalibur A p latform (BD Biosciences, USA). The instrument was calibrated with phycoerythrin (PE), and data were analyzed with appropriate negative (isotypic) controls using CellQuest and Paint-a-Gate research software (BD Biosciences). At least 10,000 cells were analyzed for each analysis. The following cell-surface epitopes were detected with anti-human antibodies: CD34-PE, CD45-PE, CD73-PE (Serotec Ltd., UK), and CD133-PE (Miltenyi Biotechnologies, USA).
Patch clamp recordings
Ionic current studies of CD34+ HSC populations were performed using a whole-cell patch clamp technique. Normal Tyrode’s (NT) solution was used as a bath and contained (in mM): 143 NaCl, 5.4 KCl, 0.5 MgCl2, 1.8 CaCl2, 10 HEPES [4-(2- hydroxyethyl)-1-piperazine ethanesulfonic acid], 0.5 NaH2PO4, and 10 glucose. Its pH was adjusted to 7.4 with NaOH. A care was taken to titrate the pH again to 7.4 when using 4-aminopyridine to block voltage-gated K+ current (Fig. 3). In the experiment shown in Fig. 4B, a K+-rich solution (Na+ replaced with K+) was used as a bath to maximize single channel recordings of pH-sensitive TASK-like K+ currents. To test the effects of acidic and alkaline pH, 5 mM of HEPES in NT solution was replaced with MES (2-N-morpholino ethanesulfonic acid, 5 mM) and THAM [tris(hydroxymethyl)aminomethane, 5 Mm], respectively. The standard K+-rich pipette solution contained (in mM): 140 K-aspartate, 5 NaCl, 1.0 MgCl2, 10 HEPES, 5.0 EGTA [2- bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid], and 3.0 Mg-ATP. Its pH was adjusted to 7.2 with KOH. The Cs+-rich pipette solution for TRPV and TRPC recordings contained (in mM): 140 Cs-aspartate, 5 KCl, 1.0 MgCl2, 10 HEPES, 5.0 EGTA, and 3.0 Mg-ATP. Its pH was adjusted to 7.2 with CsOH. The pipette solution for TRPM2 recordings contained (in mM): 87 Cs-gluconate, 38 CsCl, 10 NaCl, 1 MgSO4, 0.9 CaCl2, 1 EGTA, 10 HEPES, and 0.1 ADP-ribose (ADPR). Its pH was adjusted to 7.2 with CsOH. The external solution was replaced with equimolar N-methyl-D-glucamine (NMDG+) to induce cationfree conditions, which allowed for the examination of nonselective cation currents. Experiments were conducted at room temperature (22-24℃) except for the activation of temperaturesensitive current recordings. Solutions containing 1-Oleoyl-2- acetyl-glycerol (OAG) were sonicated for 10 min before use. All chemicals used for the patch clamp study were obtained from Sigma-Aldrich (USA).
Fig. 3. Two types of voltage-gated K+ channel (Kv) currents in CD34+ HSCs. Membrane current responses to step-like depolarization (300 ms of voltage steps between -120 and 100 mV; 20 mV interval) from -80 mV of holding voltage (see inset). Representative traces of Kv current demonstrate time-dependent inactivation (A) and sustained activity (B). The inactivating type of Kv currents were sensitive to 4-AP (5 mM, A) whereas the non-inactivating types were insensitive to 4-AP (B). The amplitudes of peak currents were normalized to the membrane capacitance (pA/pF), and the mean values are summarized as current:voltage relations [I/V curves, right panels, n = 8 and 7 for (A) and (B), respectively]. *, P < 0.05.

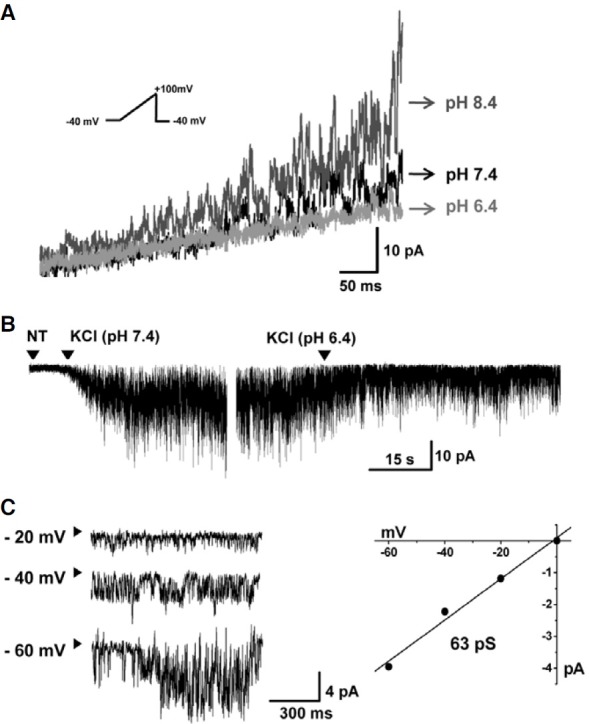
Fig. 4. Characteristics of TASK2-like K+ currents in CD34+ HSCs. (A) Representative traces of pHe- sensitive currents recorded by the ramp-like voltage change from -40 to 100 mV (see inset) in different pH solutions (pH 6.4; 7.4; and 8.4). Similar results were observed in 19 of 50 cells. (B) At -80 mV, inward currents were detected when extracellular Na+ was replaced with K+, which was inhibited by an acidic pHe of 6.4. (C) Representative traces of single-channel activity of the pHe-sensitive K+ current at negative voltages in symmetrical K+ conditions. The unitary amplitudes were plotted against the clamp voltages, and the slope conductance was 63 pS (n = 1).
RT-PCR
Total RNA was isolated from CD34+ cells using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. First-strand cDNAs were synthesized with SuperScriptII (Invitrogen), and the cDNA template was diluted 1:10 in preparation for PCR. Primer sequences used in the amplification reaction are given in Table 1. Primer sequences encompassing the intron segments were selected to exclude the contamination from genomic DNA. Also, negative results from PCR without RT were confirmed (data not shown).
Table 1.
Nucleotide sequences of the primers used for RT-PCR
| Protein (GeneBank#) | Primer | Product size | Sequence (5′ to 3′) |
|---|---|---|---|
| Human kv7.1 | Forward | 500 bp | CAGCCTGCACTTGGGGGCTC |
| (nm_000218.2) | Reverse | CCCACGCACAGTGGGCTCAG | |
| Human Nav1.7 | Forward | 446 bp | GCTCCGAGTCTTCAAGTTGG |
| (Nm_002977.3) | Reverse | GGTTGTTTGCATCAGGGTCT | |
| Human TASK2 | Forward | 567 bp | CTGCTCACCTCGGCCATCATCTTC |
| (Nm_003740.3) | Reverse | GTAGAGGCCCTCGATGTAGTTCCA | |
| Human TASK4 | Forward | 169 bp | ATAGCTGCACCCAGCCTCTA |
| (Nm_031460.3) | Reverse | GCATCTGGAACCTTCTGCTC | |
| Human TRPC4 | Forward | 317 bp | GCTGCTGGCATCTCGCTGGT |
| (Nm_016179.2) | Reverse | TGAAGCCCAGGACCCACGGT | |
| Human TRPC6 | Forward | 654 bp | CCGGCGGCAGACAGTTCTCC |
| (Nm_004621.5) | Reverse | GCCGGACTTGCCAGGCCTTT | |
| Human TRPM2 | Forward | 624 bp | CCCGGCACCTCCTCTACCCC |
| (Nm_003307.3) | Reverse | GGAAGTGGACGCTGACGGCC | |
| Human TRPV2 | Forward | 573 bp | GAGAACCCACACCAGCCCGC |
| (Nm_016113.4) | Reverse | GGCGGCCTGCTTCTTCAGGG | |
| Human Kv1.3 | Forward | 626 bp | CAGAGCATCGCGGCTTTGGCT |
| (Nm_00232.3) | Reverse | GCGGCCCCCGGACTGATAGT | |
| Human GAPDH | Forward | 323 bp | TCTTTTGCGTCGCCAGCCGAG |
| (Nm_002046.3) | Reverse | ACGTACTCAGCGCCAGCATCG | |
Data presentation and analysis
Representative ionic current traces are shown after confirming the consistent activity of corresponding ion channels. Summaries of the results are presented as mean ± SEM. Paired Student’s t-tests were used to evaluate the significance of differences between the means of two groups (Fig. 3A). P-values < 0.05 were considered statistically significant.
RESULTS
To further characterize the surface markers expressed by hematopoietic cells, we submitted the primary-cultured cells to FACS analysis before conducting an electrophysiological study. Cell purity ranged from 93 to 98% as determined using a CD34-specific antibody (Figs. 1B and 1F). CD34+ cells also were positive (> 99%) for another hematopoietic marker, CD45 (Figs. 1C and 1F). CD133 was recently identified as a marker for stem cells and progenitor cells (Guo et al., 2003; Lanza et al., 2001). FACS analysis of CD34+ HSCs revealed that the majority of cells were CD133+ (63.9 ± 8.2 %, Figs. 1D and 1F). These CD34+/CD45+/CD133high HSCs were negative (< 5%) for CD73, a marker for mesenchymal stem cells (Figs. 1E and 1F). The mean values of the percentage of marker-positive cells are represented as a bar graph (Fig. 1F; n = 3). Although HSCs were cultured with essential cytokines, the total number of cells was significantly reduced after 7 days in culture (Supplementary Fig. 1). Therefore, for the electrophysiological study and RT-PCR analysis, we used only the CD34+ HSCs cultured for 3-5 days after thawing.
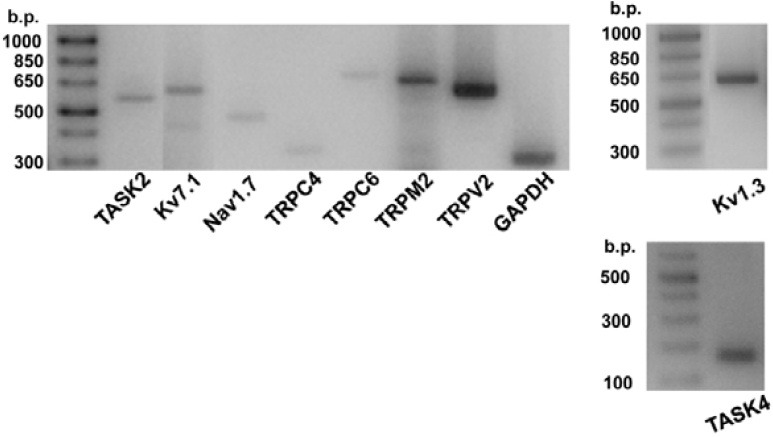
The expression of transcripts corresponding to 24 voltagegated ion channels, 14 two-pore domain background K+ channels, and 20 TRP family channels were investigated in HSCs using RT-PCR with specific primers (Supplementary Figs. 2-4). Our pilot experiments confirmed consistent expression of 10 types of mRNAs for cation channels: Kv1.3, Kv7.1, Nav1.7, TASK2, TASK4 (TALK2), TRPC4, TRPC6, TRPM2, TRPM7, and TRPV2. Because TRPM7 is ubiquitously expressed in the mammalian cells, we focused on the other nine members; Kv1.3, Kv7.1, Nav1.7, TASK2, TASK4, TRPC4, TRPC6, TRPM2, and TRPV2. The representative results of RT-PCR analysis for the eight members are shown in Fig. 2 (see Table 1 for their primer sets).
Fig. 2. Expression of ion channel transcripts. Results of RT-PCR analysis for TASK2, Kv7.1, Nav1.7, TRPC4, TRPC6, TRPM2, and TRPV2 (left panel), Kv1.3 (right upper panel), and TASK4 (right lower panel). GAPDH was used as the control.
In the whole-cell patch clamp experiment using a KCl pipette solution, voltage-gated K+ channel (Kv) currents were investigated (Fig. 3). Membrane currents were elicited using 300 ms voltage steps ranging from -120 to +100 mV from a holding potential of -80 mV. Outwardly rectifying currents were observed in concordance with the activity of Kv. The results of outward current measurements were divided into two groups depending on the sensitivity to 4-aminopyridine (4-AP; 5 mM). In 8 out of 15 cells, the outward currents were virtually abolished by 4-AP (Fig. 3A), whereas the other cells were relatively insensitive to 4-AP (Fig. 3B). Notably, the 4-AP-sensitive outward current showed time-dependent inactivation at a strong level of depolarization.
Next, the functional activity of TASK-type K+ currents was investigated. Within the two-pore domain background K+ channel KCNK family, TASK and TALK subfamilies are characterized by sensitivity to extracellular pH (pHe) (Kim, 2005). Because mRNA for TASK2 and TASK4 were observed in CD34+ HSCs, we investigated the pH-sensitive background K+ current. Ramplike pulses from 60 mV to -100 mV were applied under different pH conditions (pHe 6.4, 7.4, and 8.4, Fig. 4A). The acidic pHeinhibited current also was observed at -60 mV of holding voltage when extracellular Na+ was replaced with K+. This is consistent with voltage-independent background K+ channel activity, which also was inhibited by acidic pHe (Fig. 4B). The pHesensitive background K+ current was observed in 19 of 50 cells tested. For cases in which the channel numbers were relatively low, it was possible to discriminate the pHe-sensitive single channel current under the whole-cell configuration. The unitary slope conductance obtained in the test with various voltages was 63 pS (Fig. 4C, n = 1).
And then, we investigated the functional expression of TRPM2, TRPV2, and TRPC6 with suitable activating conditions for each type. To record the nonselective cation channel currents, a Cs+-rich pipette solution was used. TRPM2 is known to be primarily activated by cytosolic ADP-ribose (ADPR), and this activation is facilitated by Ca2+ (Kühn et al., 2005). Under the whole-cell configuration with 100 μM ADPR in the Cs+-rich pipette solution, step-like increases of inward current were observed (n = 6). The unitary current amplitude (4.78 ± 0.11 pA) and the pattern of step-like increases are consistent with the single channel conductance (~80 pS) and slow kinetics of TRPM2 (Kühn et al., 2005). The inward current was completely abolished by exchanging extracellular Na+ with NMDG+, a nonpermeable large cation (Fig. 5A).
Fig. 5. Functional activities of TRP family currents in CD34+ HSCs. (A) Representative trace of TRPM2 current in CD34+ HSCs internally dialyzed with 100 μM ADPR. Similar results were observed in 6 out of 30 cells tested. Steplike inward currents increased spontaneously after establishing the whole cell configuration (holding voltage, -60 mV) and were completely abolished by replacing extracellular Na+ with NMDG+. (B) An original chart trace of membrane currents demonstrating the high temperature (HT, 42℃) activated current in CD34+ HSCs (left panel). The heatactivated inward current was largely inhibited by replacing extracellular Na+ with NMDG+. Representative currents were obtained with a ramp pulse (from -120 to 60 mV, held at -40 mV) shown in the left panel with the I-V relationship shown in the right panel. RT; room temperature (23℃). Similar results were observed in 32 out of 46 cells tested. (C) Bath application of OAG (100 μM) increased whole-cell currents of CD34+ HSCs (left panel). Representative I/V curves were obtained with a ramp pulse (from -100 to 60 mV, held at -60 mV) shown in the left panel with the I-V relationship shown in the right panel. Similar results were observed in 3 out of 6 cells tested.
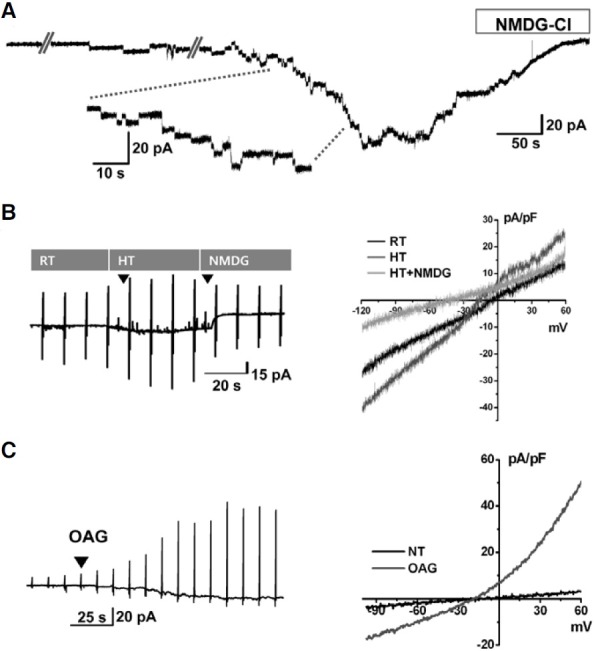
TRPV2 is expressed in mast cells, macrophages, sensory neurons, and many other cell types that can be activated by diverse stimuli such as noxious heat and arachidonic acid (Stokes et al., 2004; Vennekens et al., 2008). Temperatures above 50℃ elicit the activation of TRPV2. However, owing to the instability of whole-cell recording for CD34+ HSCs at such extreme temperatures, we could not test the effect above 42℃. Despite the limitation of applying higher temperature condition, a 42℃-activated current was consistently observed in CD34+ HSCs (n = 32, Fig. 5B). The heat-activated inward current was largely inhibited by replacing extracellular Na+ with NMDG+, indicating cationic permeability (Fig. 5B). Next, we tested the activity of TRPC6, which is known to be activated by diacylglycerol (DAG), a product of phospholipase C that acts on the plasma membrane (Hofmann et al., 1999). Bath application of CD34+ HSCs in OAG (100 μM), a membrane permeable DAG analogue, increased the membrane conductance with weakly outward rectifying voltage-dependence (n = 3, Fig. 5C).
DISCUSSION
In the present study, we provide evidence for the functional expression of three subfamilies of cation channels (Kv, K2P, and TRP) in CD34+ HSCs. CD34, a cell-surface sialomucin commonly used for HSC purification, is suggested to play a role in cellular trafficking and migration (Kronenwett et al., 2000). However, CD34 may not be essential for the physiology of HSCs because normal hematopoietic profiles were found in CD34-null mice (Cheng et al., 1996; Guo et al., 2003). Nielsen et al. reported recently that CD34 is abundantly expressed in mature mast cells (Nielsen and MaNagny, 2009). These studies suggest that CD34 might not be an appropriate marker for complete and specific isolation of HSCs. For this reason, we confirmed the coexpression of alternative markers such as CD45 and CD133 (Fig. 1). The positive results for all three markers indicate that we have investigated the ion channels of genuine HSCs from human peripheral blood. Albeit the relatively homogenous property of surface marker expression, the CD34+ HSCs showed heterogeneous properties with regard to functional expression of ion channels (see below). HSCs give rise to all types of blood cells from the myeloid (monocytes, neutrophils, erythrocytes, dendritic cells etc.), and lymphoid lineages (T cells, B cells, NK cells). Regarding to this potential, the heterogeneity of ion channels might reflect that a differentiation process might have started in vitro.
In the present study, RT-PCR analysis showed expression of Kv1.3 and Kv7.1 in CD34+ HSCs. The voltage-dependent K+ currents was inhibited by 4-AP in about half of the tested cells and showed time-dependent inactivation, that are consistent with the properties of Kv1.3. Based on the RT-PCR data, Kv7.1 might be suggested as a molecular candidate for the 4-APinsensitive outward current. However, the transient kinetic property (fast activation and inactivation, Fig. 3B), is not consistent with the well-known slow kinetics of Kv7.1 (Jespersen et al., 2005). Because the primers for K+ channels used in the present study did not cover all the members of Kv superfamily, the 4- AP-insensitive current might be encoded by one of the other Kv channels not analyzed yet.
A previous electrophysiological study of CD34+ cells from umbilical cord blood demonstrated inwardly rectifying K+ (Kir) channels (Shirihai et al., 1998). We did not detect the Kir-type current but successfully recorded voltage-gated K+ currents and background-type K+ currents. The differential expression of Kir might result from different sources of HSCs (cord blood and peripheral blood).
Unlike Kv channels that require depolarization above a threshold voltage for activation, Kir and K2P channels are active at negative Vm. Therefore, Kir and K2P exhibit the essential function of establishing the strongly hyperpolarized membrane potential of cells close to the K+ equilibrium potential. Although we did not observe Kir currents in CD34+ HSCs, the presence of TASK2, a member of K2P, would exhibit a similar function regarding the maintenance of negative membrane voltage. TASK2 activity might explain putative K+ channel-dependent cell cycle regulation (Wang et al., 1997).
In addition to background activity, some types of K2P channels exhibit sensitivity to various physicochemical stimuli. Therefore, these channels are attractive targets for regulating cellular functions (Kim, 2005). The pHe-sensitive TASK and TALK subfamilies of K2P channels potentially are important in establishing the resting membrane potential and modulating neuronal excitability. In the present study, we report the expression of TASK2 in HSCs. TASK2 is defined as TWIK-related acid sensitive K+ channel 2 and is also called K2P5.1 or KCNK5. As its name suggests, TASK2 is activated by alkaline pH and shows the largest single channel conductance (~65 pS) among the TASK and TALK subfamilies. The physiological role of TASK2 has been described in kidney proximal tubular epithelium in terms of HCO3 - reabsorption and osmotic cell volume regulation. Recently, Meuth et al. (2008) suggested that TASK2 channels have essential roles in human T-cell functions. It is important to determine whether the functional expression of TASK2 dynamically changes during the process of lymphocytic differentiation from human HSCs.
The mammalian transient receptor potential (TRP) cation channel family is composed of five subfamilies (TRPC, TRPV, TRPM, TRPA, and TRPML), and almost 30 members have been identified. Most TRP channels are permeable to Ca2+ and monovalent cations and contribute to changes in the cytosolic Ca2+ concentration. TRP channels act by providing direct Ca2+ entry pathways or by membrane depolarization (Nilius et al., 2007). Here we report that TRPM2, TRPC6, and TRPV2-like currents are functionally expressed in CD34+ HSCs. Although previous reports using CD34+ cells have discussed the expression of various TRP mRNAs and have conducted Ca2+ influx experiments (den Dekker et al., 2001; Hirschler-Laszkiewicz et al., 2009; Tong et al., 2008; Zhang et al., 2006), our direct demonstration of ion channel currents is unprecedented.
Many members of the TRPC subfamily can be activated by receptor-operated mechanisms and are regulated through phospholipase C-mediated pathways. In CD34+ erythroid cells, the erythropoietin-dependent activation of TRPC3 and the modulatory role of TRPC6 have been discussed (den Dekker et al., 2001; Hirschler-Laszkiewicz et al., 2009). It also has been reported that TRPC4 and TRPC6 proteins are upregulated in differentiated megakaryocytes compared with CD34+ stem cells and immature monocytes obtained from umbilical cord blood (den Dekker et al., 2001). In our present study, the OAGactivated cationic current and mRNAs for TRPC subtypes are partly consistent with these reports.
TRPM2 is expressed in a wide variety of cells and is involved in oxidant stress-induced cell death and inflammation (Nilius et al., 2007; Zhang et al., 2006). ADPR, the potent cytosolic activator of TRPM2, behaves as a novel hematopoietic cytokine stimulating the in vitro proliferation of HSCs (Podesta et al., 2003). This finding implies that the activation of TRPM2 might play a role in the expansion of CD34+ HSCs. In hematopoietic cells, however, the TRPM2-mediated Ca2+ signal is believed to mediate TNF-α-induced cell death (Zhang et al., 2006). It is possible that Ca2+ signaling via TRPM2 exhibits opposite roles depending on the differentiation state.
TRPV2 was initially identified as a noxious heat-activated cation channel (Stokes et al., 2004). However, recent studies suggest that TRPV2 is activated by insulin-like growth factor (Kanzaki et al., 1999). Link et al. (2010) reported that TRPV2- deficient macrophages are defective in chemoattractant-elicited motility and phagocytosis. Further investigation is required to elucidate the role of TRPV2 in terms of the proliferation of HSCs s timulated by various g rowth factors. As a whole, the CD34+ HSCs demonstrate variety of cationic conductances that are consistent with TRP family channels suggested from RTPCR analysis. However, not all the tested cells showed the corresponding currents when tested by the specific conditions (e.g. ADPR and OAG), the heterogeneity of TRP channel expression is suggested.
In summary, we provide a comprehensive depiction of functional cation channels in CD34+/CD45+/CD133high HSCs. It must be noted that not all types of ion channels were examined in the present study. For example, the anion channels, such as the ClC family, were not investigated here. Despite the limitations of molecular biological and functional assays, our results suggest a novel insight into hematopoietic regulation, differentiation, and the clinical application of CD34+ HSCs because Kv, TRP, and K2P channels are recognized to play roles in HSC-derived blood cells.
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
Acknowledgments
This study was supported by a grant from the SNUH Research Fund (No. 05-2009-0280) and by a Korean Research Foundation Grant funded by the Korean Government (Ministry of Education and Human Resources Development), Basic Research Promotion Fund, KRF-2009-0070542 and NRF 2011-0017370 and 2011-0001175).
References
- 1.Awong G., Herer E., Surh C.D., Dick J.E., La Motte-Mohs R.N., Zuniga-Pflucker J.C. Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood. (2009);114:972–982. doi: 10.1182/blood-2008-10-187013. [DOI] [PubMed] [Google Scholar]
- 2.Cheng J., Baumhueter S., Cacalano G., Carver-Moore K., Thibodeaux H., Thomas R., Broxmeyer H.E., Cooper S., Hague N., Moore M., et al. Hematopoietic defects in mice lacking the sialomucin CD34. Blood. (1996);87:479–490. [PubMed] [Google Scholar]
- 3.Civin C.I., Strauss L.C., Brovall C., Fackler M.J., Schwartz J.F., Shaper J.H. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J. Immunol. (1984);133:157–165. [PubMed] [Google Scholar]
- 4.den Dekker E., Molin D.G., Breikers G., van Oerle R., Akkerman J.W., van Eys G.J., Heemskerk J.W. xpression of transient receptor potential mRNA isoforms and Ca2+ influx in differentiating human stem cells and platelets. Biochim. Biophys. Acta. (2001);1539:243–255. doi: 10.1016/s0167-4889(01)00112-4. [DOI] [PubMed] [Google Scholar]
- 5.Guo Y., Lubbert M., Engelhardt M. CD34- hematopoietic stem cells: current concepts and controversies. Stem Cells. (2003);21:15–20. doi: 10.1634/stemcells.21-1-15. [DOI] [PubMed] [Google Scholar]
- 6.Han I.S., Ra J.S., Kim M.W., Lee E.A., Jun H.Y., Park S.K., Kwon B.S. Differentiation of CD34+ cells from human cord blood and murine bone marrow is suppressed by C6 betachemokines. Mol. Cells. (2003);15:176–180. [PubMed] [Google Scholar]
- 7.Hirschler-Laszkiewicz I., Tong Q., Conrad K., Zhang W., Flint W.W., Barber A.J., Barber D.L., Cheung J.Y., Miller B.A. TRPC3 activation by erythropoietin is modulated by TRPC6. J. Biol. Chem. (2009);284:4567–4581. doi: 10.1074/jbc.M804734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann T., Obukhov A.G., Schaefer M., Harteneck C., Gudermann T., Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. (1999);397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 9.Jespersen T., Grunnet M., Olesen S.P. The KCNQ1 pota-ssium channel: from gene to physiological function. Physiology (Bethesda) (2005);20:408–416. doi: 10.1152/physiol.00031.2005. [DOI] [PubMed] [Google Scholar]
- 10.Kanzak i M., Zhang Y.Q., Mashima H., Li L., Shibata H., Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat. Cell Biol. (1999);1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- 11.Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr. Pharm. Des. (2005);11:2717–2736. doi: 10.2174/1381612054546824. [DOI] [PubMed] [Google Scholar]
- 12.Korper S., Nolte F., Rojewski M.T., Thiel E., Schrezenmeier H. The K+ channel openers diazoxide and NS1619 induce depolarization of mitochondria and have differential effects on cell Ca2+ in CD34+ cell line KG-1a. Exp. Hematol. (2003);31:815–823. doi: 10.1016/s0301-472x(03)00199-1. [DOI] [PubMed] [Google Scholar]
- 13.Kronenwett R., Martin S., Haas R. The role of cytokines and adhesion molecules for mobilization of peripheral blood stem cells. Stem Cells. (2000);18:320–330. doi: 10.1634/stemcells.18-5-320. [DOI] [PubMed] [Google Scholar]
- 14.Kühn F.J., Heiner I., Lückhoff A. TRPM2: a calcium influx pathway regulated by oxidative stress and the novel second messenger ADP-ribose. Pflugers Arch. (2005);451:212–219. doi: 10.1007/s00424-005-1446-y. [DOI] [PubMed] [Google Scholar]
- 15.Lagasse E., Connors H., Al-Dhalimy M., Reitsma M., Dohse M., Osborne L., Wang X., Finegold M., Weissman I.L., Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. (2000);6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 16.Lanza F., Campioni D., Moretti S., Dominici M., Punturieri M., Focarile E., Pauli S., Dabusti M., Tieghi A., Bacilieri M., et al. CD34(+) cell subsets and long-term culture colonyforming cells evaluated on both autologous and normal bone marrow stroma predict long-term hematopoietic engraftment in patients undergoing autologous peripheral blood stem cell transplantation. Exp. Hematol. (2001);29:484–493. doi: 10.1016/s0301-472x(01)00726-3. [DOI] [PubMed] [Google Scholar]
- 17.Link T.M., Park U., Vonakis B.M., Raben D.M., Soloski M.J., Caterina M.J. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat. Immunol. (2010);11:232–239. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meuth S.G., Bittner S., Meuth P., Simon O.J., Budde T., Wiendl H. TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 critically influence T lymphocyte effector functions. J. Biol. Chem. (2008);283:14559–14570. doi: 10.1074/jbc.M800637200. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen J.S., McNagny K.M. CD34 is a key regulator of hematopoietic stem cell trafficking to bone marrow and mast cell progenitor trafficking in the periphery. Microcirculation. (2009);16:87–96. doi: 10.1080/10739680902941737. [DOI] [PubMed] [Google Scholar]
- 20.Nilius B., Owsianik G., Voets T., Peters J.A. Transient receptor potential cation channels in disease. Physiol. Rev. (2007);87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 21.Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S.M., Li B., Pickel J., Mckay R., Nadal-Ginard B., Bodine D.M., et al. Bone marrow cells regenerate infarcted myocardium. Nature. (2001);410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 22.Park K.S., Jung K.H., Kim S.H., Kim K.S., Choi M.R., Kim Y., Chai Y.G. Functional expression of ion channels in mesenchymal stem cells derived from umbilical cord vein. Stem Cells. (2007);25:2044–2052. doi: 10.1634/stemcells.2006-0735. [DOI] [PubMed] [Google Scholar]
- 23.Park K.S., Choi M.R., Jung K.H., Kim S., Kim H.Y., Kim K.S., Cha E.J., Kim Y., Chai Y.G. Diversity of ion channels in human bone marrow mesenchymal stem cells from amyotrophic lateral sclerosis patients. Korean J. Physiol. Pharmacol. (2008);12:337–342. doi: 10.4196/kjpp.2008.12.6.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park J.H., Park S.J., Chung M.K., Jung K.H., Choi M.R., Kim Y., Chai Y.G., Kim S.J., Park K.S. High expression of large-conductance Ca2+-activated K+ channel in the CD133+ subpopulation of SH-SY5Y neuroblastoma cells. Biochem. Biophys. Res. Commun. (2010);396:637–642. doi: 10.1016/j.bbrc.2010.04.142. [DOI] [PubMed] [Google Scholar]
- 25.Pillozzi S., Brizzi M.F., Balzi M., Crociani O., Cherubini A., Guasti L., Bartolozzi B., Becchetti A., Wanke E., Bernabei P.A., et al. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia. (2002);16:1791–1798. doi: 10.1038/sj.leu.2402572. [DOI] [PubMed] [Google Scholar]
- 26.Podesta M., Pitto A., Figari O., Bacigalupo A., Bruzzone S., Guida L., Franco L., De Flora A., Zocchi E. Cyclic ADP-ribose generation by CD38 improves human hemopoietic stem cell engraftment into NOD/SCID mice. FASEB J. (2003);17:310–312. doi: 10.1096/fj.02-0520fje. [DOI] [PubMed] [Google Scholar]
- 27.Shieh C.C., Coghlan M., Sullivan J.P., Gopalakrishnan M. Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacol. Rev. (2000);52:557–594. [PubMed] [Google Scholar]
- 28.Shirihai O., Attali B., Dagan D., Merchav S. Expression of two inward rectifier potassium channels is essential for differentiation of primitive human hematopoietic progenitor cells. J. Cell. Physiol. (1998);177:197–205. doi: 10.1002/(SICI)1097-4652(199811)177:2<197::AID-JCP1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 29.Steidl U., Kronenwett R., Rohr U.P., Fenk R., Kliszewski S., Maercker C., Neubert P., Aivado M., Koch J., Modlich O., et al. Gene expression profiling identifies significant differences between the molecular phenotypes of bone marrowderived and circulating human CD34+ hematopoietic stem cells. Blood. (2002);99:2037–2044. doi: 10.1182/blood.v99.6.2037. [DOI] [PubMed] [Google Scholar]
- 30.Stokes A.J., Shimoda L.M., Koblan-Huberson M., Adra C.N., Turner H. A TRPV2-PKA signaling module for transduction of physical stimuli in mast cells. J. Exp. Med. (2004);200:137–147. doi: 10.1084/jem.20032082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong Q., Hirschler-Laszkiewicz I., Zhang W., Conrad K., Neagley D.W., Barber D.L., Cheung J.Y., Miller B.A. TRPC3 is the erythropoietin-regulated calcium channel in human erythroid cells. J. Biol. Chem. (2008);283:10385–10395. doi: 10.1074/jbc.M710231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vennekens R., Owsianik G., Nilius B. Vanilloid transient receptor potential cation channels: an overview. Curr. Pharm. Des. (2008);14:18–31. doi: 10.2174/138161208783330763. [DOI] [PubMed] [Google Scholar]
- 33.Wang L., Xu B., White R.E., Lu L. Growth factormediated K+ channel activity associated with human myeloblastic ML-1 cell proliferation. Am. J. Physiol. (1997);273:C1657–C1665. doi: 10.1152/ajpcell.1997.273.5.C1657. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W., Hirschler-Laszkiewicz I., Tong Q., Conrad K., Sun S.C., Penn L., Barber D.L., Stahl R., Carey D.J., Cheung J.Y., et al. TRPM2 is an ion channel that modulates hematopoietic cell death through activation of caspases and PARP cleavage. Am. J. Physiol. Cell Physiol. (2006);290:C1146–C1159. doi: 10.1152/ajpcell.00205.2005. [DOI] [PubMed] [Google Scholar]


