Abstract
The ethical issues and public concerns regarding the use of embryonic stem (ES) cells in human therapy have motivated considerable research into the generation of pluripotent stem cell lines from non-embryonic sources. Numerous reports have shown that pluripotent cells can be generated and derived from germline stem cells (GSCs) in mouse and human testes during in vitro cultivation. The gene expression patterns of these cells are similar to those of ES cells and show the typical self-renewal and differentiation patterns of pluripotent cells in vivo and in vitro. However, the mechanisms underlying the spontaneous dedifferentiation of GSCs remain to be elucidated. Studies to identify master regulators in this reprogramming process are of critical importance for understanding the gene regulatory networks that sustain the cellular status of these cells. The results of such studies would provide a theoretical background for the practical use of these cells in regenerative medicine. Such studies would also help elucidate the molecular mechanisms underlying certain diseases, such as testicular germ cell tumors.
Keywords: germline, pluripotency, reprogramming, spermatogonial stem cells, stem cells
INTRODUCTION
Germ cells are unique in that they are the only cells able to transmit genetic information to the next generation. Accumulating evidence suggests that GSCs undergo spontaneous transformation into pluripotent stem cells. Accordingly, such cells hold promise as substitutes for existing pluripotent stem cells, whose use is embroiled in ethical and safety concerns. In addition to embryonic stem (ES) cells derived from the inner cell mass (ICM) of embryos, pluripotent stem cells have been derived from different mammalian germ cells, including primordial germ cells (PGCs) from embryos, gonocytes from neonatal mice, and spermatogonial stem/progenitor cells (SSCs/SPCs) from adult mice and humans. Embryonic germ cells (EGs) can be established from PGCs between 8.5 and 12.5 days postcoitum (dpc) when cultured with appropriate growth factors, including basic fibroblast growth factor (bFGF) and leukemia inhibitory factor (LIF) (Geijsen and Jones, 2008; Kerr et al., 2006).
Recently, gene regulatory mechanisms in both neonatal and adult GSCc were shown to undergo spontaneous reprogramming, resulting in the establishment of a pluripotent state under several different culture conditions. The terms ‘multipotent germline stem cells (mGSs)’ and ‘germline-derived pluripotent stem cells (gPSs)’ were coined to describe these pluripotent stem cells (Kanatsu-Shinohara et al., 2004; Ko et al., 2009). Due to the spontaneity of the reprogramming, the cellular state of GSCs is thought to be just one step away from that of ES cells. However, the potentiality of GSCs in vivo is limited, since their exclusive role is to produce functional spermatozoa in the testes.
Ko et al. (2009) showed that gPSs are more equivalent to ES cells than induced pluripotent stem (iPS) cells of somatic origin in terms of gene expression profiles. The use of mGSs in human therapeutics is free from ethical complications and can circumvent issues related to immune rejection since they use autologous cells. The genome integrity of germ cells is also thought to be superior to that of iPS cells. These observations suggest that testis-derived pluripotent stem cells could be used as a primary tool in regenerative medicine to combat various human diseases (Geijsen and Jones, 2008).
Despite the obvious advantages of using mGSs for clinical applications, there are two major obstacles that must be overcome before these cells can be used in practice. First, it remains difficult to isolate, derive, and stably culture GSCs, despite recent advances in these technologies. Second, the mechanisms that determine the spontaneous reprogramming of GSCs into testis-derived pluripotent stem cells are currently unknown. In this review, we discuss the molecular mechanisms regulating SSC maintenance. We also review the genetic and epigenetic properties of SSCs and testis-derived pluripotent stem cells. Finally, we present our current understanding of the mechanisms that could be used to induce pluripotency in GSCs.
SPERMATOGONIAL STEM CELLS
Development
Biologically active SSCs first arise from gonocytes 3-4 days after birth in the mouse testes (McLean, 2003). They maintain their self-renewal and differentiation potentials and persist throughout the life of the mouse. Adult GSCs are thought to exist only in the testes, although the existence of GSCs in the ovary is still a subject of considerable debate (Eggan et al., 2006; Gosden, 2004; Johnson et al., 2004; Telfer et al., 2005; Zou et al., 2009).
The main function of SSCs is to generate functional spermatozoa, which occurs through multiple differentiation steps referred to as spermatogenesis. Spermatogenesis consists of three major phases: proliferative mitotic divisions, meiosis, and spermiogenesis (Hermo et al., 2010). Spermatogonia are the most primitive populations in the testes, which include type A, inter mediate, and type B spermatogonia. Only the Asingle (As) population is believed to have stem cell activity. The other spermatogonial populations, which are generated from the mitotic division of type As cells, are assumed to be irreversibly differentiated and committed to sperm production (Oatley and Brinster, 2008). Notably, Nakagamwa et al. (2010) showed that the cellular states of As-Aal (Aaligned) spermatogonia cells in the testes can undergo reversible inter-conversion, even though they are heterogeneous in terms of glial cell line-derived neurotrophic factor (GDNF) family receptor alpha-1 (GFRα1)/neurogenin 3 (Ngn3) expression. Therefore, these populations are now referred to generally as ‘undifferentiated’ spermatogonia. This new nomenclature specifies that As-Aal spermatogonia share stem cell activities via functional reversibility, although their phenotypes are not synchronized at a given time.
Numerous studies have reported that the heterogeneity of mouse ES cells depends on the differential and reversible expression of Stella, Nanog, Rex1, E-cadherin, and Pecam1 (Carter et al., 2008; Toyooka et al., 2008). Thus, it is logical to assume that SSCs in vivo or in culture may also engender heterogeneous populations with variable gene expression patterns. Although a clear demarcation was not made, GFRα1 and Nanos2 are considered markers for most undifferentiated spermatogonia, and Nanos3 and Ngn3 are considered later stage markers. Promyelocytic leukemia zinc finger protein (PLZF, also known as zfp145) is expressed in most spermatogonial populations (Nakagawa et al., 2010).
As in other stem cell models, transplantation is the gold standard for assessing the identity of biologically functional SSCs (Brinster and Avarbock, 1994; Brinster and Zimmermann, 1994). This assay is commonly performed with donor testis cells and busulfan-treated or W/Wv mutant (i.e., germ cell-deficient) recipient mice.
In human, based on nuclear architecture and staining intensity with hematoxylin, SSC populations are divided into Adark (reservoir stem cell) and Apale (actively proliferating stem cell) (Hermann et al., 2009a). These populations share gene expression patterns with rodent SSCs such as α6-integrin, GFRα1, GRP125, PLZF and Thy1 (Dym et al., 2009). However, in human, very limited information is available about SSCs and the identity of the true SSCs is still unknown.
Genes important for SSC maintenance
The first gene discovered to be required for SSC self-renewal was the transcriptional repressor Plzf. Expression of Plzf is restricted to the undifferentiated spermatogonia of the mouse testes and its genetic disruption induces the progressive loss of spermatogonia. Transplantation experiments have shown that Plzf -/- recipient mice normally support self-renewal and differentiation of Plzf +/+ germ cells, implying that a lack of Plzf causes a severely degenerating phenotype only in germ cells and not in Sertoli cells (Buaas et al., 2004; Costoya et al., 2004). Plzf regulates the self-renewal of spermatogonial progenitor cells by counteracting mTORC1 via induction of the mTOR1 inhibitor Redd1 (Hobbs et al., 2010).
The transcription regulator Taf4b of the TFIID subunit is expressed uniquely in the mouse testes. Taf4b-null males gradually lose their germ cell population, resulting in infertility by 11 weeks of age (Falender, 2005). Reminiscent of Plzf -/- mice, the Taf4b-null phenotype also displays Sertoli cell-only seminiferous tubules in the adult.
Oct4, which fulfills a central role in regulating the self-renewal and pluripotency of ES cells, is also expressed in SSCs. Although the expression level is not as high as in pluripotent stem cells, Oct4 knockdown has detrimental effects on the selfrenewal and proliferation of SSCs (Dann et al., 2008). The mechanism of Oct4 regulation of SSC self-renewal appears to be independent of the Plzf-mediated pathway.
The RNA-binding protein Nanos2 also contributes to SSC maintenance. Its disruption depletes the SSC population, whereas its forced expression produces undifferentiated spermatogonia as a dominant population in the developing testes (Sada et al., 2009). In Nanos2-overexpressing testes, the number of PLZF-positive undifferentiated spermatogonia increases dramatically and the cells are resistant to differentiation. Nanos2 marks the most primitive undifferentiated spermatogonia based upon its preferential expression in As-Aal(8), whereas Plzf expression is observed throughout all stages of undifferentiated spermatogonia. These data demonstrate that Nanos2 is a master regulator of SSC maintenance, and that it probably acts upstream of Plzf in the mouse testes.
The RNA-binding protein Lin28 enhances the establishment of human induced pluripotent stem cells and stimulates the selfrenewal of ES cells by inhibiting the expression of let-7 family microRNAs and let-7-independent pathways. Lin28 is expressed exclusively in undifferentiated spermatogonia and its expression co-localizes with PLZF and GFRα1, although its cellular functions have not been defined (Zheng et al., 2009). Interestingly, it is known that LIN28 supports the expression of B-lymphocyte-induced maturation protein-1 (BLIMP1) by suppressing let-7 maturation in mouse ESC-derived primordial germ cells (West et al., 2009). Evolutionarily conserved RNAbinding proteins participate in the regulation of self-renewal and differentiation of germline stem cells in Drosophila melanogaster and C. elegans (Kimble and Crittenden, 2005; Spassov and Jurecic, 2003). Therefore, it is conceivable that other RNAbinding proteins enriched in mammalian testes are also implicated in SSC maintenance.
Surface makers
The SSC fraction in adult testes is very small, with an approximate concentration of 0.03% of total testicular cells as measured by morphometric quantification (Tegelenbosch and de Rooij, 1993). Therefore, to study the functions and applications of SSCs, it is essential to classify cells in the testes and to identify and isolate spermatogonia with stem cell activities.
A few surface markers have been identified that provide a broad spectrum of efficient SSC enrichment. The SSCs from adult testis are enriched around 8-fold and 4-fold by α6-integrin and β1-integrin, respectively (Shinohara et al., 1999). Enrichment by CD9 is comparable to enrichment by integrin-based methods in both mice and rats (Kanatsu-Shinohara, 2003). GFRα1, which is expressed preferentially in As and Apr (Apaired) spermatogonia, is theoretically one of the best SSC biomarkers. However, the enrichment of SSCs from either neonates or adults displayed < 3-fold efficiency (Ebata et al., 2005). This finding implies that the antibody capacity may be limited or that inherent barriers may exist that perturb the effectiveness of fractionation and purification. Ep-CAM is the best known marker for neonatal rats, displaying an 11-fold SSC enrichment (Ryu et al., 2004). Thy1 (also known as CD90) is a glycosyl phosphatidylinositol (GPI)-anchored glycoprotein that is expressed in both hematopoietic stem cells and spermatogonia. Thy1 efficiently enriches the SSC fraction by up to 30-fold, compared to the unsorted testis cell fraction. This protein is the best known surface marker currently available for adult mice (Kubota et al., 2003). In human, information about the identity and molecular characteristics of SSCs is very limited. Instead, a non-human primate model has been used to give insights regarding human SSCs. In rhesus monkey, stage-specific embryonic antigen-4 (SSEA-4)+ cells express SSC specific markers including PLZF, GPR125 and c-RET and represent an SSC-enriched population that can repopulate nude mouse testes following transplantation (Maki et al., 2009). About a 5-fold enrichment was observed compared to a non-sorted sample. In a separate study, Thy1 was used to enrich SSCs with a variable rhesus-to-nude mouse xenotransplant colonization efficiency of up to over 50-fold, compared to a Thy1- sample (Hermann et al., 2009b).
Fluorescence-activated cell sorting (FACS) and magneticactivated cell sorting (MACS) are the primary methods for separating the SSC population, with varying degrees of variability and effectiveness. However, because the isolated fraction remains impure, additional separation steps are often required. In some cases, isolated fractions can be further enriched by density gradient centrifugation in a Percoll solution. During in vitro culture of SSCs, extracellular matrix (ECM) is frequently used to separate germ cells from somatic adherent cells. For instance, spermatogonia have a strong affinity for laminincoated plates, while somatic cells tend to adhere exclusively to gelatin- or collagen- coated plates (Guan et al., 2009; Kanatsu-Shinohara et al., 2005; Morimoto et al., 2009).
SSCs are considered to be a promising model in the field of regenerative medicine for restoration of fertility after cancer treatment or for the generation of normal offspring by transplantation following the in vitro correction of genetic defects. Therefore, the development of definitive biomarkers of SSCs is important not only for assessing their functions in biological studies but also for therapeutic purposes when the donor cell purity is of paramount concern.
Extrinsic factors
Niches are supportive microenvironments that are crucial for the symmetric and asymmetric division of stem cells. The major contributor to the SSC niche is Sertoli cells, although the effects of Leydig and myoid cells are not negligible (Yoshida et al., 2007). Sertoli cells reside in the seminiferous tubules in close contact with spermatogonia. Sertoli cells secrete GDNF, an essential growth factor for SSC maintenance (Meng et al., 2000). As a member of the TGFβ superfamily of growth factors, GDNF controls kidney development and regulates the survival of many types of neurons (Dressler, 2006; Sariola and Saarma, 2003). Notably, GDNF binds to the GPI-linked receptor GFRα1 on the outer surface of SSCs and mediates the activation of the integral protein ‘rearranged during transfection’ (RET) tyrosine kinase receptor (Naughton et al., 2006). Activation of RET triggers the transcription of downstream genes, B cell CLL/lymphoma 6 (Bcl6b), ets variant gene 5 (Etv5), and lim homeobox protein 1 (Lhx1), the first two of which are essential for selfrenewal (Oatley et al., 2006). Mice harboring 1 null allele for GDNF are progressively depleted of spermatogonia and acquire a Sertoli-only testis phenotype (Meng et al., 2000). In contrast, undifferentiated spermatogonia accumulate in mice overexpressing GDNF. These results clearly demonstrate the biological role of GDNF in maintaining SSCs and the importance of the nurse cell, Sertoli (Meng et al., 2000). Likewise, silencing of its cognate receptor GFRa1 in mouse spermatogonia prompts the loss of stem cell activities due to the inactivation of RET tyrosine kinase (He et al., 2007).
Signaling pathways initiated by GDNF in SSCs involve the activation of the src family kinase (SFK) (He et al., 2007; Oatley et al., 2007). Blockade of SFK phosphorylation by the selective inhibitor SU6656 negatively affects self-renewal of SSCs in vitro (Oatley et al., 2007). Stimulation of SSCs by GDNF accelerates the activation of phosphoinositide 3-kinase (PI3K) (Lee et al., 2007). Selective chemical inhibition of PI3K by LY294002 results in the inactivation of its downstream effector protein kinase B (Akt), which plays a key role in SSC survival and proliferation (Lee et al., 2007). The importance of the Akt pathway was further stressed in an experiment where conditional activation of Akt by 4-hydroxytestosterone in myr-Akt-Mer GSCs sustained long-term normal self-renewal and active proliferation in the absence of GDNF (Lee et al., 2007).
A vasculature-associated niche also plays critical roles in maintaining undifferentiated spermatogonia in the mouse testes (Yoshida et al., 2007). Undifferentiated spermatogonia preferentially reside in a specific area of the basal compartment within the seminiferous tubules. This niche is located adjacent to interstitial somatic cells and blood vessels (Yoshida et al., 2007). Differentiating spermatogonia migrate out of this stem cell niche and are dispersed throughout the entire basal compartment. Since spermatogonia do not interact directly with the vasculature, Leydig or other interstitial cells might interpret unknown signals from the blood stream as stimuli for SSC maintenance in the testes.
In vitro long-term culture
The establishment of an SSC culture system would be extremely valuable for the elucidation of the molecular mechanisms underlying self-renewal and proliferation. Prolonged survival of SSCs in culture allows sufficient time to analyze their properties when they are exposed to a variety of biologically important stimuli that mimic equivalent signals in vivo. It also provides a platform for the modulation of gene expression and for the modification of DNA sequences in the genome while the cells are in culture, thus enabling a determination of the function of the gene under study in vivo (Kubota and Brinster, 2006).
Kanatsu-Shinohara et al. (2003) first reported the long-term culture of gonocytes, which the authors referred to as GSCs, from 0 dpp mouse pups. Growth factor-enriched Stempro34- based medium, which contains more than 20 additives besides the medium supplement, was shown to support GSC culture on mitotically inactivated mouse embryonic fibroblasts (MEFs) in the presence of GDNF. The culture could be maintained with exponential growth for more than 5 months (Kanatsu-Shinohara et al., 2003). LIF was initially included in the medium, until it was discovered that the downstream effector ‘signal transducer and activator of transcription 3’ (STAT3) unexpectedly promoted differentiation (Oatley et al., 2010). However, the culture conditions reported by Kanatsu-Shinohara et al. (2003) use a chemically undefined component (i.e., serum) and require a complicated recipe of expensive materials. Long-term SSC culture has, for the first time, been established in a serumfree, chemically defined medium on irradiated STO feeders in the presence of GDNF (Kubota et al., 2004). Addition of a soluble form of GFRα1 and bFGF improved the cultivation of some SSC strains. In both the Kanatsu-Shinohara et al. system and serum-free conditions, growth kinetics showed active proliferation of GSCs and a transplantation assay confirmed the stem cell activities of the cultured cells.
Technical advances in SSC cultivation allow the use of diverse culture conditions with differences in ECM composition, feeder cell choice, and medium constituents. Laminin-coated plates support adherent SSC culture without feeder layers at a decreased growth rate (Kanatsu-Shinohara et al., 2010; Morimoto et al., 2009). Non-coated dishes allow suspension culture of SSC colonies, with comparable proliferation rates to those in a feeder-supported growth environment (Kanatsu-Shinohara et al., 2006). Intriguingly, the changes in culture conditions induces subtle alterations in the growth rate as well as in gene expression and morphology, suggesting that the in vitro niche contributes to the self-renewal and differentiation capacity of SSCs and that the enrichment of a desired cell population may be possible. Progress in rodent SSC research has prompted the development of in vitro culture methods for SSCs from other mammals and their functional validation in subsequent experiments. To date, SSCs from hamster, bull, pig, and chicken have been cultured and examined for limited periods of time under similar conditions (Jung et al., 2006; Kanatsu-Shinohara et al., 2007; Kuijk et al., 2009; Lim et al., 2010; Oatley et al., 2004; Sadri-Ardekani et al., 2009; Takehashi et al., 2010). Technologies in this field are especially valuable in animals where ES cells are not yet available for transgenesis.
DEDIFFERENTIATION OF GERMLINE STEM CELLS INTO PLURIPOTENT STEM CELLS
Derivation of pluripotent stem cells from mammalian testes
Kanatsu-Shinohara et al. (2004) first reported the derivation of ES-like cells from neonatal mouse testes. The term ‘multipotent germline stem cells (mGSs)’ was coined to define these pluripotent stem cells. mGSs showed very similar morphology and gene expression patterns to mouse ES cells, and deve-loped into all 3 germ layers both in vitro and in vivo and contributed to chimera formation. Subsequent experiments showed that mGSs could also be derived from adult mice with various genetic backgrounds (Guan et al., 2006; Seandel et al., 2007). Clonal analysis in a separate experiment confirmed that they originated from SSCs (Ko et al., 2009).
In 2008, pluripotent stem cells were generated from adult human testes (Conrad et al., 2008). Although these cells were pluripotent in terms of their self-renewal and differentiation capacities, their gene expression patterns and characteristic morphologies were quite distinct from those of typical human ES cells. This finding elicited controversial opinions from the scientific community regarding the identity of these pluripotent stem cells (Geijsen and Hochedlinger, 2009; Ko et al., 2010). Regardless of the conflicting views, this result sheds light on the possibility that GSCs could be used in regenerative medicine as a substitute for ES cells. Therefore, considerable effort has been expended to derive pluripotent stem cells from cells in testes for use in human therapeutics.
Characterization of testis-derived pluripotent stem cells
Embryonic stem cells and SSCs share a high degree of similarity in gene expression such that the terms ‘ES-specific’ and ‘germline-specific’ are used indiscriminately or interchangeably when referring to particular groups of genes. Accordingly, mouse SSCs express several pluripotency marker genes, including ‘Yamanaka factors’ (Oct4, Sox2, Klf4 and c-myc), although the OCT4 and SOX2 protein expression levels are low or absent due to transcriptional repression and possibly translational inhibition (Kanatsu-Shinohara et al., 2008; Oatley et al., 2006). A low level of OCT4 expression is required for SSCs to function properly because OCT4 knockdown negatively affects SSC maintenance (Dann et al., 2008). Sox2 is expressed transcriptionally in mouse SSCs, but its protein level and functions have not yet been explicitly determined. It is believed that SSCs are the only cell type in adults that transcriptionally expresses both Oct4 and Sox2. The homeobox gene Nanog appears to be a determining factor, as it is silenced in SSCs at both the transcriptional and translational levels (Hart et al., 2005; Yamaguchi et al., 2005).
Extensive gene expression profiling of mGS, mES, iPS, and SSCs reveals that the gene expression patterns of mGS are closer to those of mES than to those of iPS, indicating that mGS cells might be better than mES cells for therapeutic purposes (Ko et al., 2009). In addition, mGS and mES cells show similar microRNA expression profiles, with pronounced expression of the miR-290 and miR-302 families and reduced expression of the let-7 family (Zovoilis et al., 2008). It is also conceivable that the reprogramming of SSCs into ES-like cells would be relatively simple compared to that of other cell types, due to the similarity in the gene expression profiles of ES cells and SSCs. On the other hand, with the exception of spontaneous reprogramming, iPS cells have yet to be established from SSCs by transgene expression or other methods involving the artificial modulation of gene expression. Thus, there might be active suppressive mechanisms that prevent the induction of pluripotency in SSCs.
Kinetics of spontaneous reprogramming
Guan et al. (2006) proposed that the niche in testes restricts SSC potential only towards sperm production. They also hypothesized that the microenvironment of in vitro cultures, which lack the in vivo microenvironment to direct SSCs to a faterestricted state, induces pluripotency by somehow modulating gene expression. In their model, cells respond to changes in the composition of the microenvironment and initiate fate conversion immediately, under proper in vitro culture conditions. In other studies, the reprogramming process is spontaneous but inefficient: reprogramming takes more than 4 weeks after derivation, depending on the experimental setup (Kanatsu-Shinohara et al., 2004; Seandel et al., 2007). Furthermore, frequent reprogramming events tend to occur in derivation steps with neonates (Kanatsu-Shinohara et al., 2004). Considering the preexistence of Oct4 and Sox2 expression in SSCs, one may speculate that the rate of SSC reprogramming into the ES-like state is faster than that from other cell types. However, for now it is almost impossible to judge the overall kinetics of the process, since what triggers the initiation of the dedifferentiation process is unknown.
Factors affecting reprogramming efficiency
The results from various studies indicate that the above-mentioned in vitro reprogramming is a general phenomenon that occurs at low efficiency with a variable derivation yield. Reprogramming appears to depend on the age and genetic background of the mice as well as on the procedures used for spermatogonium separation. Culture conditions also affect reprogramming efficiency; the successful establishment of pluripotent stem cells is found exclusively in growth factor-rich Stempro34-based medium, which is optimized for hematopoietic stem cell culture and expansion, with irradiated MEFs as a feeder layer rather than under serum-free conditions on irradiated STO feeders. However, both methods are equally efficient for SSC maintenance (Kanatsu-Shinohara et al., 2004; Seandel et al., 2007).
Isolation of a unique germ cell population appears to affect reprogramming efficiency. Seandel et al. (2007) successfully generated multipotent adult stem cells (MASCs) from GPR125+ spermatogonia using CD34+ irradiated mouse testicular stromal (CD34+ MTS) cells as feeders. Irradiated CD34+ MTS cells were effective both for the growth of GPR125+ spermatogonial progenitor cells as well as the emergence of MASCs. STRA8 was also used to enrich the spermatogonial population and subsequent conversion into pluripotent stem cells at high frequency (Guan et al., 2006). Izadyar et al. (2008) suggested that the Oct4+/c-Kit+ subset of mouse GSCs in the in vitro culture generated a distinct cell population whose properties are very similar to those of pluripotent stem cells.
Using Oct4-GFP GSCs, Ko et al. (2009) reported that the initial seeding density and culture duration are pivotal control points for the induction of pluripotency. Kim et al. (2010) proposed that an ‘intermediate state’ exists in the progression of GSCs to pluripotent stem cells. Cells in the intermediate state show characteristic expression of Nanog and have a more ESlike morphology.
At present, the tumor suppressor p53 is the only gene known to affect the dedifferentiation efficiency of GSCs. Established GSCs from p53 knockout mice generate ES-like cells at a higher rate compared to wild-type, even after long periods in culture, indicating that p53 negatively controls the reprogramming process (Kanatsu-Shinohara et al., 2004). The importance of p53 activity has been confirmed in a series of publications describing enhanced iPS formation by p53 knockout or knockdown, although SSC reprogramming may not always take the same route before pluripotency is achieved (Hong et al., 2009; Kawamura et al., 2009; Li et al., 2009; Marión et al., 2009; Utikal et al., 2009). Genetic and epigenetic alterations of other tumor suppressors and cell cycle regulators are known to affect cell reprogramming and ES cell maintenance (Boominathan, 2009; Ruiz et al., 2011). Because it is known to suppress Nanog expression, p53 also may exert its effects on reprogramming in SSCs (Kuijk et al., 2010).
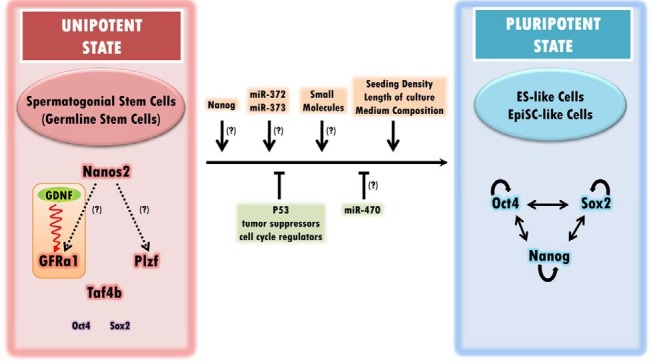
The small noncoding microRNAs mostly target the 3′ untranslated region (3′UTR) of mRNAs and either downregulate their translation or degrade them, depending on the degree of complementation between the sequences. Mir-470 recognizes a coding region of Oct4 and Nanog and negatively regulates their translation in differentiating ES cells (Tay et al., 2008). Notably, mir-470 is also expressed exclusively in the testes of adult mice, although its expression peaks in germ cells at meiotic stages (Song et al., 2009). Oct4 expression in SSCs is repressed transcriptionally, but might be regulated post-transcriptionally by the actions of mir-470, under an ES-like state threshold level, which would prevent cells in the testes from acquiring a pluripotent state in vivo. A summary of the different factors affecting the reprogramming efficiency of SSCs is indicated in Fig. 1.
Fig. 1. Factors influencing the reprogramming of unipotent SSCs into pluripotent stem cells. Nanos2 is expressed preferentially in As and Apr undifferentiated spermatogonia and positively regulates Plzf and GFRα1 when overexpressed. GDNF, an essential extrinsic growth factor, plays critical roles in self-renewal of SSCs through its cognate receptor GFRα1. It appears that SSCs maintenance by Plzf is independent of the GDNF-GFRα1 signaling pathway. Although Oct4 and Sox2 are expressed transcriptionally in SSCs, their gene expression levels are significantly lower than in ES cells. This may explain the absence of another pluripotency marker, Nanog, which is upregulated by OCT4 and SOX2. Currently, p53 is the only known gene that negatively affects the reprogramming efficiency of SSCs. Tumor suppressors and cell cycle regulators may play a similar role in the reprogramming process. MiR-470 represses Oct4 expression by targeting the coding sequences upon ES cell differentiation. The seeding density, the culture time, and medium composition are factors that influence the reversion of the cellular state of SSCs. The absence of Nanog expression may explain the unipotency of SSCs. The testicular germ cell tumor-associated microRNAs, miR-372 and miR-373, mediate a complex process involved in the establishment of the pluripotent state in the absence of p53.
Choice of pluripotent states
While ES cells are pluripotent, most adult stem cells are multipotent or unipotent and are committed to a specified lineage during development. As adult stem cells, SSCs are also dedicated exclusively to sperm production through complicated differentiation processes. In contrast, the testis-derived pluripotent stem cells established by most research groups have pluripotent properties in vitro and in vivo and do not contribute to the generation of functional spermatozoa when injected into testes. This finding indicates that testis-derived pluripotent stem cells and SSCs are two distinct populations with entirely different morphologies, growth rates, and gene expression patterns, with no functional overlap.
However, Guan et al. (2006) reported that these two mutually exclusive properties may coexist in a single type of SSC isolated from the Stra8+ population in adult mouse testes when the cells respond appropriately to their surroundings. The SSCs underwent spontaneous conversion into a pluripotent state with high efficiency in response to the in vitro culture conditions and expressed the ES cell marker Nanog. Moreover, the cells formed teratomas when they were injected subcutaneously into SCID/beige mice. However, the pluripotent stem cells, which they termed multipotent adult germline stem cells (maGSCs), became unipotent and produced only sperm when they were re-injected into seminiferous tubules in the testes. This result contradicts a recent report that extrinsic signals are dispensable for pluripotency and self-renewal in ES cells (Ying et al., 2008). Instead, it implies that the cellular environment dictates the cellular identity and predominates over the ‘intrinsic mechanism’ of the stem cells. How efficiently the cells respond to different microenvironments for switching from one cellular state to another and what mechanisms underlie this reversibility remain unclear.
One notable difference among testis-derived pluripotent stem cells is the variable epigenetic status on parent-specific imprinted loci. GSCs have complete androgenetic DNA methylation marks, as indicated by bisulfite sequencing and combined bisulfite restriction analysis (COBRA) (Kanatsu-Shinohara et al., 2004). Although a genome-wide reprogramming event reformats the epigenetic identity of the cells, reprogrammed pluripotent stem cells from GSCs in vitro show partially reconstituted imprints when the GSCs are taken from neonatal mice (Kanatsu- Shinohara et al., 2004). In separate experiments, pluripotent stem cells from adult mouse testes appeared to exhibit either an absolute androgenetic or a somatic imprinting status. Thus, there may be different routes to pluripotency that affect the epigenetic status of the resultant cells and depend on the properties of SSCs from different developmental stages (Guan et al., 2006; Ko et al., 2009).
Both mouse ES cells and epiblast stem cells (EpiSCs) are referred to as pluripotent stem cells; however, these cells represent distinct populations derived from the early and late stages of embryo development, respectively. Gene expression analysis indicates shared expression of Oct4 and SSEA1 but differential expression of a group of genes, including Stella, Rex1, Pecam1, Gbx2, and Fgf5 (Bao et al., 2009; Brons et al., 2007; Tesar et al., 2007). Differential expression of GFP by Oct4 regulatory sequences can be used to distinguish the two different pluripotent states (Han et al., 2010a; 2010b). Although EpiSCs are thought to be pluripotent, they are not incorporated properly into the ICM in the chimera formation assay because they are not compatible with the pre-implantation embryo environment. However, the previous notion that ES cells and EpiSCs represent only two possible pluripotent states was challenged by the observation that both ES cells and EpiSCs are heterogeneous in gene expression and morphology and exist in a reversible dynamic equilibrium between many possible pluripotent states (Carter et al., 2008; Hayashi et al., 2008). Further evidence indicates that either ES cells or EpiSCs could be directly derived from ICM, as the cells respond to specific culture conditions (Brons et al., 2007).
Although most testis-derived pluripotent stem cells generated so far are considered to be in an ES-like state, multipotent adult spermatogonial-derived stem cells (MASCs) from GPR125- positive germline progenitor cells show a high degree of similarity to EpiSCs. These cells exhibit a chimera formation efficiency of only 22%, express low levels of Rex1, Stra8, and Dazl, and express high levels of brachyury and Nog (Seandel et al., 2007). Earlier reports by Kanatsu-Shinohara et al. (2004) and Guan et al. (2006) clearly indicate that both ES-like and EpiSC-like colonies appear during derivation. However, the authors did not seek to uncover the identity of each population, which had a different morphology. In fact, the composition of the medium used contained both LIF and bFGF, which are key growth factors required for maintaining mouse ES cells and EpiSCs, respectively. This culture condition could probably support the survival of both types of pluripotent stem cells in the same culture, at least for a limited time. However, it remains unclear whether testes could be a definite source of two distinct types of pluripotent stem cells and whether the reprogramming preference could be directed with an adjustment in culture conditions.
Pluripotency of germ cell tumors
Although a direct correlation between testis-derived pluripotent stem cells in vitro and testicular germ cell tumors (TGCTs) in vivo has not been defined, insights into tumor development may provide clues to the molecular mechanisms promoting SSC reprogramming. Carcinoma in situ (CIS) testis cells, the preinvasive stage of both seminomas and nonseminomas, have a clear phenotypic resemblance to primordial germ cells or gonocytes (Nielsen et al., 1974). The CIS testis cells express characteristic premeiotic germ cell-specific genes, strongly suggesting their early germ cell origin. The gene expression properties in these cells have been emphasized, with the detection of Oct4 and Nanog expression. Therefore, the cells are sometimes considered to contain cancer stem cells (Kristensen et al., 2008). However, CIS cells are usually negative for Sox2 expression, which is used to discriminate these cells from Sox2-positive embryonal carcinoma (EC) cells of the nonseminomas at later stages. The EC cells are capable of self-renewal and differentiation into all three germ layers, reminiscent of ES cells (Solter, 2006). It has been suggested that acquisition of pluripotency in EC cells could be promoted by an in vivo reprogramming process accompanied by the induction of Sox2 (Almstrup et al., 2005; Skotheim, 2005).
The oncogenes miRNA-372 and miRNA-373 permit uncontrolled growth and neoplasia of primary human cells. Notably, these microRNAs contribute to the formation of TGCTs by inhibiting LATS2, allowing activation of CDK even in the presence of wild-type p53 (Voorhoeve et al., 2006). However, details of the mechanistic pathways underlying germ cell tumor formation remain to be uncovered. Environmental factors appear to contribute to the niche in the testes and, therefore, to the fate of testicular germ cells.
CONCLUSION
Since pluripotent stem cells were first derived from testes a few years ago, the analysis of their gene expression patterns and functional properties have confirmed their identity as a promising ES cell substitute and have made them critically important for the future of regenerative medicine. These cells are nonembryonic, patient-specific, and free from genetic modifications, due to the spontaneity of the reprogramming process.
Nevertheless, extra caution should also be taken before pluripotent stem cells can be used for therapy. For example, variable imprinting patterns have been observed in pluripotent stem cells derived from GSCs. Although this epigenetic difference did not affect the self-renewal or differentiation capacity of the cells, uncontrolled genomic imprinting may cause tumor formation because genes regulated in imprinted loci are often oncogenic (Lim and Maher, 2010).
A bottleneck for the practical application of these cells is that the stimuli triggering spontaneous reversion of cells into the pluripotent state are currently unknown. Although SSCs have the closest gene expression patterns to those of ES cells in adults, it could be difficult to direct SSCs towards a pluripotent state. Currently, there are only a few methods to affect the reprogramming efficiency of SSCs into pluripotent stem cells. These include p53 downregulation and manipulation of culture conditions. However, these methods only minimally increase the reprogramming efficiency; the key factors in the process remain to be uncovered. Yamanaka factors function sufficiently as master regulators to induce pluripotency in most types of somatic cells. It has been suggested that active repressive mechanisms limit SSC differentiation in vivo to cells involved in sperm production. However, it is also likely that the expression of Oct4 and Sox2 in SSCs improves the likelihood that these cells will acquire an ES-like state. The required change in Oct4 and Sox2 gene expression levels may be small compared to the requirement of the four factors in the induction of iPSs from somatic cells.
A variety of chemical compounds have been screened as active elements to sustain ES cell cultures or to help establish iPSs by increasing the efficiency or substituting transgene expression (Li and Ding, 2010). Chemicals with known functions have also been used in setups designed to support ES cell selfrenewal. In this regard, biologically active small molecules may provide an alternative approach to the modulation of gene expression towards a pluripotent state in SSCs. A similar strategy could also be used to better define the gene regulatory network in SSCs. Advances in understanding the gene regulatory mechanisms in SSCs and ES cells and the development of supportive microenvironments for their maintenance will facilitate further exploration of the plausible mechanisms responsible for switching the cellular fate of SSCs and help develop practical uses for these cells.
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
Acknowledgments
We thank Rita Vassena and Cristina Eguizabal for helpful discussions and comments. We also thank May Schwarz for critical reading of the manuscript. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0015391 and 2010-0011547) and a CIRM training grant.
References
- 1.Almstrup K., Hoei-Hansen C.E., Nielsen J.E., Wirkner U., Ansorge W., Skakkebæk N.E., Rajpert-De Meyts E., Leffers H. Genome-wide gene expression profiling of testicular carcinoma in situ progression into overt tumours. Br. J. Cancer. (2005);92:1934–1941. doi: 10.1038/sj.bjc.6602560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao S., Tang F., Li X., Hayashi K., Gillich A., Lao K., Surani M.A. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature. (2009);461:1292–1295. doi: 10.1038/nature08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boominathan L. Tumor suppressors function as a bottleneck against cellular reprogramming into iPS cells. Nature Precedings.; (2009). [Google Scholar]
- 4.Brinster R.L., Avarbock M.R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. USA. (1994);91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinster R.L., Zimmermann J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA. (1994);91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brons I.G.M., Smithers L.E., Trotter M.W.B., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S.M., Howlett S.K., Clarkson A., Ahrlund-Richter L., Pedersen R.A., et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. (2007);448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 7.Buaas F.W., Kirsh A.L., Sharma M., McLean D.J., Morris J.L., Griswold M.D., de Rooij D.G., Braun R.E. Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. (2004);36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 8.Carter M.G., Stagg C.A., Falco G., Yoshikawa T., Bassey U.C., Aiba K., Sharova L.V., Shaik N., Ko M.S.H. An in situ hybridization-based screen for heterogeneously expressed genes in mouse ES cells. Gene Expr. Patterns. (2008);8:181–198. doi: 10.1016/j.gep.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad S., Renninger M., Hennenlotter J., Wiesner T., Just L., Bonin M., Aicher W., Bühring H.-J., Mattheus U., Mack A., et al. Generation of pluripotent stem cells from adult human testis. Nature. (2008);456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 10.Costoya J.A., Hobbs R.M., Barna M., Cattoretti G., Manova K., Sukhwani M., Orwig K.E., Wolgemuth D.J., Pandolfi P.P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. (2004);36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- 11.Dann C.T., Alvarado A.L., Molyneux L.A., Denard B.S., Garbers D.L., Porteus M.H. Spermatogonial stem cell selfrenewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. (2008);26:2928–2937. doi: 10.1634/stemcells.2008-0134. [DOI] [PubMed] [Google Scholar]
- 12.Dressler G.R. The cellular basis of kidney development. Ann. Rev. Cell Dev. Biol. (2006);22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 13.Dym M., Kokkinaki M., He Z. Spermatogonial stem cells: mouse and human comparisons. Birth Defects Research Part C: Embryo Today: Reviews. (2009);87:27–34. doi: 10.1002/bdrc.20141. [DOI] [PubMed] [Google Scholar]
- 14.Ebata K.T., Zhang X., Nagano M.C. Expression patterns of cell-surface molecules on male germ line stem cells during postnatal mouse development. Mol. Rep. Dev. (2005);72:171–181. doi: 10.1002/mrd.20324. [DOI] [PubMed] [Google Scholar]
- 15.Eggan K., Jurga S., Gosden R., Min I.M., Wagers A.J. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. (2006);441:1109–1114. doi: 10.1038/nature04929. [DOI] [PubMed] [Google Scholar]
- 16.Falender A.E. Maintenance of spermatogenesis requires TAF4b, a gonad-specific subunit of TFIID. Genes Dev. (2005);19:794–803. doi: 10.1101/gad.1290105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geijsen N., Hochedlinger K. gPS navigates germ cells to pluripotency. Cell Stem Cell. (2009);5:3–4. doi: 10.1016/j.stem.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geijsen N., Jones D.L. Seminal discoveries in regenerative medicine: contributions of the male germ line to understanding pluripotency. Hum. Mol. Genet. (2008);17:R16–R22. doi: 10.1093/hmg/ddn084. [DOI] [PubMed] [Google Scholar]
- 19.Gosden R.G. Germline stem cells in the postnatal ovary: is the ovary more like a testis? Hum. Reprod. Update. (2004);10:193–195. doi: 10.1093/humupd/dmh023. [DOI] [PubMed] [Google Scholar]
- 20.Guan K., Nayernia K., Maier L.S., Wagner S., Dressel R., Lee J.H., Nolte J., Wolf F., Li M., Engel W., et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. (2006);440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 21.Guan K., Wolf F., Becker A., Engel W., Nayernia K., Hasenfuss G. Isolation and cultivation of stem cells from adult mouse testes. Nat. Protoc. (2009);4:143–154. doi: 10.1038/nprot.2008.242. [DOI] [PubMed] [Google Scholar]
- 22.Han D.W., Greber B., Wu G., Tapia N., Araúzo-Bravo M.J., Ko K., Bernemann C., Stehling M., Schöler H.R. Direct reprogramming of fibroblasts into epiblast stem cells. Nat. Cell Biol. (2010a);13:66–71. doi: 10.1038/ncb2136. [DOI] [PubMed] [Google Scholar]
- 23.Han D.W., Tapia N., Joo J.Y., Greber B., Araúzo-Bravo M.J., Bernemann C., Ko K., Wu G., Stehling M., Do J.T. Epiblast stem cell subpopulations represent mouse embryos of distinct pregastrulation stages. Cell. (2010b);143:617–627. doi: 10.1016/j.cell.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Hart A.H., Hartley L., Parker K., Ibrahim M., Looijenga L.H., Pauchnik M., Chow C.W., Robb L. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer. (2005);104:2092–2098. doi: 10.1002/cncr.21435. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi K., Lopes S., Tang F., Surani M. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell. (2008);3:391–401. doi: 10.1016/j.stem.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Z., Jiang J., Hofmann M.C., Dym M. Gfra1 silencing in mouse spermatogonial stem cells results in their differentiation via the inactivation of RET tyrosine kinase. Biol. Reprod. (2007);77:723–733. doi: 10.1095/biolreprod.107.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermann B.P., Sukhwani M., Hansel M.C., Orwig K.E. Spermatogonial stem cells in higher primates: are there differences from those in rodents? Reproduction. (2009a);139:479–493. doi: 10.1530/REP-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermann B.P., Sukhwani M., Simorangkir D.R., Chu T., Plant T.M., Orwig K.E. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum. Reprod. (2009b);24:1704–1716. doi: 10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermo L., Pelletier R.M., Cyr D.G., Smith C.E. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc. Res. Tech. (2010);73:241–278. doi: 10.1002/jemt.20783. [DOI] [PubMed] [Google Scholar]
- 30.Hobbs R.M., Seandel M., Falciatori I., Rafii S., Pandolfi P.P. Plzf regulates germline progenitor self-renewal by opposing mTORC1. Cell. (2010);142:468–479. doi: 10.1016/j.cell.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong H., Takahashi K., Ichisaka T., Aoi T., Kanagawa O., Nakagawa M., Okita K., Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. (2009);460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izadyar F., Pau F., Marh J., Slepko N., Wang T., Gonzalez R., Ramos T., Howerton K., Sayre C., Silva F. Generation of multipotent cell lines from a distinct population of male germ line stem cells. Reproduction. (2008);135:771–784. doi: 10.1530/REP-07-0479. [DOI] [PubMed] [Google Scholar]
- 33.Johnson J., Canning J., Kaneko T., Pru J.K., Tilly J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. (2004);428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 34.Jung J.G., Lee Y.M., Park T.S., Park S.H., Lim J.M., Han J.Y. Identification, culture, and characterization of germline stem cell-like cells in chicken testes. Biol. Reprod. (2006);76:173–182. doi: 10.1095/biolreprod.106.056275. [DOI] [PubMed] [Google Scholar]
- 35.Kanatsu-Shinohara M. CD9 is a surface marker on mouse and rat male germline stem cells. Biol. Reprod. (2003);70:70–75. doi: 10.1095/biolreprod.103.020867. [DOI] [PubMed] [Google Scholar]
- 36.Kanatsu-Shinohara M., Ogonuki N., Inoue K., Miki H., Ogura A., Toyokuni S., Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. (2003);69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 37.Kanatsu-Shinohara M., Inoue K., Lee J., Yoshimoto M., Ogonuki N., Miki H., Baba S., Kato T., Kazuki Y., Toyokuni S. Generation of pluripotent stem cells from neonatal mouse testis. Cell. (2004);119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Kanatsu-Shinohara M., Miki H., Inoue K., Ogonuki N., Toyokuni S., Ogura A., Shinohara T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol. Reprod. (2005);72:985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 39.Kanatsu-Shinohara M., Inoue K., Lee J., Miki H., Ogonuki N., Toyokuni S., Ogura A., Shinohara T. Anchorageindependent growth of mouse male germline stem cells in vitro. Biol. Reprod. (2006);74:522–529. doi: 10.1095/biolreprod.105.046441. [DOI] [PubMed] [Google Scholar]
- 40.Kanatsu-Shinohara M., Muneto T., Lee J., Takenaka M., Chuma S., Nakatsuji N., Horiuchi T., Shinohara T. Longterm culture of male germline stem cells from hamster testes. Biol. Reprod. (2007);78:611–617. doi: 10.1095/biolreprod.107.065615. [DOI] [PubMed] [Google Scholar]
- 41.Kanatsu-Shinohara M., Lee J., Inoue K., Ogonuki N., Miki H., Toyokuni S., Ikawa M., Nakamura T., Ogura A., Shinohara T. Pluripotency of a single spermatogonial stem cell in mice. Biol. Reprod. (2008);78:681–687. doi: 10.1095/biolreprod.107.066068. [DOI] [PubMed] [Google Scholar]
- 42.Kanatsu-Shinohara M., Inoue K., Ogonuki N., Morimoto H., Ogura A., Shinohara T. Serum- and feeder-free culture of mouse germline stem cells. Biol. Reprod. (2010);84:97–105. doi: 10.1095/biolreprod.110.086462. [DOI] [PubMed] [Google Scholar]
- 43.Kawamura T., Suzuki J., Wang Y.V., Menendez S., Morera L.B., Raya A., Wahl G.M., Belmonte J.C.I. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. (2009);460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerr C., Shamblott M., Gearhart J. Pluripotent stem cells from germ cells. Methods Enzymol. (2006);419:400–426. doi: 10.1016/S0076-6879(06)19016-3. [DOI] [PubMed] [Google Scholar]
- 45.Kim H.J., Lee H.J., Lim J.J., Kwak K.H., Kim J.S., Kim J.H., Han Y.-M., Kim K.-S., Lee D.R. Identification of an intermediate state as spermatogonial stem cells reprogram to multipotent cells. Mol. Cells. (2010);29:519–526. doi: 10.1007/s10059-010-0064-5. [DOI] [PubMed] [Google Scholar]
- 46.Kimble J., Crittenden S.L. Germline proliferation and its control. WormBook. (2005):1–14. doi: 10.1895/wormbook.1.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ko K., Tapia N., Wu G., Kim J.B., Bravo M.J.A., Sasse P., Glaser T., Ruau D., Han D.W., Greber B. Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell. (2009);5:87–96. doi: 10.1016/j.stem.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 48.Ko K., Araúzo-Bravo M.J., Tapia N., Kim J., Lin Q., Bernemann C., Han D.W., Gentile L., Reinhardt P., Greber B., et al. Human adult germline stem cells in question. Nature. (2010);465:E1–E1. doi: 10.1038/nature09089. [DOI] [PubMed] [Google Scholar]
- 49.Kristensen D., Sonne S., Ottesen A., Perrett R., Nielsen J., Almstrup K., Skakkebaek N., Leffers H., Meyts E. Origin of pluripotent germ cell tumours: the role of microenvironment during embryonic development. Mol. Cell. Endocrinol. (2008);288:111–118. doi: 10.1016/j.mce.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Kubota H., Brinster R.L. Technology Insight: in vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat. Clin. Pract. Endocrinol. Metab. (2006);2:99–108. doi: 10.1038/ncpendmet0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kubota H., Avarbock M.R., Brinster R.L. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc. Natl. Acad. Sci. USA. (2003);100:6487–6492. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubota H., Avarbock M.R., Brinster R.L. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA. (2004);101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuijk E.W., Colenbrander B., Roelen B.A.J. The effects of growth factors on in vitro-cultured porcine testicular cells. Reproduction. (2009);138:721–731. doi: 10.1530/REP-09-0138. [DOI] [PubMed] [Google Scholar]
- 54.Kuijk E.W., van Mil A., Brinkhof B., Penning L.C., Colenbrander B., Roelen B.A.J. PTEN and TRP53 independently suppress nanog expression in spermatogonial stem cells. Stem Cells Dev. (2010);19:979–988. doi: 10.1089/scd.2009.0276. [DOI] [PubMed] [Google Scholar]
- 55.Lee J., Kanatsu-Shinohara M., Inoue K., Ogonuki N., Miki H., Toyokuni S., Kimura T., Nakano T., Ogura A., Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. (2007);134:1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- 56.Li W., Ding S. Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol. Sci. (2010);31:36–45. doi: 10.1016/j.tips.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Li H., Collado M., Villasante A., Strati K., Ortega S., Cañamero M., Blasco M.A., Serrano M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. (2009);460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim D.H., Maher E.R. Genomic imprinting syndromes and cancer. Adv. Genet. (2010);70:145–175. doi: 10.1016/B978-0-12-380866-0.60006-X. [DOI] [PubMed] [Google Scholar]
- 59.Lim J.J., Sung S.Y., Kim H.J., Song S.H., Hong J.Y., Yoon T.K., Kim J.K., Kim K.S., Lee D.R. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif. (2010);43:405–417. doi: 10.1111/j.1365-2184.2010.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maki C.B., Pacchiarotti J., Ramos T., Pascual M., Pham J., Kinjo J., Anorve S., Izadyar F. Phenotypic and molecular characterization of spermatogonial stem cells in adult primate testes. Hum. Reprod. (2009);24:1480–1491. doi: 10.1093/humrep/dep033. [DOI] [PubMed] [Google Scholar]
- 61.Marión R.M., Strati K., Li H., Murga M., Blanco R., Ortega S., Fernandez-Capetillo O., Serrano M., Blasco M.A. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. (2009);460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLean D.J. Characterization of spermatogonial stem cell maturation and differentiation in neonatal mice. Biol. Reprod. (2003);69:2085–2091. doi: 10.1095/biolreprod.103.017020. [DOI] [PubMed] [Google Scholar]
- 63.Meng X., Lindahl M., Hyvonen M.E., Parvinen M., de Rooij D.G., Hess M.W., Raatikainen-Ahokas A., Sainio K., Rauvala H., Lakso M., et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. (2000);287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 64.Morimoto H., Kanatsu-Shinohara M., Takashima S., Chuma S., Nakatsuji N., Takehashi M., Shinohara T. Phenotypic plasticity of mouse spermatogonial stem cells. PLoS ONE. (2009);4:e7909. doi: 10.1371/journal.pone.0007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakagawa T., Sharma M., Nabeshima Y.i., Braun R.E., Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. (2010);328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naughton C.K., Jain S., Strickland A.M., Gupta A., Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol. Reprod. (2006);74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- 67.Nielsen H., Nielsen M., Skakkebaek N.E. The fine structure of possible carcinoma-in-situ in the seminiferous tubules in the testis of four infertile men. Acta Pathol. Microbiol. Scand. A. (1974);82:235–248. doi: 10.1111/j.1699-0463.1974.tb03848.x. [DOI] [PubMed] [Google Scholar]
- 68.Oatley J.M., Brinster R.L. Regulation of spermatogonial stem cell self-renewal in mammals. Ann. Rev. Cell Dev. Biol. (2008);24:263–286. doi: 10.1146/annurev.cellbio.24.110707.175355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oatley J.M., Reeves J.J., McLean D.J. Biological activity of cryopreserved bovine spermatogonial stem cells during in vitro culture. Biol. Reprod. (2004);71:942–947. doi: 10.1095/biolreprod.104.028894. [DOI] [PubMed] [Google Scholar]
- 70.Oatley J.M., Avarbock M.R., Telaranta A.I., Fearon D.T., Brinster R.L. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc. Natl. Acad. Sci. USA. (2006);103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oatley J.M., Avarbock M.R., Brinster R.L. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J. Biol. Chem. (2007);282:25842–25851. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oatley J.M., Kaucher A.V., Avarbock M.R., Brinster R.L. Regulation of mouse spermatogonial stem cell differentiation by STAT3 signaling. Biol. Reprod. (2010);83:427–433. doi: 10.1095/biolreprod.109.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruiz S., Panopoulos A.D., Herrerías A., Bissig K.-D., Lutz M., Berggren W.T., Verma I.M., Izpisua Belmonte J.C. A high proliferation rate is required for cell reprogramming and maintenance of human embryonic stem cell identity. Curr. Biol. (2011);21:45–52. doi: 10.1016/j.cub.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ryu B.-Y., Orwig K.E., Kubota H., Avarbock M.R., Brinster R.L. Phenotypic and functional characteristics of spermatogonial stem cells in rats. Dev. Biol. (2004);274:158–170. doi: 10.1016/j.ydbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Sada A., Suzuki A., Suzuki H., Saga Y. The RNAbinding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. (2009);325:1394–1398. doi: 10.1126/science.1172645. [DOI] [PubMed] [Google Scholar]
- 76.Sadri-Ardekani H., Mizrak S.C., van Daalen S.K., Korver C.M., Roepers-Gajadien H.L., Koruji M., Hovingh S., de Reijke T.M., de la Rosette J.J., van der Veen F., et al. Propagation of human spermatogonial stem cells in vitro. JAMA. (2009);302:2127–2134. doi: 10.1001/jama.2009.1689. [DOI] [PubMed] [Google Scholar]
- 77.Sariola H., Saarma M. Novel functions and signalling pathways for GDNF. J. Cell Sci. (2003);116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 78.Seandel M., James D., Shmelkov S.V., Falciatori I., Kim J., Chavala S., Scherr D.S., Zhang F., Torres R., Gale N.W., et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. (2007);449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shinohara T., Avarbock M.R., Brinster R.L. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA. (1999);96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Skotheim R.I. Differentiation of human embryonal carcinomas in vitro and in vivo reveals expression profiles relevant to normal development. Cancer Res. (2005);65:5588–5598. doi: 10.1158/0008-5472.CAN-05-0153. [DOI] [PubMed] [Google Scholar]
- 81.Solter D. From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nat. Rev. Genet. (2006);7:319–327. doi: 10.1038/nrg1827. [DOI] [PubMed] [Google Scholar]
- 82.Song R., Ro S., Michaels J.D., Park C., McCarrey J.R., Yan W. Many X-linked microRNAs escape meiotic sex chromosome inactivation. Nat. Genet. (2009);41:488–493. doi: 10.1038/ng.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spassov D., Jurecic R. The PUF family of RNA-binding proteins: does evolutionarily conserved structure equal conserved function? IUBMB Life. (2003);55:359–366. doi: 10.1080/15216540310001603093. [DOI] [PubMed] [Google Scholar]
- 84.Takehashi M., Kanatsu-Shinohara M., Shinohara T. Generation of genetically modified animals using spermatogonial stem cells. Dev. Growth Differ. (2010);52:303–310. doi: 10.1111/j.1440-169X.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 85.Tay Y., Zhang J., Thomson A.M., Lim B., Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. (2008);455:1124–1128. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 86.Tegelenbosch R.A., de Rooij D.G. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat. Res. (1993);290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 87.Telfer E.E., Gosden R.G., Byskov A.G., Spears N., Albertini D., Andersen C.Y., Anderson R., Braw-Tal R., Clarke H., Gougeon A. On regenerating the ovary and generating controversy. Cell. (2005);122:821–822. doi: 10.1016/j.cell.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 88.Tesar P.J., Chenoweth J.G., Brook F.A., Davies T.J., Evans E.P., Mack D.L., Gardner R.L., McKay R.D.G. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. (2007);448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 89.Toyooka Y., Shimosato D., Murakami K., Takahashi K., Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. (2008);135:909–918. doi: 10.1242/dev.017400. [DOI] [PubMed] [Google Scholar]
- 90.Utikal J., Polo J.M., Stadtfeld M., Maherali N., Kulalert W., Walsh R.M., Khalil A., Rheinwald J.G., Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. (2009);460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Voorhoeve P.M., le Sage C., Schrier M., Gillis A.J.M., Stoop H., Nagel R., Liu Y.-P., van Duijse J., Drost J., Griekspoor A. A genetic screen implicates miRNA-372 and miRNA-373 As oncogenes in testicular germ cell tumors. Cell. (2006);124:1169–1181. doi: 10.1016/j.cell.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 92.West J.A., Viswanathan S.R., Yabuuchi A., Cunniff K., Takeuchi A., Park I.-H., Sero J.E., Zhu H., Perez-Atayde A., Frazier A.L., et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature. (2009);460:909–913. doi: 10.1038/nature08210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamaguchi S., Kimura H., Tada M., Nakatsuji N., Tada T. Expression in mouse germ cell development. Gene Expr. Patterns. (2005);5:639–646. doi: 10.1016/j.modgep.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 94.Ying Q.-L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. (2008);453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoshida S., Sukeno M., Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. (2007);317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 96.Zheng K., Wu X., Kaestner K.H., Wang P. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev. Biol. (2009);9:38. doi: 10.1186/1471-213X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zou K., Yuan Z., Yang Z., Luo H., Sun K., Zhou L., Xiang J., Shi L., Yu Q., Zhang Y., et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. (2009);11:631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 98.Zovoilis A., Nolte J., Drusenheimer N., Zechner U., Hada H., Guan K., Hasenfuss G., Nayernia K., Engel W. Multipotent adult germline stem cells and embryonic stem cells have similar microRNA profiles. Mol. Hum. Reprod. (2008);14:521–529. doi: 10.1093/molehr/gan044. [DOI] [PubMed] [Google Scholar]


