Abstract
In plants, cell-to-cell communication is pivotal for the orchestration of cell fate determination, organ development, and the integration of whole plant physiology. One of the strategies for intercellular communication uses symplasmic communication channels, called plasmodesmata (PD). These PD establish unique cytoplasmic channels for the intercellular exchange not only of metabolites and small signaling molecules, but also of regulatory proteins and RNAs to allow for local orchestration of development and physiology. A number of non-cell-autonomous transcription factors (NCATFs) have been shown to function in the coordination of specific regulatory networks. To further explore the potential of such NCATFs, a genome-wide screen was performed on the transcription factor (TF) families in Arabidopsis. We here report that, among the 76 TFs examined, 22 were shown to move beyond their sites of transcription in the root apex; these NCATFs belonged to 17 TF families, including homeobox, GRAS, and MYB. Expression studies performed on variously-sized mCherry constructs identified a range of PD size exclusion limits within tissues of the root. In addition, our studies showed that actual protein level was an important factor controlling the range of TF intercellular movement. Interestingly, our studies on CAPRICE movement revealed tissue-specificity with respect to the mode of intercellular trafficking. These findings are discussed with respect to the regulation between cell-autonomous or non-cell-autonomous action.
Keywords: cell-to-cell communication, intercellular protein trafficking, non-cell-autonomous transcription factor (NCATF), plasmodesmata (PD)
INTRODUCTION
Transcription factors (TFs) are proteins that contain a DNA-binding domain(s) that recognizes specific sequences, thus controlling gene expression, a first step for the flow of genetic information from DNA to mRNA (Karin, 1990; Latchman, 1997; Mitchell and Tjian, 1989; Ptashne and Gann, 1997). Thus, transcriptional regulatory networks controlled by TFs play pivotal roles in regulating cellular and biological activities. Activity of a TF in a certain cell/tissue, at a specific developmental stage, can be regulated at the transcriptional, post-transcriptional, translational and post-translational levels. Some important post-transcriptional regulatory mechanisms include splicing, nucleocytoplasmic mRNA export and miRNA- or nonsense-mediated RNA degradation (Conti and Izaurralde, 2005; Dhandapani et al., 2011; Hyun et al., 2011; Kidner and Martienssen, 2005; Sommer and Nehrbass, 2005; 2006; Wienholds and Plasterk, 2005). Once an mRNA expression pattern is established, regulation of protein translation (Mignone et al., 2002), cell-to-cell trafficking (Gallagher and Benfey, 2005; Kim, 2005), and nuclear localization (Schuller and Ruis, 2002) can control the protein level at the target site(s).
In plants, intercellular and long-distance communication are essential processes for the coordination of plant development and physiology (Chen and Kim, 2006; Lough and Lucas, 2006). Plants have evolved various sophisticated strategies for intercellular communication. Symplasmic intercellular trafficking of small signaling molecules, regulatory proteins and RNAs through plasmodesmata (PD) represents an important mechanism to allow for the non-cell-autonomous control of plant development (Cilia and Jackson, 2004; Gallagher and Benfey, 2005; Lucas and Lee, 2004; Lucas et al., 1993; 2009; Maule, 2008; Oparka, 2004; Zambryski and Crawford, 2000).
Non-cell-autonomous transcription factors (NCATFs) can play an important role as intercellular messengers that coordinate specific cellular activities. For example, the maize homeobox transcription factor, KNOTTED1 (KN1), traffics cell to cell and acts non-cell-autonomously to control cell fate within the maize vegetative meristem (Freeling and Hake, 1985; Jackson et al., 1994; Kim et al., 2002; 2003; 2005; Lucas et al., 1995; Vollbrecht et al., 1991). Furthermore, molecular studies performed on KN1 and its Arabidopsis homologs KNAT1/BP and STM established that the intercellular trafficking machinery required for cell-to-cell movement is conserved between maize (monocots) and the eudicots (Kim et al., 2002; 2003; 2005; Lucas et al., 1995; Rim et al., 2009; Xu et al., 2011). Trafficking of KNAT1/BP proteins from their site of expression into neighboring tissues was shown to be essential to maintain epidermal differentiation that is required for normal plant architecture (Rim et al., 2009).
Other well-characterized NCATFs include the floral homeotic proteins, LEAFY (Sessions et al., 2000; Wu et al., 2003), AGAMOUS (Urbanus et al., 2010), DEFICIENS and GLOBOSA (Perbal et al., 1996), as well as TFs involved in root development, such as the Arabidopsis GRAS family member, SHORT ROOT (SHR) (Nakajima et al., 2001), CAPRICE (CPC), a Myb-like DNA-binding domain protein (Kurata et al., 2005; Wada et al., 2002), basic helix-loop-helix proteins GLABRA 3 (GL3) and ENHANCED GLABRA 3 (EGL3) (Bernhardt et al., 2005).
In a study conducted on Arabidopsis roots, Lee et al. (2006) reported that among 24 TFs tested, 6 showed protein domains that extended beyond their sites of transcription. The Arabidopsis genome contains some 2000 TFs (1922 loci, 2290 gene models) that have been classified into 64 families based on DNA binding domain homologies (Guo et al., 2005). Given the extent and diversity of these Arabidopsis TFs, the question arises as to whether individual families of TFs evolved the capacity to function non-cell-autonomously, or whether specific members within each family gained (or lost) this ability.
To address this question, we performed a genome-wide screen to identify NCATFs using the GAL4-UAS trans-activation expression system, in combination with dual fluorescent reporter proteins, GFP and mCherry (RFP). In the current study, transgenic lines expressing more than 270 TFs were developed to screen for NCATFs; 76 transgenic lines exhibited detectable dual fluorescent signals and were used for further analysis. Based on these studies, 22 TFs, belonging to 17 TF families, were classified as NCATFs and sorted into three groups, depending on the patterns of red fluorescence distribution within roots. Thus, approx. 29% of the tested TFs have the capacity to move beyond the cells in which they were transcribed. In addition, our studies indicated that protein size, subcellular localization and relative protein level are important factors controlling intercellular movement of TFs.
MATERIALS AND METHODS
Plant material, culture conditions and plant transformation
The J0571 enhancer trap plant line (GAL4/UAS::GFPer, Arabidopsis thaliana ecotype C24) was used in all experiments (Birnbaum et al., 2003). Arabidopsis thaliana transcription factor fusion gateway constructs were introduced into Agrobacterium tumefaciens GV3101, and transformed into the J0571 enhancer trap line using the floral dip technique (Clough and Bent, 1998). Seeds were surface-sterilized for 5 min in a 20% (v/v) bleach solution and then placed on Petri dishes with 1x Murashige and Skoog (MS) medium, pH 5.7, and 0.6% (w/v) plant agar. Plates were kept at 4℃ for 5 days in the dark and then moved to a growth chamber where they were grown under long-day conditions (16:8 light:dark regime) at 20-24℃ under fluorescent lighting and 55-65% relative humidity. Transgenic T1 seedlings were screened on MS medium containing 50 μg/ml hygromycin B. Inserted TFs were identified by sequencing after PCR amplification using mCherry-mid-r primer (Bioneer, Supplementary Table S1).
Gateway library construction and isolation of independent clones
In order to express TF-mCherry fusions in the J0571 line, a basic gateway destination vector, pCCL293 carrying UAS::GW-mCherry, was used (Rim et al., 2009). An entry clone library, including some 1,500 TFs, was employed to produce a UAS:: TF-mCherry destination vector library that was then used to transform Escherichia coli DH10B. Plasmids isolated from the E. coli library were transformed into Agrobacterium GV3101. Amplified DNA restriction analysis (ADRA) was used to obtain independent TF Agrobacterium clones. PCR products, amplified using attB1/attB2 primers, were digested using HaeIII and HhaI and digestion patterns were compared after agarose-gel electrophoresis.
Plasmid cloning
Construction of the UAS::mCherry (pCCL276) was as previously reported (Rim et al., 2009). UAS::mCherry-NLS (pRYG239) was produced by self-ligation after BsrGI digestion of pRYG240. UAS::mCherry-H2B (pCCL284) was produced by insertion of H2B ORF into the AvrII/KpnI sites of pCCL276. UAS::2emCherry (pRYG237) was constructed by insertion of an mCherry fragment, produced by PCR, into the AvrII/SacI sites of pCCL276 (UAS::mCherry). UAS::2xmCherry-NLS (pRYG240) was made by insertion of an mCherry ORF fragment into the AvrII/KpnI sites of pCCL284. UAS::GW-mCherry-H2B (pCCL306) was produced by insertion of the Gateway recombinant XhoI fragment from pEarlygate103 at the SalI site of pCCL284. UAS:: 2xmCherry-H2B (pRYG243) was constructed by LR reaction between pCCL306 and pEntry-mCherry. UAS::CPC-mCherry (pCCL281) was produced by insertion of the CPC ORF at the SalI site of pCCL276. UAS::CPCM78A-mCherry (pCCL282) was produced by site-specific mutation using the appropriate primers (Supplementary Table S1). All primers used for plasmid constructions are listed in Supplementary Table S1.
Confocal laser scanning microscopy
Fluorescent images of GFP and mCherry were obtained using a FluoView FV1000 confocal microscope (Olympus, Japan). Excitation and emission spectra in the GFP channel were at 488 nm and 510-540 nm, respectively, and those for mCherry and propidium iodide (PI) were at 543 nm and 587-625 nm, respectively. Seven- to ten-day-old seedlings of the various transgenic plant lines were used for imaging.
Software and web resources
Classification of Arabidopsis TFs families was performed using the Database of Arabidopsis Transcription Factors (DATF) (http://datf.cbi.pku.edu.cn/). In silico expression analysis was performed using the Bio-Array Resource for Arabidopsis Functional Genomics (BAR, http://142.150.214.117).
RESULTS
Genome-wide screening for non-cell-autonomous transcription factors
To explore the extent to which Arabidopsis TFs have the potential for cell-to-cell movement, we conducted a large-scale screen involving TFs selected to cover the majority of families in the Arabidopsis genome. Open reading frames (ORFs) of TF cDNAs were cloned, in-frame, upstream of mCherry and these fusions were then expressed under the control of the Upstream Activation Sequence (UAS) of the GAL4 TF in a background of the Haseloff J0571 enhancer trap Arabidopsis line (Birnbaum et al., 2003; http://www.arexdb.org/db). The J0571 line shows trans-activation of ER-localized GFP (GFPer) by a chimeric GAL4/VP16 TF under the control of enhancer(s) that drive expression in stomata and mesophyll cells in leaves (Supplementary Fig. S1) and cortical and endodermal cells in roots (Fig. 1, left panels). Thus, green fluorescent cells indicate the cellular origin of TF-mCherry expression. Any TF-mCherry fusion that moved beyond the cortical and endodermal cells was defined as a NCATF in this experimental system.
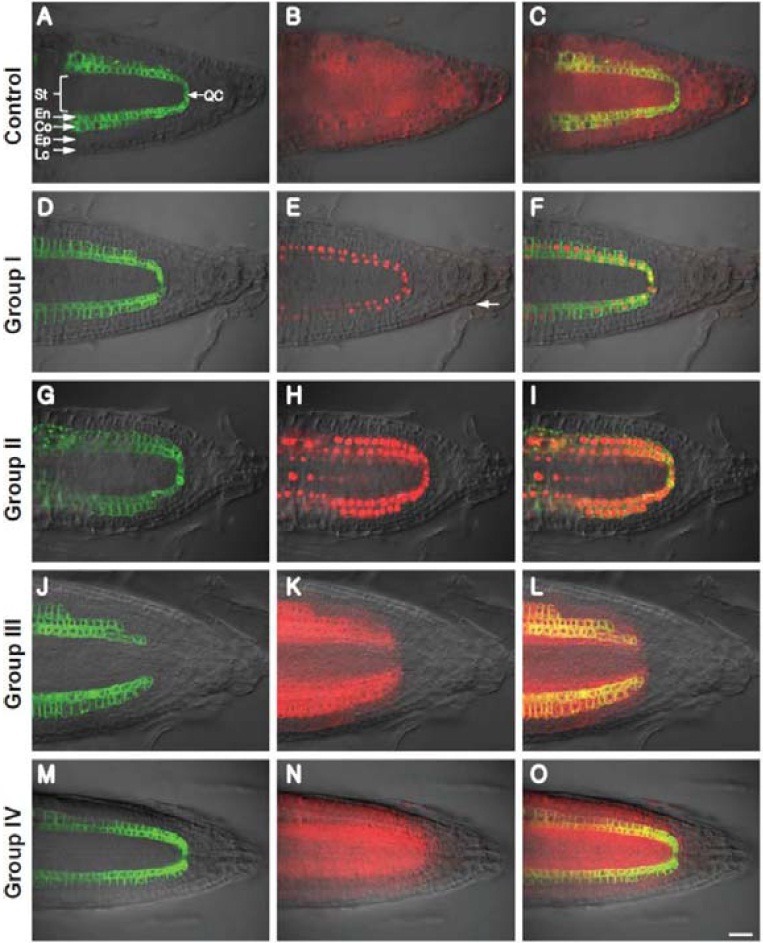
Fig. 1. Three cellular patterns observed for TFmCherry movement in Arabidopsis root tissues. (A-C) Fluorescent pattern observed when mCherry was expressed in cortical/endodermal cells. Note that in this control experiment, mCherry was able to move throughout all root tissues (Rim et al., 2009). (D-F) Group I TFs were classified as cell-autonomous since the cellular patterns of mCherry and GFPer signals were identical. (G-I) Group II TFs showed multi-celllayered movement into the stele, but movement outward into the epidermal or lateral root cap cells was not observed. (J-L) Group III TFs were detected in the entire root tip except for the distal root cap cells. (MO) Group IV TFs were detected in the entire root tip, including the lateral and distal root cap cells. Co, cortex; En, endodermis; Ep, epidermis; Lc, lateral cap cell; QC, quiescent center; St, stele. Bar in (O) is common to all images and equals 20 μm.
Over 1500 TF ORFs, in Gateway pEntry plasmids, were used to generate a plant expression library (UAS::TF-mCherry); more than 800 independent Agrobacterium clones, sorted by ADRA, were used for transformation of the Arabidopsis J0571 line. UAS::mCherry was used as a control in these experiments. At least 10 independent transgenic T1 plants for over 270 independent clones were examined for TF-mCherry expression in the root tip. Transgenic lines originating from 76 TF clones showed measurable level of mCherry fluorescence and were further evaluated in the current study. This reduction in number of TFs from 270 to 76 likely reflects the nature of these proteins in terms of their overall level and stability.
First, we examined whether the mCherry (27 kDa) reporter could move out from the cortical/endodermal layers in the root tip. The J0571 line carrying a UAS::mCherry reporter construct showed GFPer expression only in the cortical and endodermal layers, but red fluorescent signal throughout the entire root tip, including the epidermis, stele and root cap (Figs. 1A-1C; Rim et al., 2009). Thus, the free form of mCherry could move from its sites of synthesis (Fig. 1A) into all tissue types throughout the root tip. This finding suggested that the PD interconnecting the majority of root cells have size exclusion limits (SELs) that are greater than 27 kDa.
Based on this root screening system, TFs were classified into four groups depending on the observed TF-mCherry fluorescent patterns. Group I represented cell-autonomous TFs, as they had red fluorescent signal confined to the same cells as that for GFPer. A representative image (AT2G02820) and list of group I TFs is shown in Figs. 1D-1F, Supplementary Fig. S2 and Table S2, respectively. Groups II-IV contained 22 NCATFs belonging to 17 TF families (Table 1), including ERF/AP2, DOF zinger finger, homeobox, Alfin-like, MADS-box, ARF, CCCHtype zinc finger, MBF1, GATA, TCP, zinc finger-HD, GRAS, AS2, myc/bHLH, MYB, bZIP, and MYB-related. The theoretical molecular weights for these NCATFs range from 11 to 49.
Table 1.
Non-cell-autonomous transcription factors identified in this study.
| Locus (AGI ID) | Alias (Gene name) | Family | MW (kDa)a | Group |
|---|---|---|---|---|
| AT1G62360 | STM, SHL, BUM1 | HB | 42.8 | II |
| AT5G63260 | MDC12.23 | C3H | 48.6 | II |
| AT1G51600 | ZML2, TIFY2A, | ZIM | 33.1 | II |
| AT5G41920 | SCL23 | GRAS | 44.9 | II |
| AT1G31320 | LBD4 | AS2 | 18.7 | II |
| AT5G67110 | ALC, bHLH073 | bHLH | 23.0 | II |
| AT5G26210 | AL4 | Alfin | 28.8 | II |
| AT5G65670 | IAA9 | AUX-IAA | 36.4 | II |
| AT1G16060 | ADAP | AP2-EREBP | 39.2 | III |
| AT4G00940 | AtDof4,1 | C2C2-Dof | 33.4 | III |
| AT4G35550 | HB-4, WOX13 | HB | 29.7 | III |
| AT5G65050 | AGL31, MAF2 | MADS | 22.1 | III |
| AT1G08970 | HAP5C, NF-YC9 | CCAAT-HAP5 | 25.6 | III |
| AT5G65410 | AtHB25, ZFHD2 | ZF-HD | 31.1 | III |
| AT5G65060 | FCL3, MAF3, AGL70 | MADS | 22.1 | III |
| AT1G51700 | AtDof1. 7, ADOF1 | C2C2-Dof | 21.7 | III |
| AT2G36450 | HRD | AP2-EREBP | 19.8 | III |
| AT1G58100 | TCP8 | TCP | 42.5 | III |
| AT3G62420 | AtbZIP53 | bZIP | 16.8 | III |
| AT2G46410 | CPC | MYB-related | 11.4 | IV |
| AT1G22810 | T22J18.2 | AP2-EREBP | 15.8 | IV |
| AT2G42680 | MBF1A | MBF1 | 15.6 | IV |
aMW, calculated theoretical molecular weight
The group II TFs displayed red fluorescence in the inner stellar cells as well as signal in the cortex and endodermis (ground tissues). However, red fluorescence was not detected in either the epidermis or the root cap, including the lateral cap cells (Figs. 1G-1I, Supplementary Fig. S3). Group II TFs included STM, AL4, IAA9, MSC12.23, ZML2, SCL23, LBD4, and ALC. Group III TFs exhibited red fluorescent signal on both sides of the ground tissue, including the epidermis and stele. In these cases, the signal showed a sharp gradient between lateral root cap cells and the epidermis; but, at a low frequency, a weak signal could be detected within some lateral root cap cells. However, red fluorescence was not detected in the lower root cap cells; in some cases, signal was observed in the columellar initials located immediately beneath the cells of the quiescent center (QC) (Figs. 1J-1L, Supplementary Fig. S3). Group III TFs included ADAP, AtDof1.7, HRD, AtDof4.1, WOX13, AGL31, HAP5C, TCP8, AtHB25, AtbZIP53, and AGL70. Group IV TFs behaved like free cytosolic mCherry, with red fluorescent signal being detected throughout the entire region of the root tip (Figs. 1M-1O, Supplementary Fig. S3). Group IV TFs included T22J18.2, MBF1A and CPC that have relatively low molecular mass (11- 16 kDa).
Protein size and subcellular localization affect intercellular movement
Within the list of identified NCATFs were STM, CPC and SCL23 that were earlier suggested to move through PD by a selective trafficking pathway (Gallagher and Benfey, 2009; Kim et al., 2005; Kurata et al., 2005). However, other NCATFs, like LFY have been suggested to move cell to cell via a diffusionbased non-selective mode in which the NCATF does not need a direct interaction with PD components (Wu et al., 2003). In this regard, mCherry is an example of non-selective/diffusionbased movement. The size of the non-cell-autonomous protein and its subcellular localization were proposed as important factors controlling such diffusion-based cell-to-cell protein movement (Crawford and Zambryski, 2000).
To ascertain the extent to which the identified NCATFs might move via the diffusion-based pathway, we next employed various mCherry fusions carrying different molecular masses, a nuclear localization sequence and combinations therein. In contrast to mCherry which could move throughout all tissues of the root (Figs. 2A-2C), 2xmCherry behaved as Group III NCATFs, in that red fluorescent signal was detected in epidermal and stellar cells (Figs. 2D-2F). However, signal intensity was much higher in the stele compared to the epidermis, consistent with TF-mCherry patterns (compare group II and group III TFs; Figs. 1G-1I and Figs. 1J-1L). The absence of 2xmCherry from the distal root cap likely reflects a PD imposed limit on the diffusion of this reporter.
Fig. 2. Protein size and subcellular localization affect intercellular movement. Capacity of mCherry to move symplasmically within the root apex was probed using differently-sized reporters, with or without a nuclear localization signal (NLS). (A-C) mCherry, (D-F) 2x mCherry, (G-I) 2xmCherry-NLS, (J-L) mCherry-NLS, (M-O) mCherry- H2B, (P-R) 2x mCherry-H2B. Co, cortex; En, endodermis; Ep, epidermis; Lc, lateral cap cells; St, stele. Bar in (R) is common to all images and equals 20 μm.
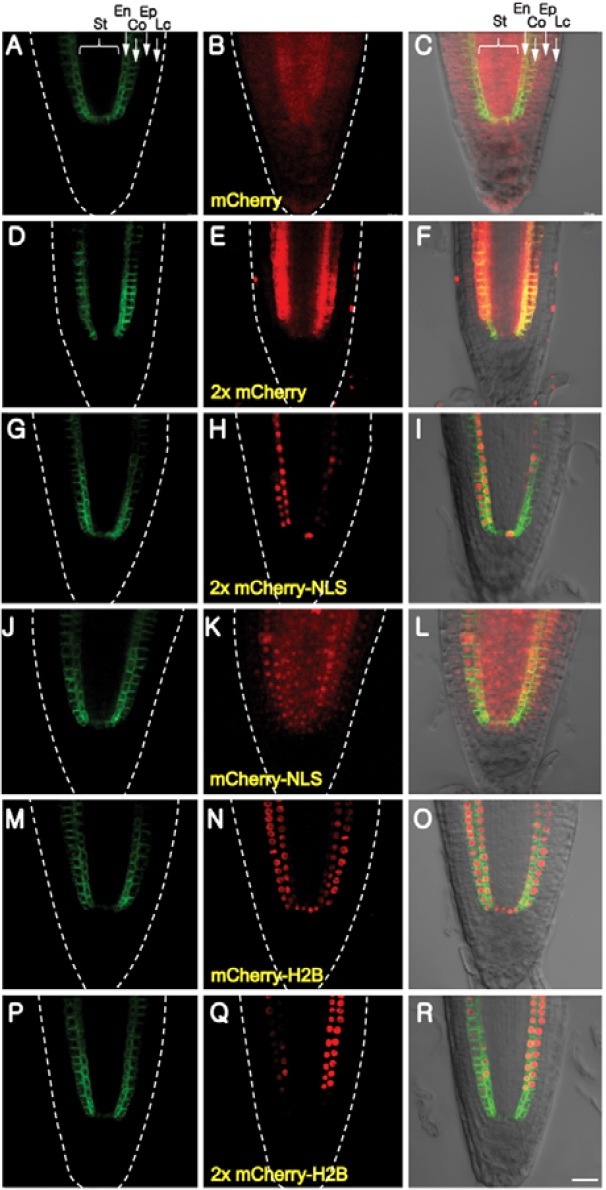
The influence of sub-cellular compartmentalization on mCherry movement was next investigated using reporters carrying a nuclear localization signal (NLS). The cellular distribution of mCherry-NLS was affected only in terms of movement into the distal root cap cells (Figs. 2J-2L). In contrast, the 2xmCherry-NLS displayed a pattern similar to that of group I NCATFs, except that weak signal could still be detected in the pericycle (Figs. 2G-2I). The role of protein anchoring was next explored using an H2B fusion protein; this would result in sequestration of mCherry within nuclei. Interestingly, both mCherry-H2B and 2xmCherry-H2B displayed fluorescent patterns equivalent to the group I TFs (Figs. 2M-2R); i.e, strong binding to H2B reduced the level of mCherry within the cytoplasm to such an extent that movement was prevented (compare Figs. 2L and 2O).
Identified NCATFs are expressed in root tissues
The Arabidopsis J0571 line used in the current study drives expression of the GFPer reporter in cortical and endodermal cells of the root. Ectopic expression of a TF might result in artificial intercellular movement of the protein because of a lack of endogenous tethering agents, such as protein-protein interaction, or incorrect subcellular localization. To strengthen the biological significance of the NCATFs identified in our screen, we next examined whether they were expressed in root tissues, using available expression data (Supplementary Fig. S5 and Table S3).
As STM, a class I KNOTTED-like protein, is expressed specifically in the shoot apical meristem (Long et al., 1996), we reasoned that the expression value measured for STM in the root could be used as a base line for gene expression in this tissue (Supplementary Table S3). Only AGL31 showed lower values than those for STM in the endodermis+cortex. Expression patterns and intensities for the other identified NCATFs were variable, but they all had higher levels of expression relative to STM. We noted that, in particular, T22J18.2, AtDof4.1 and SCL23 showed the highest expression levels in endodermal and cortical cells compared to the neighboring tissues of the epidermis and stele, which was recapitulated by our J0571 expression system. Based on these comparative studies, we concluded that the NCATFs identified using our current screening system most likely represent authentic movement capacity within the context of their endogenous transcriptional networks.
Protein level as a factor controlling extent of intercellular movement
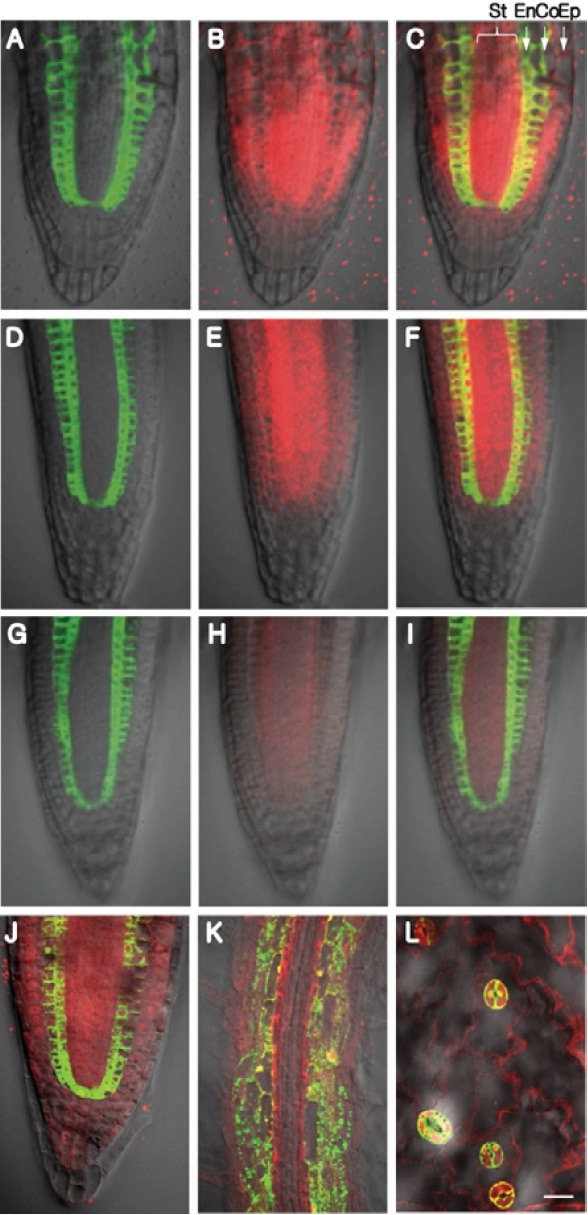
The J0571 is a stable enhancer trap line that drives strong expression of reporters; however, its retransformation with UAS:: CPC-mCherry produced transgenic lines having a range of different fluorescent signal strengths. Careful inspection of the pattern of red fluorescent signal in such transgenic lines established a correlation between expression level and extent of movement. For example, transgenic lines showing strong levels of CPC-mCherry expression had red fluorescent signal in all tissues, including root cap cells (Figs. 3A-3C), whereas lines with lower levels of expression had little or no detectable signal in the root cap cells (Figs. 3D-3I). Interestingly, under low levels of CPC-mCherry expression, movement occurred preferentially into the stele (Figs. 3G-3I). These results clearly established that the protein level can act as an important factor in determining both the range and preferred direction of NCATF movement.
Fig. 3. Protein expression level acts as an important factor in determining the extent of intercellular NCATF movement. (A-C) High level of CPC-mCherry expression in the cortex/endodermis was correlated with extensive movement into epidermal and stellar tissues, with less movement into the distal root cap. (D-F) Intermediate level of CPC-mCherry expression gave an equivalent pattern, except that CPC-mCherry was absent from cells of the distal root cap. (G-I) Low level of CPC-mCherry expression appeared to favor movement into the stele. (J-L) CPCM78A-mCherry, a mutant that cannot move between epidermal cells, was observed to move throughout the root apex (J), mature zone of the root (K) and young leaves (L). Co, cortex; En, endodermis; Ep, epidermis; St, stele. Bar in (L) is common to all images and equals 20 μm.
Tissue specificity in protein intercellular movement
Earlier studies demonstrated that CPC traffics in a tissue-specific manner, in that CPC-GFP was shown to move between epidermal cells; it can move from atrichoblasts to trichoblasts and vice versa. However, when expressed in cells of the stele, using the SHR promoter, CPC-GFP was unable to traffic into endodermal/cortical cells (Kurata et al., 2005). This suggested a selectivity of CPC movement between different cell layers as well as a directional movement in the same endodermal and pericycle cell boundary. Furthermore, a selective mode for CPC movement between epidermal cells was supported by the fact that CPC carrying a specific mutation (CPCM78A) retained its function but was defective in cell-to-cell movement (Kurata et al., 2005). To test whether CPCM78A was also dysfunctional in movement from endodermal/cortical cells, UAS::CPCM78A-mCherry was introduced into J0571 plants. Surprisingly, we detected extensive CPCM78A-mCherry movement throughout the root tip, mature root and between leaf epidermal cells (Figs. 3J-3L). This finding suggests that strong expression of CPCM78A-mCherry may have overcome its defect in terms of interacting with the PD machinery required for its cell-to-cell trafficking. Alternatively, CPCM78A-mCherry might move cell to cell by diffusion when expressed in the J0571 plant line (cortex, endodermis, QC). In support of this notion, we observed that CPCM78A-mCherry still moved out from its site of synthesis even when it was being expressed at relatively low levels (Supplementary Fig. S4).
DISCUSSION
World of NCATFs: Genome-wide screening of NCATFs
Using the J0571 line, in combination with the GAL4-UAS expression system allowed us to screen a population of Arabidopsis TFs to identify those capable of movement beyond their sites of synthesis. Based on our screen, some 22 among 76 TFs were found to be non-cell-autonomous in nature (Fig. 1, Supplementary Fig. S3 and Table 1). Interestingly, these data indicate that, of the 76 TFs investigated, 29% are NCATFs, a value similar to that reported by Lee et al. (2006); i.e., 6 NCATFs out of a total of 24, or 25%. It is important to note that Lee et al. (2006) employed endogenous promoters to analyze the movement potential of their 24 TFs. Although we used an enhancer trap system, our in silico expression analysis indicated that 20 of the 22 identified NCATFs are express within the endodermis and cortex (Supplementary Fig. S5 and Table S3). This indicates that these NCATFs likely function within the system employed for our screen.
A clear possibility exists that we failed to identify additional NCATFs whose movement may be regulated in a tissuespecific tissuespecific or development-specific manner. For example, some NCATFs may move only in leaf tissues or in the shoot apical meristem. Furthermore, TFs whose stability or level of protein expression may be differentially regulated within specific cell types could easily have escaped identification as NCATFs. In contrast, a number of the identified NCATFs may have moved cell to cell due to the strong promoter used in the current study, as exemplified by our CPC-mCherry results (Fig. 3).
Patterns of NCATF movement do not correlate with protein size
Our findings, using variously-sized mCherry reporters, allowed us to establish a map of PD SELs for the different tissues of the root tip (Fig. 4A). The cellular boundaries having the highest PD SEL appear to be located between the endodermal/pericycle, pericycle/inner stellar cells, and cortical/epidermal cells, where the limit for diffusion appears to be on the order of 60 kDa. Interestingly, group II-IV NCATFs were able to move across these same cellular boundaries; however, 7 out of the 22 NCATFs had molecular weights above this upper limit of the PD SEL. Hence, these 7 NCATFs most probably move using the selective cell-to-cell trafficking pathway, whereas the remaining 15 NCATFs could traffic either by diffusion or on this same selective pathway. It is also of interest to note that all 22 NCATFs have predicted molecular mass in the range of 11-49 kDa and, hence, in their endogenous settings, potentially they could all move into the epidermal and stellar cells via the diffusion pathway. Further experiments will be required to ascertain the actual mode used by each of these NCATFs.
Fig. 4. Schematic illustrating a map of plasmodesmal size exclusion limits and NCATF trafficking domains within the Arabidopsis root apex. (A) High plasmodesmal SELs (54 kDa) present between the stellar/endodermal and cortical/epidermal cellular boundaries. Based on the movement of 2x mCherry-NLS, the plasmodesmal SEL at the endodermis/ pericycle cell boundary is ~60 kDa, whereas between cells of the cortex and QC and the distal root cap cells the SEL is >27 but <54 kDa. (B) Trafficking domains established for group II-IV NCATFs. Arrows indicate the direction and thickness reflects the level of signal crossing the indicated cellular boundaries. En, endodermis; Ep, epidermis; Lc, lateral cap cell; Pe, pericycle; QC, quiescent center; St, stele.
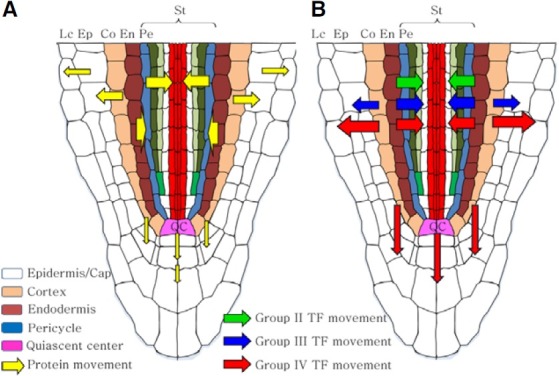
In contrast to the group III and group IV NCATFs, those in group II appeared to engage in preferential movement from the cortex/endodermis into the cells of the stele (Figs. 1G-1I). Again, these group II NCATFs varied in size from 45.7 to 75.6 kDa (TF-mCherry fusions), and 4 were above the ~60 kDa PD SEL, thus these larger NCATFs would likely move via the selective trafficking pathway. However, although the remaining members have molecular weights below this PD SEL, the most plausible explanation for their directional movement would be that they too move cell to cell via the selective trafficking pathway. Finally, it is interesting to note that the group IV NCATFs were all relatively small (11.4 to 15.8 kDa) and had the greatest range of movement (Figs. 1M-1O). These characteristics are consistent with a diffusion based mechanism of cell-to-cell movement; however, one member, CPC was earlier shown to engage in selective trafficking within the epidermal cell layer (Kurata et al., 2005). So again, the size of a NCATF cannot be used as a predictor of the mechanism underlying cell-to-cell movement.
Evolutionary aspect related to key factors controlling intercellular TF movement
Plants have evolved TFs that function cell-autonomously as well as those that have the capacity to move through PD, via either a diffusion-based, non-selective mechanism or a selective pathway. We have reasoned that, based on the steps that occurred during the process of evolving from simple algal PD (lacking a centrally-located ER) to highly complex PD, initially, all TFs were likely non-cell-autonomous in nature (Lucas et al., 2009). Although the ER insertion in the center of PD would have dramatically diminished the capacity of TFs to diffuse through this cytoplasmic sleeve, to further develop cell-auto-nomous TFs, several strategies likely were employed.
First, protein size, or more precisely the Stokes radius of proteins may have undergone an increase to block their free diffusion through PD. As with earlier studies based on GFP, increasing the molecular weight of mCherry, through the formation of fusion proteins, dramatically reduced cell-to-cell movement of these reporter proteins (Fig. 2; Crawford and Zambryski, 2000; Kim et al., 2002). Next, complex formation by proteinprotein interaction could also have resulted in a size increase, or tethering within the cytoplasm to prevent trafficking through PD.
The SCR is an example of a TF that may well have undergone the transition from a non-cell-autonomous to cell-autonomous protein. During the present study, we identified a SCL23 putative TF (group II, AT5G41920) that has the capacity to move cell to cell within the root (Supplementary Fig. S2). This 44.9 kDa protein is comprised almost entirely of the GRAS domain that is conserved between SHR and SCR. Importantly, this GRAS domain was shown to be essential for non-cell-autonomous movement (Gallagher and Benfey, 2009). Compared to either SHR or this SCL23 protein, SCR has an N-terminal extension that is reported to be involved in preventing protein movement. This block on protein trafficking could be related either to an increase in size or to the formation of a protein whose structure cannot interact with the PD machinery involved in SHR/SCL23 trafficking.
Strong protein sequestration within a cellular compartment, such as the nucleoplasm, may have been another mechanism to reduce the probability for TF cell-to-cell movement. In the current study, we clearly demonstrated that attaching an NLS to mCherry reduced the extent of intercellular movement within the root tip (Figs. 2I and 2L). Further, addition of a functional H2B drastically reduced the cell-to-cell movement of the mCherry-H2B, due to its binding to DNA (Fig. 2O).
Other mechanisms that could have contributed to cell-autonomous TF function are controlling the protein level through either promoter activity or protein turnover. In this scenario, although the TF may be able to move, its low level within neighboring cells may not be sufficient to activate gene transcription. In this regard, it is interesting to note that of the 276 TFs tested in this study, only 76 showed detectable levels of mCherry signal. This could reflect either the intrinsic instability of the majority of these TFs or a lack of the protein machinery necessary for function. Here, it is important to note that most of the 55 group I cell-autonomous TFs were found to have very low levels of TF-mCherry fluorescent signal (Supplementary Fig. S2). Further, there was no apparent correlation between the observed levels of protein and endogenous gene expression in the root. For example, AT1G01010 mRNA level was almost 10 fold higher than that of AT5G16560, but its protein level was even lower (Supplementary Fig. S2 and Table S4). These findings suggest that the proteins for at least some members among these group I TFs are being rapidly turned over. In terms of regulating the TF level, closely controlling the turnover of these proteins may be an additional important evolutionary strategy to convert NCATFs into functional cell-autonomous TFs. Finally, as the identified NCATFs were not confined to specific gene family, but rather were broadly distributed across most of families examined in this study, it would appear this capacity arose independently on many occasion, or it was the common mode of function in early land plants.
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
Acknowledgments
We thank Sunseon Kim and Munawar Ahmad for technical assistance, Roger Tsien for providing the mCherry construct and Jim Haseloff for seeds of the J0571 plant line. This work was supported by World Class University program grant R33- 10002 through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology, and a grant from the Next-Generation BioGreen 21 Program (SSAC, grant PJ008109), Rural Development Administration, Republic of Korea.
References
- 1.Bernhardt C., Zhao M., Gonzalez A., Lloyd A., Schiefelbein J. The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development. (2005);132:291–298. doi: 10.1242/dev.01565. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum K., Shasha D.E., Wang J.Y., Jung J.W., Lambert G.M., Galbraith D.W., Benfey P.N. A gene expression map of the Arabidopsis root. Science. (2003);302:1956–1960. doi: 10.1126/science.1090022. [DOI] [PubMed] [Google Scholar]
- 3.Chen X.Y., Kim J.Y. Transport of macromolecules through plasmodesmata and the phloem. Physiol. Plant. (2006);126:560–571. [Google Scholar]
- 4.Cilia M.L., Jackson D. Plasmodesmata form and function. Curr. Opin. Cell Biol. (2004);16:500–506. doi: 10.1016/j.ceb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Conti E., Izaurralde E. Nonsense-mediated mRNA decay: Molecular insights and mechanistic variations across species. Curr. Opin. Cell Biol. (2005);17:316–325. doi: 10.1016/j.ceb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Crawford K.M., Zambryski P.C. Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr. Biol. (2000);10:1032–1040. doi: 10.1016/s0960-9822(00)00657-6. [DOI] [PubMed] [Google Scholar]
- 7.Dhandapani V., Ramchiary N., Paul P., Kim J., Choi S.H., Lee J., Hur Y., Lim Y.P. Identification of potential microRNAs and their targets in Brassica rapa L. Mol. Cells. (2011);32:21–37. doi: 10.1007/s10059-011-2313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeling M., Hake S. Developmental genetics of mutants that specify knotted leaves in maize. Genetics. (1985);111:617–634. doi: 10.1093/genetics/111.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher K.L., Benfey P.N. Not just another hole in the wall: Understanding intercellular protein trafficking. Genes Dev. (2005);19:189–195. doi: 10.1101/gad.1271005. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher K.L., Benfey P.N. Both the conserved GRAS domain and nuclear localization are required for SHORT-ROOT movement. Plant J. (2009);57:785–797. doi: 10.1111/j.1365-313X.2008.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo A., He K., Liu D., Bai S., Gu X., Wei L., Luo J. DATF: A database of Arabidopsis transcription factors. Bioinformatics. (2005);21:2568–2569. doi: 10.1093/bioinformatics/bti334. [DOI] [PubMed] [Google Scholar]
- 12.Hyun T.K, Uddin M.N., Rim Y., Kim J.Y. Cell-to-cell trafficking of RNA and RNA silencing through plasmodesmata. Protoplasma. (2011);248:191–203. doi: 10.1007/s00709-010-0225-6. [DOI] [PubMed] [Google Scholar]
- 13.Jackson D., Veit B., Hake S. Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development. (1994);120:405–413. [Google Scholar]
- 14.Karin M. Too many transcription factors: positive and negative interactions. New Biol. (1990);2:126–131. [PubMed] [Google Scholar]
- 15.Kidner C.A., Martienssen R.A. The developmental role of microRNA in plants. Curr. Opin. Plant Biol. (2005);8:38–44. doi: 10.1016/j.pbi.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.Y. Regulation of short-distance transport of RNA and protein. Curr. Opin. Plant Biol. (2005);8:45–52. doi: 10.1016/j.pbi.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.Y., Yuan Z., Cilia M., Khalfan-Jagani Z., Jackson D. Intercellular trafficking of a KNOTTED1 green fluorescent protein fusion in the leaf and shoot meristem of Arabidopsis. Proc. Natl. Acad. Sci. USA. (2002);99:4103–4108. doi: 10.1073/pnas.052484099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J.Y., Yuan Z., Jackson D. Developmental regulation and significance of KNOX protein trafficking in Arabidopsis. Development. (2003);130:4351–4362. doi: 10.1242/dev.00618. [DOI] [PubMed] [Google Scholar]
- 19.Kim J.Y., Rim Y., Wang J., Jackson D. A novel cellto- cell trafficking assay indicates that the KNOX homeodomain is necessary and sufficient for intercellular protein and mRNA trafficking. Genes Dev. (2005);19:788–793. doi: 10.1101/gad.332805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurata T., Ishida T., Kawabata-Awai C., Noguchi M., Hattori S., Sano R., Nagasaka R., Tominaga R., Koshino-Kimura Y., Kato T., et al. Cell-to-cell movement of the CAPRICE protein in Arabidopsis root epidermal cell differentiation. Development. (2005);132:5387–5398. doi: 10.1242/dev.02139. [DOI] [PubMed] [Google Scholar]
- 21.Latchman D.S. Transcription factors: an overview. Int. J. Biochem. Cell Biol. (1997);29:1305–1312. doi: 10.1016/s1357-2725(97)00085-x. [DOI] [PubMed] [Google Scholar]
- 22.Lee J.Y., Colinas J., Wang J.Y., Mace D., Ohler U., Benfey P.N. Transcriptional and posttranscriptional regulation of transcription factor expression in Arabidopsis roots. Proc. Natl. Acad. Sci. USA. (2006);103:6055–6060. doi: 10.1073/pnas.0510607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long J.A., Moan E.I., Medford J.I., Barton M.K. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature. (1996);379:66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- 24.Lough T.J., Lucas W.J. Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annu. Rev. Plant Biol. (2006);57:203–232. doi: 10.1146/annurev.arplant.56.032604.144145. [DOI] [PubMed] [Google Scholar]
- 25.Lucas W.J., Lee J.Y. Plasmodesmata as a supracellular control network in plants. Nat. Rev. Mol. Cell Biol. (2004);5:712–726. doi: 10.1038/nrm1470. [DOI] [PubMed] [Google Scholar]
- 26.Lucas W.J., Ding B., van der Schoot C. Plasmodesmata and the supracellular nature of plants. New Phytol. (1993);125:435–476. doi: 10.1111/j.1469-8137.1993.tb03897.x. [DOI] [PubMed] [Google Scholar]
- 27.Lucas W.J., Bouché-Pillon S., Jackson D.P., Nguyen L., Baker L., Ding B., Hake S. Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science. (1995);270:1980–1983. doi: 10.1126/science.270.5244.1980. [DOI] [PubMed] [Google Scholar]
- 28.Lucas W.J., Ham B.K., Kim J.Y. Plasmodesmata - bridging the gap between neighboring plant cells. Trends Cell Biol. (2009);19:495–503. doi: 10.1016/j.tcb.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Maule A.J. Plasmodesmata: structure, function and biogenesis. Curr. Opin. Plant Biol. (2008);11:680–686. doi: 10.1016/j.pbi.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Mignone F., Gissi C., Liuni S., Pesole G. Untranslated regions of mRNAs. Genome Biol. (2002);3:0004.1–0004.10. doi: 10.1186/gb-2002-3-3-reviews0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell P.J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. (1989);245:371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima K., Sena G., Nawy T., Benfey P.N. Intercellular movement of the putative transcription factor SHR in root patterning. Nature. (2001);413:307–311. doi: 10.1038/35095061. [DOI] [PubMed] [Google Scholar]
- 33.Oparka K.J. Getting the message across: how do plant cells exchange macromolecular complexes? Trends Plant Sci. (2004);9:33–41. doi: 10.1016/j.tplants.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Perbal M.C., Haughn G., Saedler H., Schwarz-Sommer Z. Non-cell-autonomous function of the Antirrhinum floral homeotic proteins DEFICIENS and GLOBOSA is exerted by their polar cell-to-cell trafficking. Development. (1996);122:3433–3441. doi: 10.1242/dev.122.11.3433. [DOI] [PubMed] [Google Scholar]
- 35.Ptashne M., Gann A. Transcriptional activation by recruitment. Nature. (1997);386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- 36.Rim Y., Jung J.H., Chu H., Cho W.K., Kim S.W., Hong J.C., Jackson D., Datla R., Kim J.Y. A non-cell-autonomous mechanism for the control of plant architecture and epidermal differentiation involves intercellular trafficking of BREVIPEDICELLUS protein. Funct. Plant Biol. (2009);36:280–289. doi: 10.1071/FP08243. [DOI] [PubMed] [Google Scholar]
- 37.Schüller C., Ruis H. Regulated nuclear transport. Results Probl. Cell Differ. (2002);35:169–189. doi: 10.1007/978-3-540-44603-3_9. [DOI] [PubMed] [Google Scholar]
- 38.Sessions A., Yanofsky M.F., Weigel D. Cell-cell signaling and movement by the floral transcription factors LEAFY and APETALA1. Science. (2000);289:779–781. doi: 10.1126/science.289.5480.779. [DOI] [PubMed] [Google Scholar]
- 39.Sommer P., Nehrbass U. Quality control of messenger ribonucleoprotein particles in the nucleus and at the pore. Curr. Opin. Cell Biol. (2005);17:294–301. doi: 10.1016/j.ceb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Sommer P., Nehrbass U. Erratum: quality control of messenger ribonucleoprotein particles in the nucleus and at the pore. Curr. Opin. Cell Biol. (2006);18:125. doi: 10.1016/j.ceb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Urbanus S.L., Martinelli A.P., Dinh Q.D., Aizza L.C.B., Dornelas M.C., Angenent G.C., Immink R.G.H. Intercellular transport of epidermis-expressed MADS domain transcription factors and their effect on plant morphology and floral transition. Plant J. (2010);63:60–72. doi: 10.1111/j.1365-313X.2010.04221.x. [DOI] [PubMed] [Google Scholar]
- 42.Vollbrecht E., Veit B., Sinha N., Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. (1991);350:241–243. doi: 10.1038/350241a0. [DOI] [PubMed] [Google Scholar]
- 43.Wada T., Kurata T., Tominaga R., Koshino-Kimura Y., Tachibana T., Goto K., Marks M.D., Shimura Y., Okada K. Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development. (2002);129:5409–5419. doi: 10.1242/dev.00111. [DOI] [PubMed] [Google Scholar]
- 44.Wienholds E., Plasterk R.H.A. MicroRNA function in animal development. FEBS Lett. (2005);579:5911–5922. doi: 10.1016/j.febslet.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 45.Wu X., Dinneny J.R., Crawford K.M., Rhee Y., Citovsky V., Zambryski P.C., Weigel D. Modes of intercellular transcription factor movement in the Arabidopsis apex. Development. (2003);130:3735–3745. doi: 10.1242/dev.00577. [DOI] [PubMed] [Google Scholar]
- 46.Xu X.M., Wang J., Xuan Z., Goldshmidt A., Borrill P.G., Hariharan N., Kim J.Y., Jackson D. Chaperonins facilitate KNOTTED1 cell-to-cell trafficking and stem cell function. Science. (2011);333:1141–1144. doi: 10.1126/science.1205727. [DOI] [PubMed] [Google Scholar]
- 47.Zambryski P., Crawford K. Plasmodesmata: gatekeepers for cell-to-cell transport of developmental signals in plants. Annu. Rev. Cell Dev. Biol. (2000);16:393–421. doi: 10.1146/annurev.cellbio.16.1.393. [DOI] [PubMed] [Google Scholar]


