Abstract
Jolkinolide B, a bioactive diterpene isolated from the roots of Euphorbia fischeriana Steud, is known to induce apoptosis in cancer cells. However, the molecular mechanism of its anti-cancer activity has not been fully elucidated. In the present study, we found that Jolkinolide B reduced cell viability and induced apoptosis in a dose- and timedependent manner in human leukemic U937. The induction of apoptosis was also accompanied by the downregulation of PI3K/Akt and the inhibitor of apoptosis protein (IAP) family proteins. Moreover, we observed that Jolkinolide B treatment resulted in activation of caspase-3 and -9, which may partly explain the anti-cancer activity of Jolkinolide B. Taken together, our study for the first time suggest that Jolkinolide B is able to enhance apoptosis of U937 cells, at least in part, through downregulation of PI3K/Akt and IAP family proteins. Moreover, triggering of caspase-3 and -9 activation mediated apoptotic induction.
Keywords: apoptosis, caspase, Euphorbia fischeriana Steud, Jolkinolide B, U937
INTRODUCTION
Medicinal plants are widely used in the treatment of various cancers in many Asian countries (Xie et al., 2009). Euphorbia fischeriana Steud (Euphorbiaceae) is a perennial herbaceous plant with a milky juice, distributed mainly in North China (Zhou et al., 2003). Euphorbia fischeriana Steud has long been used as a folk medicine to treat cancer in China. Early chemical investigation showed that the plant contains diterpenoids, triterpenoids and steroids including two ent-abietane diterpenoids, jolkinolide A and B, which exhibit significant antitumour activities against several tumour lines such as Sarcoma 180 and Ehrlich ascites carcinoma in mice (Wang et al., 2009). Previous study has shown that Jolkinolide B from the roots of Euphorbia fischeriana Steud could induce apoptosis in human chronic myeloid leukemia K562 cells (Luo, 2006). However, the exact mechanism and signaling pathway involved in Jolkinolide Binduced apoptosis have not been fully elucidated.
The regulation of apoptosis in both normal and malignant cells has become an area of extensive study in cancer research. Twenty years ago, several cell lines derived from patients with leukemia and blocked at various stages of differentiation were intensively used to study proliferation, apoptosis, and differentiation processes (Ilgar and Arican, 2009; Ovadje et al., 2011). U937 cell line is a human monocytic cell line,which derived from malignant cells obtained from the pleural effusion of a patient with histiocytic lymphoma.
The cellular decision to undergo either cell death or cell survival is a very complex process, which depends on the integration of multiple survival and death signals (Szliszka et al., 2011). Phosphatidylinositol 3-kinases (PI3K) is a family of related intracellular signal transducer enzymes that have been linked to a diversity of cellular functions, including cell growth, proliferation, differentiation, motility, survival and intracellular trafficking (Guan et al., 2009). Active Akt inhibits apoptosis by regulating the expression of Bcl-2 and Bax (Lu et al., 2011). In addition to Akt, inhibitor of apoptosis protein (IAP) families of proteins also plays a critical role. X-linked inhibitor of apoptosis protein (XIAP) belongs to a group of inhibitors of apoptosis that block the activation of specific caspases and prevent caspasemediated cell degradation (Wu et al., 2005). Specifically, XIAP inhibits caspase 3, 7 and 9 and therefore blocks both intrinsic and extrinsic apoptotic pathways (Kosarac et al., 2011). The inhibitor of apoptosis (IAP) family of proteins is potent natural factors that function by directly inhibiting the activity of caspase, the principal effectors of apoptosis (Turner et al., 2007).
In this study, we have evaluated the antitumor potential of Jolkinolide B from the roots of Euphorbia fischeriana Steud in U937 cells. Aimed to clarify the mechanisms underlying Jolkinolide B cell growth inhibition activity, we analyzed the effect of the drug on cell death and apoptosis. The contribution of caspase, PI3K/AKT pathway and XIAP families in the Jolkinolide B from the roots of Euphorbia fischeriana Steud-induced cell death was also investigated.
MATERIALS AND METHODS
Reagents
Monoclonal anti-β-actin antibodies were purchased from Santa Cruz Biotechnology, Inc. (USA). Anti-Phospho-specific Akt and Akt; Anti-XIAP; Anti-cIAP1; Anti-cIAP2; Anti-Smac; Anti-survivn (New England Biolabs, USA); Stocks of the selective PI3K/Akt inhibitor LY294002; stocks of the selective XIAP inhibitor Embelin (Calbio-chem-Behring, USA). RPMI-1640 and fatal bovine serum (FBS) were purchased from Gibco BIL Company (Gibco, USA). An annexin V apoptosis detection kit was purchased from R&D Systems (UK). Cell isolation and tissue culture reagents were obtained from Invitrogen life Technologies (Sweden). All other reagents were obtained from Sigma-Aldrich (USA).
Cell culture
U937 cells obtained from Sun Yat-sen University were used in all experiments, and they were grown in RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutanine, 100 U/ml penicillin, and 100 g/ml streptomycin (RPMI medium) incubating at 37℃ in a 5% CO2, 95% air humidified atmosphere. The exponentially growing U937 cells appeared large, round, and single cells. Cell counts were determined by hemacytometer and the cells density were modified as 5 × 105/ml, 5 × 105 cells were inoculated in each well in the 24-well culture plate. The cells were collected at different treated-time points. Before induction with Jolkinolide B, the cells were washed once with PBS to remove dead cells, and incubated in tissue-culture plates for 30 min at 37℃. U937 cells in 24-well flat-bottomed plates were incubated with Jolkinolide B at different concentrations (0, 25, 50, and 100 μg/ml) for 48 h or at a concentration of 50 μg/ml for 0, 24, 48, and 72 h respectively. In some experiments, 50 μM LY294002 (PI3K inhibitor) or 20 μM Embelin (XIAP inhibitor) were used at 30 min before Jolkinolide B induction.
Cell viability
To assess the overall viability of U937 cells following Jolkinolide B treatment, the cells were treated as described above. At particular time points, the U937 cells were washed two times with PBS and treated with a 0.4% solution of trypan blue and be visualized as clear cells under the microscope. U937 cells that are no longer viable, which have damaged membranes that allow entry of the dye, stain blue. Assays were performed in triplicate and repeated at least three times. The number of intact viable cells was expressed as a percentage of total cells and was assessed at different time points. Calculated the percentage of viable cells as follows: viable cells (%) = (total number of viable cells per ml of aliquot/total number of cells per ml of aliquot) × 100.
Hoechst 33258 staining
The cells were stained with Hoechst 33258 (Molecular Probes Inc., USA) with a dilution of 1:600 (stock solution: 1 mg/ml) for 5 min in dark. The samples were observed under a fluorescence microscope. The 500 cells were counted from each coverslip in turns, and the results were confirmed by visualizing the apoptotic nuclei. There were five coverslips in each group.
Flow cytometry analysis
U937 apoptosis was quantified by flow cytometry using FITCconjugated annexin V and PI. Specific binding of annexin V was achieved by incubating 106 cells in 60 μl of binding buffer saturated with annexin V for 15 min at 4℃ in the dark. To discriminate between early apoptosis and necrosis, the cells were simultaneously stained with annexin V and PI before analysis. The binding of annexin V-FITC and PI to the cells was measured by flow cytometry (FACS Calibur, BD Biosciences) using CellQuest software. At least 10 000 cells were counted in each sample. Experiments were performed and interpreted as follows: cells that were Annexin V (-)/ PI (-) (lower left quadrant) were considered as living cells, the Annexin V (+)/ PI (-) cells (lower right quadrant) as apoptotic cells, Annexin V (+)/ PI (+) (upper right quadrant) as necrotic or advanced apoptotic cells, and Annexin V (-) / PI (+) (upper left quadrant) may be bare nuclei, cells in late necrosis, or cellular debris.
Western blot analysis
U937 cells were treated with 50 μg/ml Jolkinolide B for 0, 24, 48, or 72 h respectively, and then cells were washed once with icecold phosphate buffered saline containing 1 mM Na2VO4 and extracted with lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM EDTA, 5% Glycerol, 1% Triton X-100, 25 mM NaF, 2 mM Na2VO4, 10 μg/ml of each aprotinin, leupeptin and pepstatin). The preparation of cytoplasmic was conducted using the NEPER cytoplasmic extraction reagents (Pierce). The cell lysates were frozen and thawed three times and were further centrifuged at 14,000 × g for 10 min at 4℃ to pellet insoluble material. The supernatant of cell extracts was analyzed for protein concentration by a DC protein assay kit based on the Lowry method (Bio-Rad, USA). Equal amounts of protein (50 μg) from each sample were separated on 10% sodium dodecyl sulfatepolyacrylamide gels and transferred to PVDF membranes (MSI, USA). Membranes were blocked in 5% nonfat dry milk in Trisbuffered saline containing 0.05% Tween-20 (TBST) and then incubated with rabbit polyclonal for Phospho-specific Akt and Akt (1:2000 dilution); XIAP (1:2000 dilution); cIAP1 (1:2000 dilution); cIAP2 (1:2000 dilution); Smac (1:2000 dilution) and Survivin (1:2000 dilution). The β-actin (1:2000) was used to control for equal protein loading. The immunoblots were then washed three times with TBS-T buffer, incubated with a horseradish peroxidaseconjugated secondary antibody (goat antirabbit IgM, USA), and developed using chemiluminescent substrate (Pierce, USA).
Measurement of caspase-3 and -9 activity
U937 Cells were harvested and centrifuged at 1500 rpm for 10 min. Cells were washed two times with PBS (pH 7.4) and then resuspended with 50 μl lysis buffer at 4℃ and incubated on ice for 10 min. All subsequent steps were performed on ice. After centrifugation, cell extracts were transferred to fresh tubes, and protein concentrations were measured. Each 50 μl cell extract containing 100 μg of protein were combined with equal volumes of 2 × reaction buffer in a microplate followed by the addition of 5 μl of peptide substrates of caspase-3 and -9. After overnight incubation in dark at 37℃, samples were read in a microplate reader at 405 nm. Caspase-3 and -9 activity were evaluated by the absorbance ratio of treated/control samples. In some experiments, caspase-3 (Z-DEVD-FMK) or caspase-9 inhibitor (Z-LEHD-FMK) were added into fresh medium of U937 cells at 1 h before Jolkinolide B was added.
Real-time reverse transcription (RT)-PCR
Total RNA was extracted from U937 using Trizol reagent (Invitrogen), treated with DNase I (Ambion) to remove potential genomic DNA contamination and purified using RNeasy Mini Kit (Qiagen). Total RNA concentration was measured and the purity of the samples was estimated by the OD ratios (A260/A280, ranging within 1.8-2.2). cDNA was synthesized from 2 μg of DNA-free total RNA in a 25 μl reaction volume using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega, USA). cDNA samples were diluted 10-fold for real-time PCR reactions. Gene-specific transcription levels were determined in a 20 μl reaction volume in duplicate using SYBR Green and an ABI 7500 real-time PCR system (Applied BioSystems). The sense primer for XIAP is 5′ AAATTGGGAAC CTTGTGATCGT 3′, and the antisence primer is 5′ GGCCCAAAACAAAGA AGCAA 3′; the sence primer for cIAP2 (James et al., 2006) is 5′ CCTCCTGGGTTGAAGCA 3′ and the antisence primer is 5′ GACTCAGTTCTTGTGTGGA 3′; the sence primer for Smac is GACCATGGCACAAAACTGTGA; the antisence primer AAG ACACTGCTCTCCTCATCAATG; GAPDH was chosen as internal controls. The sense primer for GAPDH is 5′ ACCCACT CCTCCACCTTTGA 3′; and the antisence primer is 5′ TGT TGCTGTAGCCAAATTCGTT 3′ (James et al., 2006). Real- time PCR products were analyzed on agarose gel electrophoresis and were verified by DNA sequencing.
Statistical analysis
Each experiment was carried out in duplicate or triplicate and three or four independent experiments were performed. Results are expressed as means ± standard deviation (SD) and analyzed with SPSS 11.5 software. Results were compared using analysis of variance (ANOVA). When ANOVA showed a statistically significant difference, a group-by-group comparison was performed using a t-test with Tukey’s correction for multiple comparisons. Statistical significance was set at P < 0.05.
RESULTS
Jolkinolide B induces apoptosis in human U937 cells
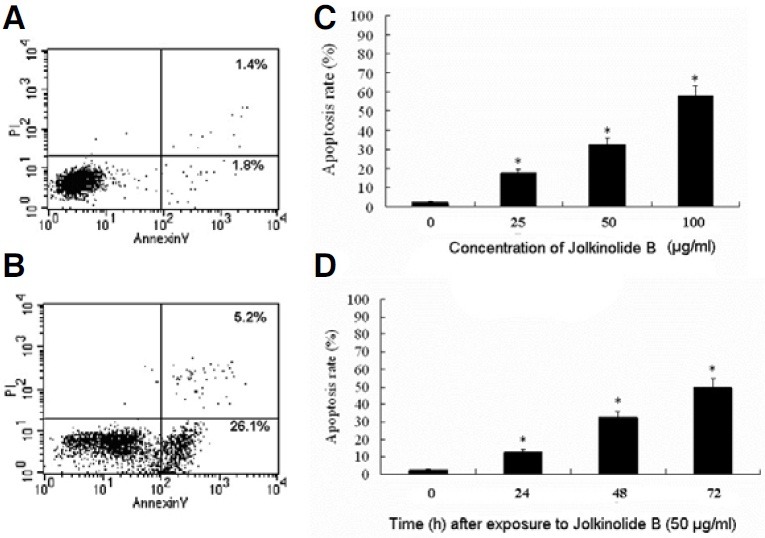
We determined that Jolkinolide B can induce apoptosis in U937 cells. In order to detect the U937 viability, we performed a trypan blue exclusion assay. Trypan blue stainings showed that 98.01% ± 1.74% of the U937 cells incubated with medium retained an integrated cell membrane (i.e., resisted trypan blue staining). The percentage of cells viability was decreased with the increasing of time and the concentrations of Jolkinolide B (Fig. 1).
Fig. 1. Effect of Jolkinolide B on U937 cells. U937 were incubated with different concentrations of Jolkinolide B for 0, 24, 48 and 72 h respectively. Viability of U937 was determined by trypan blue assay as described in “Materials and Methods”.

Microscopy of Jolkinolide B-inducted U937 cells revealed morphological changes (Fig. 2B) compared to the control (Fig. 2A). Apoptotic cells were characterized by membrane blebbing and nuclear condensation, while necrotic cells were typically larger and lighter with plasma membrane lesions. The percentage of apoptotic cells was calculated through observing 500 cells (Fig. 2C).
Fig. 2. Morphology of U937 cells undergoing apoptosis. U937 were cultured with (B) or without (A) 50 μg/ml Jolkinolide B and then cells were stained with Hoechst 33258 staining. 500 cells were counted from each coverslip in turns. The percentage of apoptotic cells was calculated through observing 500 cells (C). **P < 0.01.

Flow cytometry using FITC-conjugated annexin V revealed that U937 cells exposed to Jolkinolide B underwent rapid apoptosis (Fig. 3B) compared to control (Fig. 3A). This effect was positively correlated with the concentrations of Jolkinolide B (Fig. 3C) and exposure time (Fig. 3D), and excessive apoptosis was associated with loss of membrane integrity in an increased portion of U937, which indicates necrosis or late apoptosis.
Fig. 3. Jolkinolide B-induced apoptosis in U937 cells. U937 were challenged with 50 μg/ml Jolkinolide B (B) compared with that of U937 alone (A). U937 cells were harvested for 48 h with different concentration of Jolkinolide B (C) or 50 μg/ml Jolkinolide B for different time (D). After treatment, cells were incubated with FITC-conjugated annexin V (AV) and propidium iodide (PI) double staining. Flow cytometric analysis was performed, and the data shown are representative of three separate experiments. The lower right quadrants represent early apoptotic cells that were stained by annexin V but not by propidium iodide. The upper right quadrants represent late apoptotic cells that were stained by both annexin V and propidium iodide. *P < 0.01.
Involvement of PI3K in the apoptosis of U937 cells
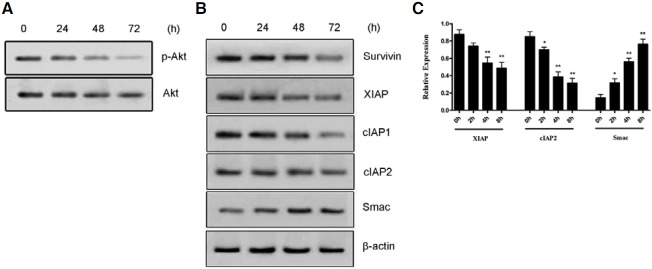
It was found that Jolkinolide B treatment significantly decreased Phospho-Akt (P-Akt) production in a time-dependent manner (Fig. 4A). These experiments support the conclusion that Jolkinolide B-induced apoptosis is mediated by P-Akt down-regulation.
Fig. 4. PI3K and XIAP family proteins are involved in U937 apoptosis induced by Jolkinolide B. The expression of phosphorylated and total Akt protein (A) or XIAP family proteins (B) in U937 challenged with 50 μg/ml Jolkinolide B for the indicated time points. The β-actin was used as a loading control. The data are from one representative experiment. Real-time RCR was used to detect the mRNA expression of the target genes (C). *P < 0.05, **P < 0.01.
Jolkinolide B induces U937 apoptotic cell death via modulation of XIAP family proteins
In order to discuss the apoptosis mechanism of U937 induced by Jolkinolide B, we examined the anti-apoptotic proteins expression. Members of the mammalian IAP family mainly include: XIAP, cIAP-1, cIAP-2. The results showed that the transcription and expressions of cIAP1/2, XIAP and survivin were decreased in a time-dependent manner after challenged with Jolkinolide B (Figs. 4B and 4C). However, the transcription and expressions of Smac (second mitochondria derived activator of caspase), which is an intrinsic antagonist of XIAP, was increased in a time-dependent manner (Figs. 4B and 4C). These results suggest that the changes in expression of cIAP1/2, Survivin, Smac and XIAP may contribute to Jolkinolide B-induced apoptogenesis in U937 cells.
Effects of inhibitors of Akt or XIAP on Jolkinolide B-induced U937 apoptosis
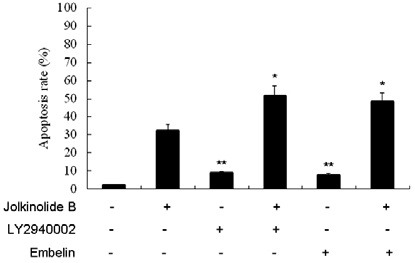
To identify the relevance of Akt and XIAP signaling pathways in controlling the apoptotic cell death by Jolkinolide B, inhibition assays were performed with LY294002 (a specific inhibitor of PI3K) or Embelin (a specific inhibitor of XIAP). The percentage of apoptosis was determined by flow cytometery. U937 cells were pretreated with 50 μM LY294002 or 20 μM Embelin for 30 min, and then cultured with 50 μg /ml Jolkinolide B for 48 h. The results showed that LY294002 or Embelin significantly increased the apoptosis rate (Fig. 5).
Fig. 5. Effect of PI3K or XIAP inhibitors on Jolkinolide B-induced U937 apoptosis. U937 were treated with 50 μg/ml Jolkinolide B and incubated for 48 h with the indicated concentrations of PI3K and XIAP inhibitors: 50 μM LY294002 or 20 μM Embelin for 30 min. After treatment, cells were incubated with FITC-conjugated annexin V (AV) and PI double staining. Flow cytometric analysis was performed. Values represent means ± SD of five experiments performed in duplicate. *P < 0.05, **P < 0.001 compared with that of Jolkinolide B alone.
Expression of caspase-3 and -9 activity
The expression of caspase-3 and -9 activity in U937 cells incubated in the presense of Jolkinolide B is presented in Fig. 6. Treatment of U937 cells with Jolkinolide B for 48 h at concentrations of 0, 25, 50, and 100 μg/ml respectively (Fig. 6A) or for different hours at a concentration of 50 μg/ml (Fig. 6B) showed marked increase of caspase-3 and -9 activation. Activity of caspase-3 and -9 in U937 cells with Jolkinolide B treatment showed dose- and time-dependent up-regulation. Inhibition of PI3K pathway with LY294002 or XIAP with Embelin potentiated the Jolkinolide B-induced caspase-3 and -9 activity (Fig. 6C). To characterize the pathway of apoptosis execution, experiments were carried out using the caspase inhibitor Z-DEVDFMK (specific for caspase-3) or Z-LEHD-FMK (specific for caspase- 9). Apoptosis was greatly decreased by Z-DEVD and ZLEHD- FMK (Fig. 7). Whereas, caspase inhibitors had no effect on PI3K, XIAP and Smac activation in U937 and THP-1 cells (Data not shown). Together, our data demonstrated that caspase- 3 and -9 mediates Jolkinolide B-induced U937 apoptosis.
Fig. 6. Effect of Jolkinolide B on the activity of caspase-3 and -9 activity of U937. (A) Dose-dependency of Jolkinolide B-induced caspase-3 and -9 activity; (B) Time-dependency of Jolkinolide B-induced caspase-3 and -9 activity; (C) U937 cells were treated with 50 μg/ml Jolkinolide B and incubated for 48 h, with the indicated concentrations of LY294002 or Embelin. Values represent means ± SD of five experiments performed in duplicate (A, B) *, # P < 0.05, **, ## P < 0.001 compared with that of control; (C) *, # P < 0.05, **, ## P < 0.001 compared with that of Jolkinolide B alone.

Fig. 7. Effect of caspase-3 or -9 inhibitors on Jolkinolide B-induced U937 apoptosis.U937 cells were pretreated with caspase-3 or -9 inhibitors ZDEVD- FMK or Z-LEHD-FMK for 1 h. U937 cells were harvested for 48 h at a concentration of 50 μg/ml after Jolkinolide B induction and incubated with FITC-conjugated annexin V (AV) and PI double staining. Flow cytometric analysis was performed as described in the materials and methods section. Values represent means ± SD of five experiments performed in duplicate. *P < 0.05 compared with that of Jolkinolide B alone.

DISCUSSION
Previous studies have demonstrated that Jolkinolide B induces apoptosis in human chronic myeloid leukemia K562 cells. Jolkinolide B induced tumor cells apoptosis via multiple signaling pathways. The 17-Acetoxyjolkinolide B is a novel type NF-κB pathway inhibitor. It irreversibly inhibits IκB kinase and induces apoptosis of tumor cells (Yan et al., 2008). Furthermore, 17- Hydroxy-jolkinolide B strongly inhibits IL-6-induced as well as constitutive STAT3 activation, which inhibits growth and induces apoptosis of tumor cells (Wang et al., 2009). In the current study, we investigated the in vitro effects of Jolkinolide B on cell growth and death in U937 cells, human histiocytic lymphoma cell lines, and examined the mechanisms underlying its actions. To the best of our knowledge, this study for the first time demonstrated that Jolkinolide B induced U937 cell apoptosis in a time- and dose-dependent manner.
Apoptosis is an important defense mechanism that plays a key role in cancer prevention and treatment (Kim and Hong, 2011). Apoptosis signaling is regulated by various pro- and antiapoptotic proteins (Ahn et al., 2010). Akt promotes cell survival by inhibiting apoptosis, and its phosphorylation has been considered a critical factor in the aggressiveness of cancer (Lee et al., 2008). Although the precise anti-apoptotic effects of Akt are still unclear, Akt directly phosphorylates and inactives procaspase- 9 and blocks caspase-9-mediated apoptosis. Therefore, we investigated whether Jolkinolide B induces downregulation of PI3K/Akt and whether pretreatment with LY294002 could enhance Jolkinolide B-induced apoptosis in U937 cells. The data presented here suggest that Jolkinolide B treatment resulted in a dose- and time-dependent decrease in the level of phosphorylated Akt in U937 cells, contributing to the promotion of apoptosis. Specific inhibition of abnormally expressed or activated kinases is a popular strategy for the development of anticancer drugs (Dan et al., 2004). In the present study, we have also found that the pharmacological inhibitor of PI3K, LY294002, dramatically exert caspase-3 and -9 activity under Jolkinolide B treatment condition.
Another family of apoptosis-regulatory proteins, inhibitors of apoptosis proteins (IAPs), are recently considered a valuable target to modulate apoptotic cell death in many cancer cells (Ahn et al., 2010). Members of the mammalian IAP family include: XIAP, cIAP-1, cIAP-2, and others, which directly inhibit caspase-3, caspase -7, and caspase-9 (Roy et al., 1997). The main antagonist of XIAP is Smac. Dimeric Smac sterically and/or competitively occludes the caspase 3, 7 and 9 binding sites of XIAP and thereby can pave the way for efficient cell death execution (Hao et al., 2004; Wu et al., 2000). Smac also binds to other IAPs, such as livin/ML-IAP, Bruce/Apollon, cIAPs 1 and 2 (Bartke et al., 2004; Vucic et al., 2002), which interfere with caspase-mediated cell death and/or have additional roles in pro-survival and proliferation signalling. Some studies have shown that XIAP is one of the target molecules in the PI3K/Akt pathway. Nevertheless, it is not currently known whether Jolkinolide B-induced apoptosis is related to alteration of the IAP family proteins. In this study, there were a tendency of alterations with a decreased expression level of cIAP1/2, Survivin and XIAP, and also with an increased expression level of Smac and activation of caspase-3 and -9.
During apoptosis, a class of proteases, called caspases (cysteineaspartate- specific proteases), is activated. Caspases are an evolutionarily conserved family of cell death proteases, and their activation during apoptosis is an important underlying theme in cancer therapy (Nuñez et al., 1998). Caspase-3 in particular, when activated, has many cellular targets that, when served and/or activated, produce the morphologic features of apoptosis (Cohen, 1997). Recent studies have revealed that the modulation of caspases is complex process and involves a number of regulatory proteins, including PI3K and IAP family proteins (Chauhan et al., 2007; Roccaro et al., 2010). Study of caspases, which are also important regulators of apoptosis, revealed that exposure of U937 cells to Jolkinolide B increased caspase-3 and -9 activity. To more directly link PI3K/Akt and XIAP signaling pathway with caspase-3 activation, we examined Jolkinolide B-mediated caspase-3 and -9 activation in cells treated with PI3K/Akt or XIAP inhibitors LY294002 or Embelin. Inhibition of PI3K/Akt or XIAP significantly increased caspase-3 and -9 activitiy. In our studies, pretreatment with caspase-3 or caspase-9 specific inhibitor significantly decreased apoptosis rate. These results suggest that in Jolkinolide B-treated U937 cells, LY294002 or Embelin induced apoptosis, which are dependent on the activation of caspase-3 and -9.
In conclusion, data presented here support the hypothesis that Jolkinolide B are potential and selective anti-human monocytic leukemia agents by modulating apoptosis, proliferation and drug resistance in leukemia cells. Further studies should test the usefulness (efficacy and toxicity) of these compounds in other cell lines.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China [81101224].
References
- 1.Ahn Q., Jeong S.J., Lee H.J., Kwon H.Y., Han I., Kim H.S., Lee H.J., Lee E.O., Ahn K.S., Jung M.H., et al. Inhibition ocyclooxygenase-2-dependent survivin mediates decursin-induced apoptosis in human KBM-5 myeloid leukemia cells. Cancer Lett. (2010);298:212–221. doi: 10.1016/j.canlet.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartke T., Pohl C., Pyrowolakis G., Jentsch S. Dual role of BRUCE as an antiapoptotic IAP and a chimeric E2/E3 ubiquitin ligase. Mol. Cell. (2004);14:801–811. doi: 10.1016/j.molcel.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan D., Neri P., Velankar M., Podar K., Hideshima T., Fulciniti M., Tassone P., Raje N., Mitsiades C., Mitsiades N., et al. Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM). Blood. (2007);109:1220–1227. doi: 10.1182/blood-2006-04-015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen G.M. Caspases: the executioners of apoptosis. Biochem. J. (1997);326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dan H.C., Sun M., Kaneko S., Feldman R.I., Nicosia S.V., Wang H.G., Tsang B.K., Cheng J.Q. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J. Biol. Chem. (2004);279:5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 6.Guan S.M., Zhang M., He J.J., Wu J.Z., Guan S.M., Zhang M., He J.J., Wu J.Z. Mitogen-activated protein kinases and phosphatidylinositol 3-kinase are involved in Prevotella intermedia- induced proin.ammatory cytokines expression in human periodontal ligament cells. Biochem. Biophys. Res. Commun. (2009);386:471–476. doi: 10.1016/j.bbrc.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 7.Hao Y., Sekine K., Kawabata A., Nakamura H., Ishioka T., Ohata H., Katayama R., Hashimoto C., Zhang X., Noda T., et al. Apollon ubiquitinates SMAC and caspase-9, and has an essential cytoprotection function. Nat. Cell. Biol. (2004);6:849–860. doi: 10.1038/ncb1159. [DOI] [PubMed] [Google Scholar]
- 8.Ilgar N.N., Arican G.O. Induction of apoptosis and cell proliferation inhibition by paclitaxel in FM3A cell cultures. Afr. J. Biotechnol. (2009);8:547–555. [Google Scholar]
- 9.James M.A., Lee J.H., Klingelhutz A.J. Human papillomavirus type 16 E6 activates NF-kappaB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motifdependent manner. J. Virol. (2006);80:5301–5307. doi: 10.1128/JVI.01942-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim B.M., Hong S.H. Sequential caspase-2 and caspase- 8 activation is essential for saikosaponin a-induced apoptosis of human colon carcinoma cell lines. Apoptosis. (2011);16:184–197. doi: 10.1007/s10495-010-0557-x. [DOI] [PubMed] [Google Scholar]
- 11.Kosarac O., Takei H., Zhai Q.J., Schwartz M.R., Mody D.R. S100P and XIAP expression in pancreatic ductal adenocarcinoma: potential novel biomarkers as a diagnostic adjunct to fine needle aspiration cytology. Acta Cytol. (2011);55:142–148. doi: 10.1159/000320913. [DOI] [PubMed] [Google Scholar]
- 12.Lee D.H., Szczepanski M., Lee Y.J., Lee D.H., Szczepanski M., Lee Y.J. Role of Bax in quercetin-induced apoptosis in human prostate cancer cells. Biochem. Pharmacol. (2008);75:2345–2355. doi: 10.1016/j.bcp.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu S., Lu C., Han Q., Li J., Du Z., Liao L., Zhao R.C. Adipose-derived mesenchymal stem cells protect PC12 cells from glutamate excitotoxicity-induced apoptosis by upregulation of XIAP through PI3-K/Akt activation. Toxicology. (2011);279:189–195. doi: 10.1016/j.tox.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Luo H., Wang A. Induction of apoptosis in K562 cells by jolkinolide B. Can. J. Physiol. Pharmacol. (2006);84:959–965. doi: 10.1139/y06-045. [DOI] [PubMed] [Google Scholar]
- 15.Nuñez G., Benedict M.A., Hu Y., Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. (1998);17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 16.Ovadje P., Chatterjee S., Griffin C., Tran C., Hamm C., Pandey S. Selective induction of apoptosis through activation of caspase-8 in human leukemia cells (Jurkat) by dandelion root extract. J. Ethnopharmacol. (2011);133:86–91. doi: 10.1016/j.jep.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Roccaro A.M., Sacco A., Husu E.N., Pitsillides C., Vesole S., Azab A.K., Azab F., Melhem M., Ngo H.T., Quang P., et al. Dual targeting of the PI3K/Akt/mTOR pathway as an antitumor strategy in Waldenstrom macroglobulinemia. Blood. (2010);115:559–569. doi: 10.1182/blood-2009-07-235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy N., Deveraux Q.L., Takahashi R., Salvesen G.S., Reed J.C., Roy N., Deveraux Q.L., Takahashi R., Salvesen G.S., Reed J.C. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. (1997);16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szliszka E., Zydowicz G., Janoszka B., Dobosz C., Kowalczyk- Ziomek G., Krol W. Ethanolic extract of Brazilian green propolis sensitizes prostate cancer cells to TRAIL-induced apoptosis. Int. J. Oncol. (2011);38:941–953. doi: 10.3892/ijo.2011.930. [DOI] [PubMed] [Google Scholar]
- 20.Turner D.J., Alaish S.M., Zou T., Rao J.N., Wang J.Y., Strauch E.D. Bile salts induce resistance to apoptosis through NF-κB-mediated XIAP expression. Ann. Surg. (2007);245:415–425. doi: 10.1097/01.sla.0000236631.72698.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vucic D., Deshayes K., Ackerly H., Pisabarro M.T., Kadkhodayan S., Fairbrother W.J., Dixit V.M. SMAC negatively regulates the anti-apoptotic activity of melanoma inhibitor of apoptosis (ML-IAP). J. Biol. Chem. (2002);277:12275–12279. doi: 10.1074/jbc.M112045200. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Ma X., Yan S., Shen S., Zhu H., Gu Y., Wang H., Qin G., Yu Q. 17-Hydroxy-jolkinolide B inhibits signal transducers and activators of transcription signaling by covalently cross-Linking Janus kinases and induces apoptosis of human cancer cells. Cancer Res. (2009);69:7302–7310. doi: 10.1158/0008-5472.CAN-09-0462. [DOI] [PubMed] [Google Scholar]
- 23.Wu G., Chai J., Suber T.L., Wu J.W., Du C., Wang X., Shi Y. Structural basis of IAP recognition by Smac/DIABLO. Nature. (2000);408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 24.Wu M., Yuan S., Szporn A.H., Gan L., Shtilbans V., Burstein D.E. Immunocytochemical detection of XAIP in body cavity effusions and washes. Mod. Pathol. (2005);18:1618–1622. doi: 10.1038/modpathol.3800478. [DOI] [PubMed] [Google Scholar]
- 25.Xie H., Qin Y.X., Zhou Y.L., Tong L.J., Lin L.P., Geng M.Y., Duan W.H., Ding J. GA3, a new gambogic acid derivative, exhibits potent antitumor activities in vitro via apoptosisinvolved mechanisms. Acta Pharmacol. Sin. (2009);30:346–354. doi: 10.1038/aps.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan S.S., Li Y., Wang Y., Shen S.S., Gu Y., Wang H.B., Qin G.W., Yu Q. 17-Acetoxyjolkinolide B irreversibly inhibits IkappaB kinase and induces apoptosis of tumor cells. Mol. Cancer Ther. (2008);7:1523–1532. doi: 10.1158/1535-7163.MCT-08-0263. [DOI] [PubMed] [Google Scholar]
- 27.Zhou T.X., Bao G.H., Ma Q.G., Qin G.W., Che C.T., Lv Y., Wang C., Zheng Q.T. Langduin C, a novel dimeric diterpenoid from the roots of Euphorbia fischeriana. Tetrahedron. Lett. (2003);44:135–137. [Google Scholar]


